Sex Hormones as Key Modulators of the Immune Response in Multiple Sclerosis: A Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
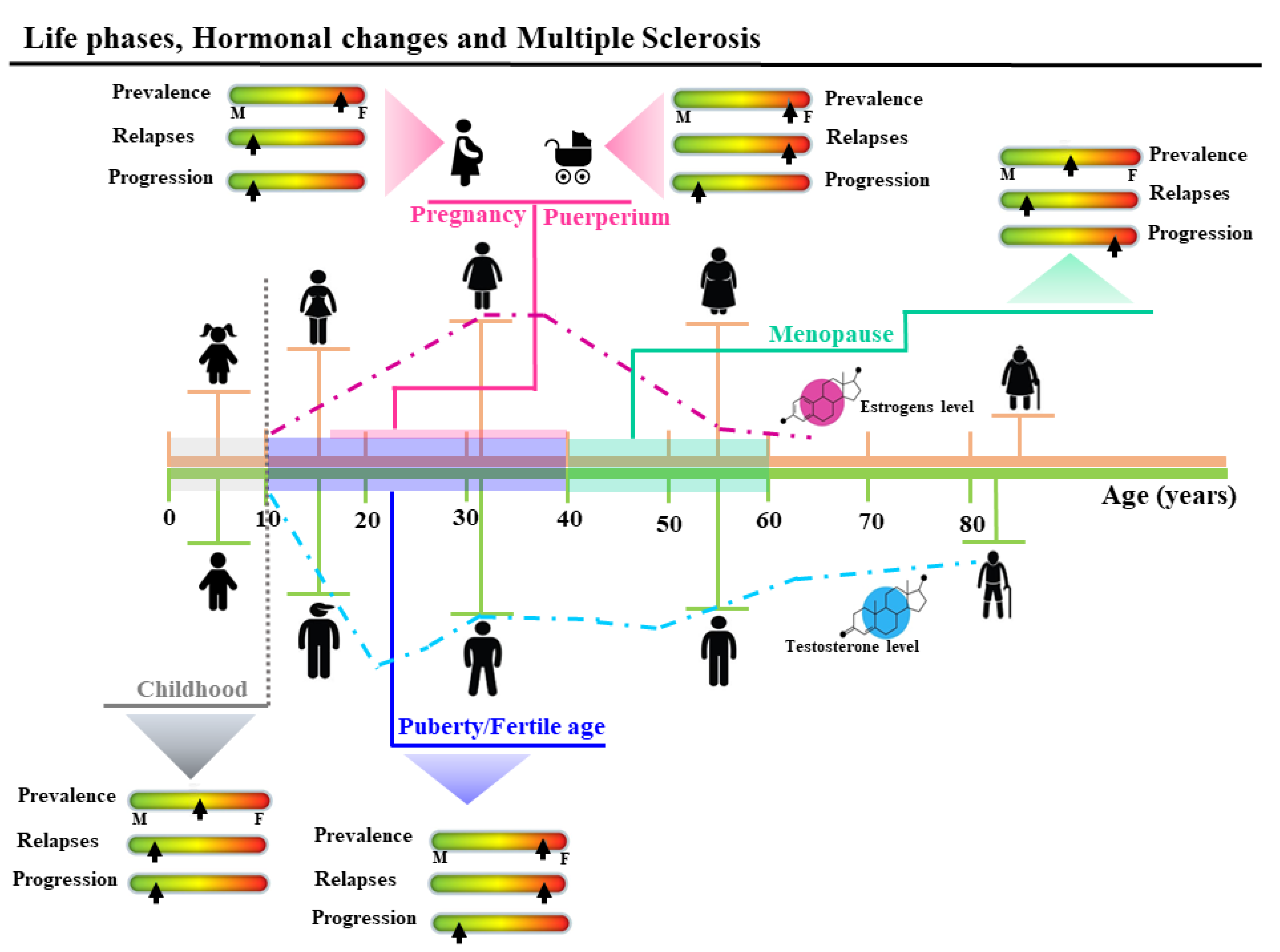
3.1. Gender and Multiple Sclerosis
3.2. Clinical Trials with the Use of Testosterone
3.3. Women’s Life Reproductive Phases and MS
3.3.1. Puberty
3.3.2. Pregnancy and Post-Partum
3.3.3. Menopause
3.4. Clinical Trials with the Use of Estrogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garg, N.; Smith, T.W. An Update on Immunopathogenesis, Diagnosis, and Treatment of Multiple Sclerosis. Brain Behav. 2015, 5, e00362. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.; Trevick, S.; Younger, D.S. Epidemiology of Multiple Sclerosis. Neurol. Clin. 2016, 34, 919–939. [Google Scholar] [CrossRef] [PubMed]
- Gonsette, R.E. Self-Tolerance in Multiple Sclerosis. Acta Neurol. Belg. 2012, 112, 133–140. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Ysrraelit, M.C.; Correale, J. Impact of Sex Hormones on Immune Function and Multiple Sclerosis Development. Immunology 2019, 156, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Fish, E.N. The X-Files in Immunity: Sex-Based Differences Predispose Immune Responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Berthenet, K.; Garlanda, C. Sexual Dimorphism in Innate Immunity. Clin. Rev. Allergy Immunol. 2019, 56, 308–321. [Google Scholar] [CrossRef]
- Kovats, S. Estrogen Receptors Regulate Innate Immune Cells and Signaling Pathways. Cell Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef]
- Sicotte, N.L.; Giesser, B.S.; Tandon, V.; Klutch, R.; Steiner, B.; Drain, A.E.; Shattuck, D.W.; Hull, L.; Wang, H.-J.; Elashoff, R.M.; et al. Testosterone Treatment in Multiple Sclerosis: A Pilot Study. Arch. Neurol. 2007, 64, 683–688. [Google Scholar] [CrossRef]
- Gold, S.M.; Chalifoux, S.; Giesser, B.S.; Voskuhl, R.R. Immune Modulation and Increased Neurotrophic Factor Production in Multiple Sclerosis Patients Treated with Testosterone. J. Neuroinflammation 2008, 5, 32. [Google Scholar] [CrossRef]
- Bove, R.; Musallam, A.; Healy, B.C.; Raghavan, K.; Glanz, B.I.; Bakshi, R.; Weiner, H.; De Jager, P.L.; Miller, K.K.; Chitnis, T. Low Testosterone Is Associated with Disability in Men with Multiple Sclerosis. Mult. Scler. 2014, 20, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Kurth, F.; Luders, E.; Sicotte, N.L.; Gaser, C.; Giesser, B.S.; Swerdloff, R.S.; Montag, M.J.; Voskuhl, R.R.; Mackenzie-Graham, A. Neuroprotective Effects of Testosterone Treatment in Men with Multiple Sclerosis. Neuroimage Clin. 2014, 4, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Metzger-Peter, K.; Kremer, L.D.; Edan, G.; Loureiro De Sousa, P.; Lamy, J.; Bagnard, D.; Mensah-Nyagan, A.-G.; Tricard, T.; Mathey, G.; Debouverie, M.; et al. The TOTEM RRMS(Testosterone Treatment on Neuroprotection and Myelin Repair in Relapsing Remitting Multiple Sclerosis) Trial: Study Protocol for a Randomized, Double-Blind, Placebo-Controlled Trial. Trials 2020, 21, 591. [Google Scholar] [CrossRef] [PubMed]
- Sicotte, N.L.; Liva, S.M.; Klutch, R.; Pfeiffer, P.; Bouvier, S.; Odesa, S.; Wu, T.C.J.; Voskuhl, R.R. Treatment of Multiple Sclerosis with the Pregnancy Hormone Estriol. Ann. Neurol. 2002, 52, 421–428. [Google Scholar] [CrossRef]
