Gangliosides and Their Role in Multilineage Differentiation of Mesenchymal Stem Cells
Abstract
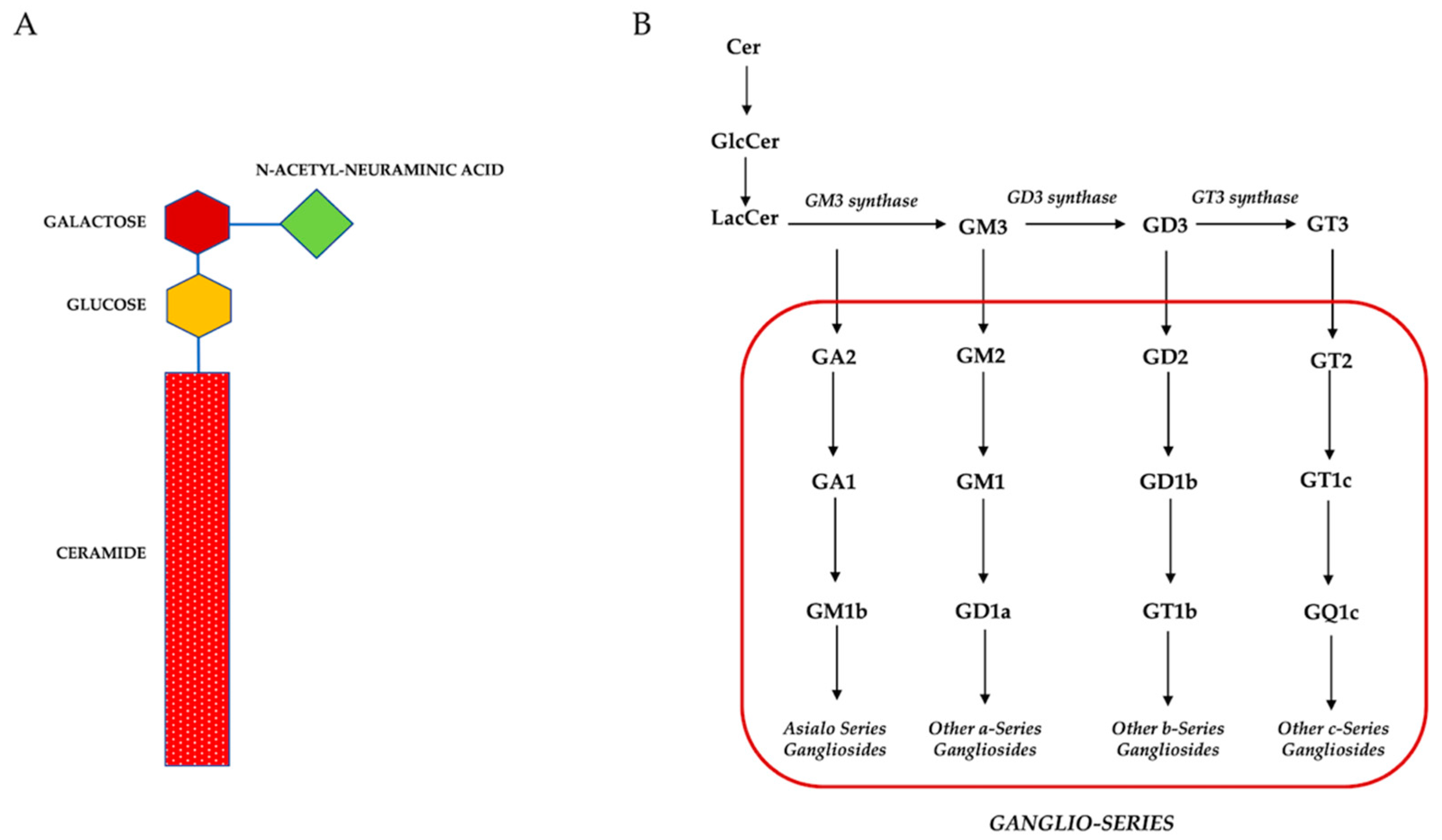
1. Introduction
2. Main MSCs’ Properties
3. Role of Gangliosides during Multilineage Differentiation of MSCs Isolated from Different Sources
3.1. Umbilical Cord Derived Mesenchymal Stem Cells (UC-MSCs)
3.2. Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs)
3.3. Dental Pulp Mesenchymal Stem Cells (DPSCs)
3.4. Adipose Mesenchymal Derived Stem Cells (ADSCs)
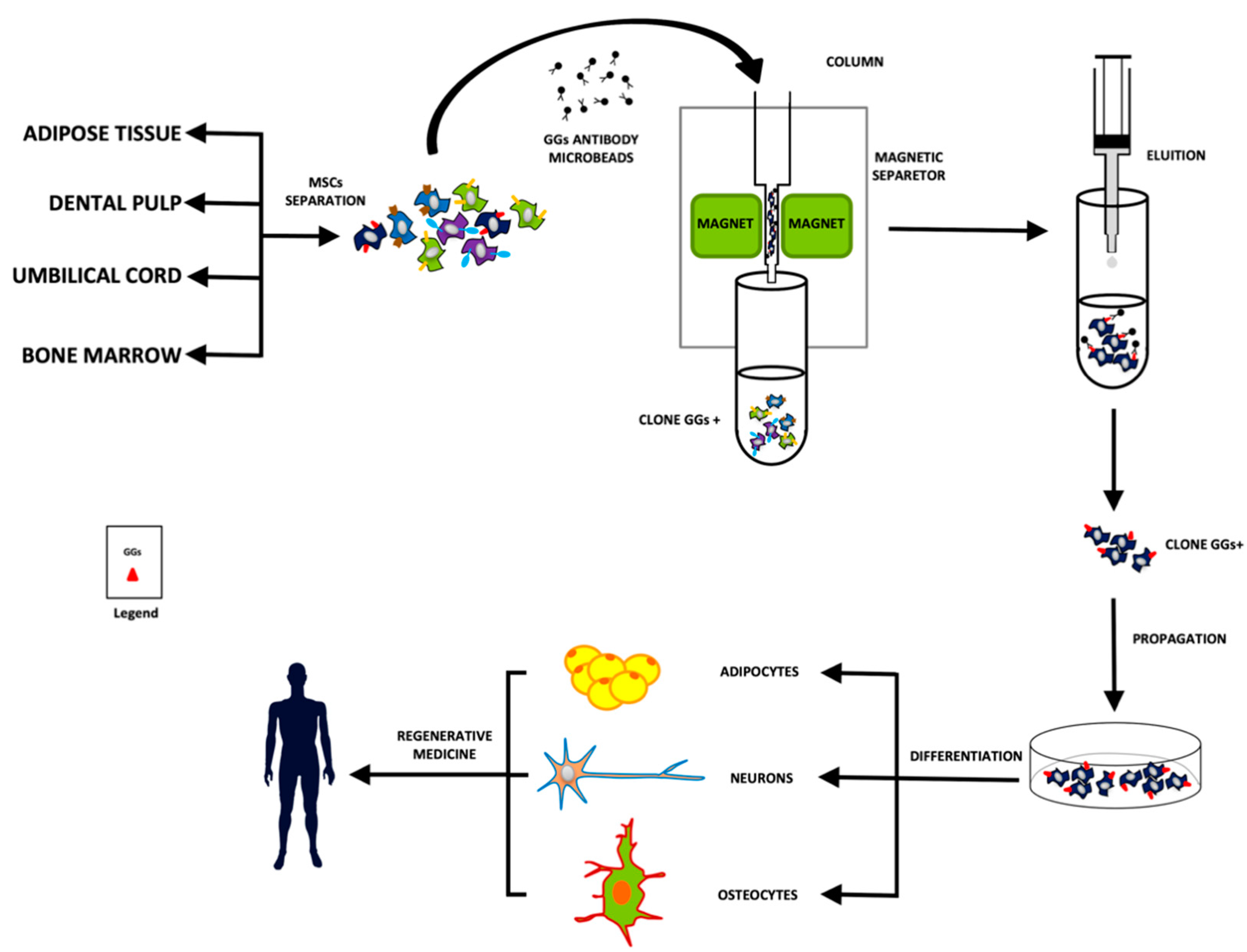
4. Gangliosides as a Potential New Class of Stem Cell Markers
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147, Erratum in Science 1998, 282, 1827. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.L.; Stavridis, M.; Griffiths, D.; Li, M.; Smith, A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 2003, 21, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Gorskaja, J.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar] [PubMed]
- Mattei, V.; Santacroce, C.; Tasciotti, V.; Martellucci, S.; Santilli, F.; Manganelli, V.; Piccoli, L.; Misasi, R.; Sorice, M.; Garofalo, T. Role of lipid rafts in neuronal differentiation of dental pulp-derived stem cells. Exp. Cell Res. 2015, 339, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, S.; Manganelli, V.; Santacroce, C.; Santilli, F.; Piccoli, L.; Sorice, M.; Mattei, V. Role of prion protein-EGFR multimolecular complex during neuronal differentiation of human dental pulp-derived stem cells. Prion 2018, 12, 117–126. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Rohban, R.; Pieber, T.R. Mesenchymal Stem and Progenitor Cells in Regeneration: Tissue Specificity and Regenerative Potential. Stem Cells Int. 2017, 2017, 5173732. [Google Scholar] [CrossRef]
- He, X.; Guan, F.; Lei, L. Structure, and function of glycosphingolipids on small extracellular vesicles. Glycoconj. J. 2022, 39, 197–205. [Google Scholar] [CrossRef]