- Soldan, S.S.; Alvarez Retuerto, A.I.; Sicotte, N.L.; Voskuhl, R.R. Immune Modulation in Multiple Sclerosis Patients Treated with the Pregnancy Hormone Estriol. J. Immunol. 2003, 171, 6267–6274. [Google Scholar] [CrossRef]
- Gold, S.M.; Sasidhar, M.V.; Morales, L.B.; Du, S.; Sicotte, N.L.; Tiwari-Woodruff, S.K.; Voskuhl, R.R. Estrogen Treatment Decreases Matrix Metalloproteinase (MMP)-9 in Autoimmune Demyelinating Disease through Estrogen Receptor Alpha (ERalpha). Lab. Investig. 2009, 89, 1076–1083. [Google Scholar] [CrossRef]
- MacKenzie-Graham, A.; Brook, J.; Kurth, F.; Itoh, Y.; Meyer, C.; Montag, M.J.; Wang, H.-J.; Elashoff, R.; Voskuhl, R.R. Estriol-Mediated Neuroprotection in Multiple Sclerosis Localized by Voxel-Based Morphometry. Brain Behav. 2018, 8, e01086. [Google Scholar] [CrossRef]
- Pozzilli, C.; De Giglio, L.; Barletta, V.T.; Marinelli, F.; Angelis, F.D.; Gallo, V.; Pagano, V.A.; Marini, S.; Piattella, M.C.; Tomassini, V.; et al. Oral Contraceptives Combined with Interferon β in Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e120. [Google Scholar] [CrossRef]
- De Giglio, L.; Marinelli, F.; Barletta, V.T.; Pagano, V.A.; De Angelis, F.; Fanelli, F.; Petsas, N.; Pantano, P.; Tomassini, V.; Pozzilli, C. Effect on Cognition of Estroprogestins Combined with Interferon Beta in Multiple Sclerosis: Analysis of Secondary Outcomes from a Randomised Controlled Trial. CNS Drugs 2017, 31, 161–168. [Google Scholar] [CrossRef]
- Confavreux, C.; Hutchinson, M.; Hours, M.M.; Cortinovis-Tourniaire, P.; Moreau, T. Rate of Pregnancy-Related Relapse in Multiple Sclerosis. N. Engl. J. Med. 1998, 339, 285–291. [Google Scholar] [CrossRef]
- Vukusic, S.; Hutchinson, M.; Hours, M.; Moreau, T.; Cortinovis-Tourniaire, P.; Adeleine, P.; Confavreux, C.; Pregnancy In Multiple Sclerosis Group. Pregnancy and Multiple Sclerosis(the PRIMS Study): Clinical Predictors of Post-Partum Relapse. Brain 2004, 127 Pt 6, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Airas, L. Hormonal and Gender-Related Immune Changes in Multiple Sclerosis. Acta Neurol. Scand. 2015, 132, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Lorefice, L.; Fronza, M.; Fenu, G.; Frau, J.; Coghe, G.; D’Alterio, M.N.; Barracciu, M.A.; Murgia, F.; Angioni, S.; Cocco, E. Effects of Pregnancy and Breastfeeding on Clinical Outcomes and MRI Measurements of Women with Multiple Sclerosis: An Exploratory Real-World Cohort Study. Neurol. Ther. 2022, 11, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Holmqvist, P.; Wallberg, M.; Hammar, M.; Landtblom, A.-M.; Brynhildsen, J. Symptoms of Multiple Sclerosis in Women in Relation to Sex Steroid Exposure. Maturitas 2006, 54, 149–153. [Google Scholar] [CrossRef]
- Ladeira, F.; Salavisa, M.; Caetano, A.; Barbosa, R.; Sá, F.; Correia, A.S. The Influence of Menopause in Multiple Sclerosis Course: A Longitudinal Cohort Study. Eur. Neurol. 2018, 80, 223–227. [Google Scholar] [CrossRef]
- Bove, R.; Chitnis, T. The Role of Gender and Sex Hormones in Determining the Onset and Outcome of Multiple Sclerosis. Mult. Scler. 2014, 20, 520–526. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Gold, S.M. Sex-Related Factors in Multiple Sclerosis Susceptibility and Progression. Nat. Rev. Neurol. 2012, 8, 255–263. [Google Scholar] [CrossRef]
- Pozzilli, C.; Tomassini, V.; Marinelli, F.; Paolillo, A.; Gasperini, C.; Bastianello, S. “Gender Gap” in Multiple Sclerosis: Magnetic Resonance Imaging Evidence. Eur. J. Neurol. 2003, 10, 95–97. [Google Scholar] [CrossRef]
- Schoonheim, M.M.; Popescu, V.; Lopes, F.C.R.; Wiebenga, O.T.; Vrenken, H.; Douw, L.; Polman, C.H.; Geurts, J.J.G.; Barkhof, F. Subcortical Atrophy and Cognition: Sex Effects in Multiple Sclerosis. Neurology 2012, 79, 1754–1761. [Google Scholar] [CrossRef]
- Kalincik, T.; Vivek, V.; Jokubaitis, V.; Lechner-Scott, J.; Trojano, M.; Izquierdo, G.; Lugaresi, A.; Grand’maison, F.; Hupperts, R.; Oreja-Guevara, C.; et al. Sex as a Determinant of Relapse Incidence and Progressive Course of Multiple Sclerosis. Brain 2013, 136 Pt 12, 3609–3617. [Google Scholar] [CrossRef]
- Malik, M.T.; Healy, B.C.; Benson, L.A.; Kivisakk, P.; Musallam, A.; Weiner, H.L.; Chitnis, T. Factors Associated with Recovery from Acute Optic Neuritis in Patients with Multiple Sclerosis. Neurology 2014, 82, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Renoux, C.; Vukusic, S.; Mikaeloff, Y.; Edan, G.; Clanet, M.; Dubois, B.; Debouverie, M.; Brochet, B.; Lebrun-Frenay, C.; Pelletier, J.; et al. Natural History of Multiple Sclerosis with Childhood Onset. N. Engl. J. Med. 2007, 356, 2603–2613. [Google Scholar] [CrossRef] [PubMed]
- Belman, A.L.; Krupp, L.B.; Olsen, C.S.; Rose, J.W.; Aaen, G.; Benson, L.; Chitnis, T.; Gorman, M.; Graves, J.; Harris, Y.; et al. Characteristics of Children and Adolescents with Multiple Sclerosis. Pediatrics 2016, 138, e20160120. [Google Scholar] [CrossRef] [PubMed]
- Sloka, J.S.; Pryse-Phillips, W.E.; Stefanelli, M. The Relation between Menarche and the Age of First Symptoms in a Multiple Sclerosis Cohort. Mult. Scler. 2006, 12, 333–339. [Google Scholar] [CrossRef]
- Ramagopalan, S.V.; Valdar, W.; Criscuoli, M.; DeLuca, G.C.; Dyment, D.A.; Orton, S.-M.; Yee, I.M.; Ebers, G.C.; Sadovnick, A.D.; for the Canadian Collaborative Study Group. Age of Puberty and the Risk of Multiple Sclerosis: A Population Based Study. Eur. J. Neurol. 2009, 16, 342–347. [Google Scholar] [CrossRef]
- Lulu, S.; Graves, J.; Waubant, E. Menarche Increases Relapse Risk in Pediatric Multiple Sclerosis. Mult. Scler. 2016, 22, 193–200. [Google Scholar] [CrossRef]
- D’hooghe, M.B.; Haentjens, P.; Nagels, G.; D’Hooghe, T.; De Keyser, J. Menarche, Oral Contraceptives, Pregnancy and Progression of Disability in Relapsing Onset and Progressive Onset Multiple Sclerosis. J. Neurol. 2012, 259, 855–861. [Google Scholar] [CrossRef]
- Avila, M.; Bansal, A.; Culberson, J.; Peiris, A.N. The Role of Sex Hormones in Multiple Sclerosis. Eur. Neurol. 2018, 80, 93–99. [Google Scholar] [CrossRef]
- Yoshinaga, K. Review of Factors Essential for Blastocyst Implantation for Their Modulating Effects on the Maternal Immune System. Semin. Cell Dev. Biol. 2008, 19, 161–169. [Google Scholar] [CrossRef]