- Moran, A.B.; Gardner, R.A.; Wuhrer, M.; Lageveen-Kammeijer, G.S.M.; Spencer, D.I.R. Sialic Acid Derivatization of Fluorescently Labeled N-Glycans Allows Linkage Differentiation by Reversed-Phase Liquid Chromatography-Fluorescence Detection-Mass Spectrometry. Anal. Chem. 2022, 94, 6639–6648. [Google Scholar] [CrossRef]
- Martellucci, S.; Santacroce, C.; Santilli, F.; Manganelli, V.; Sorice, M.; Mattei, V. Prion Protein in Stem Cells: A Lipid Raft Component Involved in the Cellular Differentiation Process. Int. J. Mol. Sci. 2020, 21, 4168. [Google Scholar] [CrossRef]
- Codini, M.; Garcia-Gil, M.; Albi, E. Cholesterol and Sphingolipid Enriched Lipid Rafts as Therapeutic Targets in Cancer. Int. J. Mol. Sci. 2021, 22, 726. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191, Erratum in Nat. Rev. Mol. Cell Biol. 2018, 19, 673. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qin, Y.; Yu, X.; Xu, X.; Yu, W. Lipid raft involvement in signal transduction in cancer cell survival, cell death and metastasis. Cell Prolif. 2022, 55, e13167. [Google Scholar] [CrossRef] [PubMed]
- Mattei, V.; Manganelli, V.; Martellucci, S.; Capozzi, A.; Mantuano, E.; Longo, A.; Ferri, A.; Garofalo, T.; Sorice, M.; Misasi, R. A multimolecular signaling complex including PrPC and LRP1 is strictly dependent on lipid rafts and is essential for the function of tissue plasminogen activator. J. Neurochem. 2020, 152, 468–481. [Google Scholar] [CrossRef]
- Sorice, M.; Mattei, V.; Tasciotti, V.; Manganelli, V.; Garofalo, T.; Misasi, R. Trafficking of PrPC to mitochondrial raft-like microdomains during cell apoptosis. Prion 2012, 6, 354–358. [Google Scholar] [CrossRef]
- Sorice, M.; Mattei, V.; Matarrese, P.; Garofalo, T.; Tinari, A.; Gambardella, L.; Ciarlo, L.; Manganelli, V.; Tasciotti, V.; Misasi, R.; et al. Dynamics of mitochondrial raft-like microdomains in cell life and death. Commun. Integr. Biol. 2012, 5, 217–219. [Google Scholar] [CrossRef]
- Manganelli, V.; Capozzi, A.; Recalchi, S.; Signore, M.; Mattei, V.; Garofalo, T.; Misasi, R.; Degli Esposti, M.; Sorice, M. Altered Traffic of Cardiolipin during Apoptosis: Exposure on the Cell Surface as a Trigger for Antiphospholipid Antibodies. J. Immunol. Res. 2015, 2015, 847985. [Google Scholar] [CrossRef]
- Yanagisawa, M. Stem cell glycolipids. Neurochem. Res. 2011, 36, 1623–1635. [Google Scholar] [CrossRef]
- Yu, R.K.; Suzuki, Y.; Yanagisawa, M. Membrane glycolipids in stem cells. FEBS Lett. 2010, 584, 1694–1699. [Google Scholar] [CrossRef]
- Kannagi, R.; Cochran, N.A.; Ishigami, F.; Hakomori, S.; Andrews, P.W.; Knowles, B.B.; Solter, D. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983, 2, 2355–2361. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Fujitani, N.; Furukawa, J.; Araki, K.; Fujioka, T.; Takegawa, Y.; Piao, J.; Nishioka, T.; Tamura, T.; Nikaido, T.; Ito, M.; et al. Total cellular glycomics allows characterizing cells and streamlining the discovery process for cellular biomarkers. Proc. Natl. Acad. Sci. USA 2013, 110, 2105–2110. [Google Scholar] [CrossRef]
- Nakatani, Y.; Yanagisawa, M.; Suzuki, Y.; Yu, R.K. Characterization of GD3 ganglioside as a novel biomarker of mouse neural stem cells. Glycobiology 2010, 20, 78–86. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Yoshimura, S.; Yu, R.K. Expression of GD2 and GD3 gangliosides in human embryonic neural stem cells. ASN Neuro 2011, 3, e00054. [Google Scholar] [CrossRef]
- Martinez, C.; Hofmann, T.J.; Marino, R.; Dominici, M.; Horwitz, E.M. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: A novel surface marker for the identification of MSCs. Blood 2007, 109, 4245–4248, Erratum in Blood 2007, 110, 826. [Google Scholar] [CrossRef]