- Papenfuss, T.L.; Powell, N.D.; McClain, M.A.; Bedarf, A.; Singh, A.; Gienapp, I.E.; Shawler, T.; Whitacre, C.C. Estriol Generates Tolerogenic Dendritic Cells in Vivo That Protect against Autoimmunity. J. Immunol. 2011, 186, 3346–3355. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Wang, H.; Wu, T.C.J.; Sicotte, N.L.; Nakamura, K.; Kurth, F.; Itoh, N.; Bardens, J.; Bernard, J.T.; Corboy, J.R.; et al. Estriol Combined with Glatiramer Acetate for Women with Relapsing-Remitting Multiple Sclerosis: A Randomised, Placebo-Controlled, Phase 2 Trial. Lancet Neurol. 2016, 15, 35–46. [Google Scholar] [CrossRef]
- Gold, S.M.; Voskuhl, R.R. Estrogen Treatment in Multiple Sclerosis. J. Neurol. Sci. 2009, 286, 99–103. [Google Scholar] [CrossRef] [PubMed]
- McCombe, P.A.; Greer, J.M. Female Reproductive Issues in Multiple Sclerosis. Mult. Scler. 2013, 19, 392–402. [Google Scholar] [CrossRef]
- Bove, R.; Secor, E.; Healy, B.C.; Musallam, A.; Vaughan, T.; Glanz, B.I.; Greeke, E.; Weiner, H.L.; Chitnis, T.; Wicks, P.; et al. Evaluation of an Online Platform for Multiple Sclerosis Research: Patient Description, Validation of Severity Scale, and Exploration of BMI Effects on Disease Course. PLoS ONE 2013, 8, e59707. [Google Scholar] [CrossRef]
- Jacobsen, B.K.; Heuch, I.; Kvåle, G. Age at Natural Menopause and All-Cause Mortality: A 37-Year Follow-up of 19,731 Norwegian Women. Am. J. Epidemiol. 2003, 157, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Bove, R.; Chitnis, T.; Houtchens, M. Menopause in Multiple Sclerosis: Therapeutic Considerations. J. Neurol. 2014, 261, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Christianson, M.S.; Mensah, V.A.; Shen, W. Multiple Sclerosis at Menopause: Potential Neuroprotective Effects of Estrogen. Maturitas 2015, 80, 133–139. [Google Scholar] [CrossRef]
- Molnár, I.; Bohaty, I.; Somogyiné-Vári, É. High Prevalence of Increased Interleukin-17A Serum Levels in Postmenopausal Estrogen Deficiency. Menopause 2014, 21, 749–752. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Lambrinoudaki, I.; Goulis, D.G. Menopause in Women with Multiple Sclerosis: A Systematic Review. Maturitas 2020, 135, 68–73. [Google Scholar] [CrossRef]
- Bove, R.; Okai, A.; Houtchens, M.; Elias-Hamp, B.; Lugaresi, A.; Hellwig, K.; Kubala Havrdová, E. Effects of Menopause in Women With Multiple Sclerosis: An Evidence-Based Review. Front. Neurol. 2021, 12, 554375. [Google Scholar] [CrossRef]
- Musella, A.; Gentile, A.; Rizzo, F.R.; De Vito, F.; Fresegna, D.; Bullitta, S.; Vanni, V.; Guadalupi, L.; Stampanoni Bassi, M.; Buttari, F.; et al. Interplay Between Age and Neuroinflammation in Multiple Sclerosis: Effects on Motor and Cognitive Functions. Front. Aging Neurosci. 2018, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, R.L.; De Groot, C.J.; Montagne, L.; Freitag, P.; van der Valk, P.; Kappos, L.; Leppert, D. The Expression Profile of Matrix Metalloproteinases(MMPs) and Their Inhibitors(TIMPs) in Lesions and Normal Appearing White Matter of Multiple Sclerosis. Brain 2001, 124 Pt 9, 1743–1753. [Google Scholar] [CrossRef]
- Selter, R.C.; Hemmer, B. Update on Immunopathogenesis and Immunotherapy in Multiple Sclerosis. Immunotargets Ther. 2013, 2, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar] [PubMed]
- Olsen, N.J.; Olson, G.; Viselli, S.M.; Gu, X.; Kovacs, W.J. Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology 2001, 142, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Benten, W.P.M.; Becker, A.; Schmitt-Wrede, H.-P.; Wunderlich, F. Developmental Regulation of Intracellular and Surface Androgen Receptors in T Cells. Steroids 2002, 67, 925–931. [Google Scholar] [CrossRef]
- Benten, W.P.M.; Stephan, C.; Wunderlich, F. B Cells Express Intracellular but Not Surface Receptors for Testosterone and Estradiol. Steroids 2002, 67, 647–654. [Google Scholar] [CrossRef]
- Olsen, N.J.; Gu, X.; Kovacs, W.J. Bone Marrow Stromal Cells Mediate Androgenic Suppression of B Lymphocyte Development. J. Clin. Investig. 2001, 108, 1697–1704. [Google Scholar] [CrossRef]
- Mierzejewska, K.; Borkowska, S.; Suszynska, E.; Suszynska, M.; Poniewierska-Baran, A.; Maj, M.; Pedziwiatr, D.; Adamiak, M.; Abdel-Latif, A.; Kakar, S.S.; et al. Hematopoietic Stem/Progenitor Cells Express Several Functional Sex Hormone Receptors-Novel Evidence for a Potential Developmental Link between Hematopoiesis and Primordial Germ Cells. Stem. Cells Dev. 2015, 24, 927–937. [Google Scholar] [CrossRef]
- Białek, M.; Zaremba, P.; Borowicz, K.K.; Czuczwar, S.J. Neuroprotective Role of Testosterone in the Nervous System. Pol. J. Pharm. 2004, 56, 509–518. [Google Scholar]
- Chisu, V.; Manca, P.; Zedda, M.; Lepore, G.; Gadau, S.; Farina, V. Effects of Testosterone on Differentiation and Oxidative Stress Resistance in C1300 Neuroblastoma Cells. Neuro Endocrinol. Lett. 2006, 27, 807–812. [Google Scholar]
- Rasika, S.; Alvarez-Buylla, A.; Nottebohm, F. BDNF Mediates the Effects of Testosterone on the Survival of New Neurons in an Adult Brain. Neuron 1999, 22, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Ansar Ahmed, S. The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases. Front. Immunol. 2015, 6, 635. [Google Scholar] [CrossRef] [PubMed]
- Maglione, A.; Rolla, S.; Mercanti, S.F.D.; Cutrupi, S.; Clerico, M. The Adaptive Immune System in Multiple Sclerosis: An Estrogen-Mediated Point of View. Cells 2019, 8, 1280. [Google Scholar] [CrossRef] [PubMed]
- Kipp, M.; Amor, S.; Krauth, R.; Beyer, C. Multiple Sclerosis: Neuroprotective Alliance of Estrogen–Progesterone and Gender. Front. Neuroendocrinol. 2012, 33, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Prieto, G.A.; Rosenstein, Y. Oestradiol Potentiates the Suppressive Function of Human CD4 CD25 Regulatory T Cells by Promoting Their Proliferation. Immunology 2006, 118, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kebir, H.; Ifergan, I.; Alvarez, J.I.; Bernard, M.; Poirier, J.; Arbour, N.; Duquette, P.; Prat, A. Preferential Recruitment of Interferon-Gamma-Expressing TH17 Cells in Multiple Sclerosis. Ann. Neurol. 2009, 66, 390–402. [Google Scholar] [CrossRef]
- Hill, L.; Jeganathan, V.; Chinnasamy, P.; Grimaldi, C.; Diamond, B. Differential Roles of Estrogen Receptors α and β in Control of B-Cell Maturation and Selection. Mol. Med. 2011, 17, 211–220. [Google Scholar] [CrossRef]
- Lehmann-Horn, K.; Kinzel, S.; Weber, M.S. Deciphering the Role of B Cells in Multiple Sclerosis-Towards Specific Targeting of Pathogenic Function. Int. J. Mol. Sci. 2017, 18, 2048. [Google Scholar] [CrossRef]
- Fettke, F.; Schumacher, A.; Costa, S.-D.; Zenclussen, A.C. B Cells: The Old New Players in Reproductive Immunology. Front. Immunol. 2014, 5, 285. [Google Scholar] [CrossRef]
- Singh, M.; Su, C. Progesterone and neuroprotection. Horm Behav. 2013, 63, 284–290. [Google Scholar] [CrossRef]
- Piccinni, M.P.; Giudizi, M.G.; Biagiotti, R.; Beloni, L.; Giannarini, L.; Sampognaro, S.; Parronchi, P.; Manetti, R.; Annunziato, F.; Livi, C. Progesterone Favors the Development of Human T Helper Cells Producing Th2-Type Cytokines and Promotes Both IL-4 Production and Membrane CD30 Expression in Established Th1 Cell Clones. J. Immunol. 1995, 155, 128–133. [Google Scholar] [PubMed]
- Druckmann, R.; Druckmann, M.-A. Progesterone and the Immunology of Pregnancy. J. Steroid Biochem. Mol. Biol. 2005, 97, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Leitner, H. Multiple Sclerosis and Progestins: A Comment to POPART’MUS. J. Neurol. Sci. 2011, 300, 198, author reply 198–199. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M.; Ffrench-Constant, C. Remyelination in the CNS: From Biology to Therapy. Nat. Rev. Neurosci. 2008, 9, 839–855. [Google Scholar] [CrossRef]

| Authors | Title | Year | Sample Size | Study Group | Group of Control | Result |
|---|---|---|---|---|---|---|
| Testosterone | ||||||
| Sicotte N.L. et al. [9] | Testosterone treatment in multiple sclerosis | 2007 | 10 men with RR-MS | 10 men with RR-MS treated daily with gel containing 100 mg of testosterone. | Each patient served as his own control | Treatment with testosterone gel for 12 months was associated with improved cognition and slowing of brain atrophy |
| Gold S.M. et al. [10] | Immune modulation and increased neurotrophic factor production in Multiple Sclerosis patients treated with testosterone | 2008 | Blood samples from 10 men with RR-MS | 10 men with RR-MS treated daily with gel containing 100 mg of testosterone. | Each patient served as his own control | Increased production of BDNF and PDGF-BB suggests a potential neuroprotective effect. |
| Bove R. et al. [11] | Low testosterone is associated with disability in men with multiple sclerosis | 2014 | 96 men with RR-MS | 96 men with RR-MS | - | Lower testosterone levels were correlated with higher EDSS. |
| Kurth F. et al. [12] | Neuroprotective effects of testosterone treatment in men with multiple sclerosis | 2014 | 10 men with RR-MS | 10 men with RR-MS treated daily with gel containing 100 mg of testosterone. | Each patient served as his own control | These observations may reflect the potential of testosterone treatment to reverse gray matter atrophy associated with MS |
| Metzger P.K. et al. [13] | The TOTEM RR-MS (testosterone treatment on neuroprotection and myelin repair in RR-MS) trial | 2020 | 40 testosterone-deficent men with RR-MS | Patients treated with Natalizumab + testosterone | Patients treated with Natalizumab + placebo | Study still ongoing |
| Estriol | ||||||
| Sicotte L. et al. [14] | Treatment of multiple sclerosis with the pregnancy hormone estriol | 2002 | 10 female patients with clinically definite MS (6 RR/4SP) | All the patients were treated with estriol (8 mg) | Each patient served as her own control | The total volume and number of enhancing lesions decreased during the treatment |
| Soldan S. et al. [15] | Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol | 2003 | 10 female patients with clinically definite MS (6 RR/4 SP) | All the patients were treated with estriol (8 mg) | Each patient served as her own control | Increased production of IL-5 and IL-10 and decreased TNF-alpha during estriol treatment |
| Gold S.M. et al. [16] | Estrogen treatment decreases matrix metalloproteinase (MMP)-9 in autoimmune demyelinating disease through estrogen receptor alpha (ERα) | 2009 | 3 premenopausal female patients with RR-MS | All the patients were treated with estriol (8 mg/die) | Each patient served as her own control | RR-MS patients treated with pregnancy levels of estriol showed a decrease in MMP-9 levels and activity and in enhancing lesions on MRI. |
| MacKenzie-Graham A. et al. [17] | Estriol-mediated neuroprotection in multiple sclerosis localized by voxel-based morphometry | 2018 | 164 patients | 83 patients treated with glatiramer acetate + estriol (8 mg/die) | 81 patients treated with glatirameracetate + placebo | Voxel-based morphometry analysis of gray matter revealed that it was significantly preserved in estriol + GA-treated subjects, but not in placebo + GA-treated subjects. |
| Estradiol | ||||||
| Pozzilli C. et al. [18] | Oral contraceptives combined with interferon-β in multiple sclerosis | 2015 | 150 women with RR-MS | Patients treated with IFN-β-1a + ethinylestradiol (20 mg) and desogestrel (150 mg) Patients treated with IFN-β-1a + ethinylestradiol (40 mg) and desogestrel (125 mg) | Patients treated with IFN-β-1a only | The anti-inflammatory effect of treatment, as measured by MRI activity, was more pronounced in patients receiving high-dose estrogens than in those receiving IFN-β alone. |
| De Giglio L. et al. [19] | Effect on cognition of estroprogestins combined with interferon-β in multiple sclerosis: analysis of secondary outcomes from a randomized controlled trial | 2016 | 150 women with RR-MS | Patients treated with IFN-β-1a + ethinylestradiol (20 mg) and desogestrel (150 mg) Patients treated with IFN-β-1a + ethinylestradiol (40 mg) and desogestrel (125 mg) | Patientstreated with IFN-b-1a only | A significant proportion of patients in the group treated with high-dose estrogens improved their cognitive status compared with those treated with IFN-β alone. |
| Pregnancy and post-partum | ||||||
| Confavreux C. et al. [20] | Rate of pregnancy- related relapse in multiple sclerosis | 1998 | 254 women with MS | 254 women with MS | Mean rate of relapse per year before pregnancy = 0.7 Mean rate of relapse during the third trimester of pregnancy = 0.2 Mean rate of relapse during the first three months post-partum = 1.2 | |
| S.Vukusic et al. [21] | Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse | 2004 | 227 women with MS | 227 women with MS | Three prognostic factors of high post-partum relapse rate: (1) increased relapse rate in the pre-pregnancy year, (2) an increased relapse rate during pregnancy, (3) higher DSS score at pregnancy onset, significantly correlated with the occurrence of a post-partum relapse | |
| L. Airas et al. [22] | Immunoregulatory factors in multiple sclerosis patients during and after pregnancy: relevance of natural killer cells | 2007 | 42 pregnant (RR-MS) | 42 pregnant (RR-MS) | Reduced disease activity during the last trimester was associated with a significant increase in the percentage of circulating CD56-bright natural killer cells | |
| Lorefice L. et al. [23] | Effects of pregnancy and breastfeeding on clinical outcomes and MRI measurements of women with multiple sclerosis: an exploratory real-world cohort study | 2021 | 210 women with MS | 210 women with MS | A higher annualized relapse rate in the post-partum year versus the pre-conception year was observed. Pregnancy during MS was associated with a lower EDSS score, while no relationships were reported with MRI measurements. | |
| Menopause | ||||||
| Holmqvist P. et al. [24] | Symptoms of multiple sclerosis in women in relation to sex steroid exposure | 2005 | 128 women with MS | 128 women with MS | 39% of the women reported worsening of MS symptoms related to menopause, whereas 56% reported no change of symptoms and 5% reported decreased symptoms. | |
| Ladeira F. et al. [25] | The influence of menopause in multiple sclerosis course: a longitudinal cohort study | 2018 | 37 women with MS | 37 women with MS | Following menopause, a reduction in the relapse rate was observed, but the disability progression continued at a similar rate, compared to the pre-menopausal period. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murgia, F.; Giagnoni, F.; Lorefice, L.; Caria, P.; Dettori, T.; D’Alterio, M.N.; Angioni, S.; Hendren, A.J.; Caboni, P.; Pibiri, M.; et al. Sex Hormones as Key Modulators of the Immune Response in Multiple Sclerosis: A Review. Biomedicines 2022, 10, 3107. https://doi.org/10.3390/biomedicines10123107
Murgia F, Giagnoni F, Lorefice L, Caria P, Dettori T, D’Alterio MN, Angioni S, Hendren AJ, Caboni P, Pibiri M, et al. Sex Hormones as Key Modulators of the Immune Response in Multiple Sclerosis: A Review. Biomedicines. 2022; 10(12):3107. https://doi.org/10.3390/biomedicines10123107
Chicago/Turabian StyleMurgia, Federica, Florianna Giagnoni, Lorena Lorefice, Paola Caria, Tinuccia Dettori, Maurizio N. D’Alterio, Stefano Angioni, Aran J. Hendren, Pierluigi Caboni, Monica Pibiri, and et al. 2022. "Sex Hormones as Key Modulators of the Immune Response in Multiple Sclerosis: A Review" Biomedicines 10, no. 12: 3107. https://doi.org/10.3390/biomedicines10123107
APA StyleMurgia, F., Giagnoni, F., Lorefice, L., Caria, P., Dettori, T., D’Alterio, M. N., Angioni, S., Hendren, A. J., Caboni, P., Pibiri, M., Monni, G., Cocco, E., & Atzori, L. (2022). Sex Hormones as Key Modulators of the Immune Response in Multiple Sclerosis: A Review. Biomedicines, 10(12), 3107. https://doi.org/10.3390/biomedicines10123107