- Gang, E.J.; Bosnakovski, D.; Figueiredo, C.A.; Visser, J.W.; Perlingeiro, R.C. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood 2007, 109, 1743–1751. [Google Scholar] [CrossRef]
- Lee, D.H.; Koo, D.B.; Ko, K.; Ko, K.; Kim, S.M.; Jung, J.U.; Ryu, J.S.; Jin, J.W.; Yang, H.J.; Do, S.I.; et al. Effects of daunrubcin on ganglioside expression and neuronal differentiation of mouse embryonic stem cells. Biochem. Biophys. Res. Commun. 2007, 362, 313–318. [Google Scholar] [CrossRef]
- Ryu, J.S.; Ko, K.; Lee, J.W.; Park, S.B.; Byun, S.J.; Jeong, E.J.; Ko, K.; Choo, Y.K. Gangliosides are involved in neural differentiation of human dental pulp-derived stem cells. Biochem. Biophys. Res. Commun. 2009, 387, 266–271. [Google Scholar] [CrossRef]
- Klenk, E. Über die Ganglioside, eine neue Gruppe von zuckerhaltigen Gehirnlipoiden. Biol. Chem. 1942, 273, 76–86. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, J.; Yang, L.; Zhao, S.; Liu, X.; Su, Y.; Zhang, J.; Zhao, M. Gangliosides play important roles in the nervous system by regulating ion concentrations. Neurochem. Res. 2022, 47, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L. The gangliosides. J. Lipid. Res. 1964, 5, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L. Chromatographic separation of human brain gangliosides. J. Neurochem. 1963, 10, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Jung, K.Y.; Kwak, D.H.; Lee, S.H.; Ryu, J.S.; Kim, J.S.; Chang, K.T.; Lee, J.W.; Choo, Y.K. Inhibition of ganglioside GD1a synthesis suppresses the differentiation of human mesenchymal stem cells into osteoblasts. Dev. Growth Differ. 2011, 53, 323–332. [Google Scholar] [CrossRef]
- Moussavou, G.; Kwak, D.H.; Lim, M.U.; Kim, J.S.; Kim, S.U.; Chang, K.T.; Choo, Y.K. Role of gangliosides in the differentiation of human mesenchymal-derived stem cells into osteoblasts and neuronal cells. BMB Rep. 2013, 46, 527–532. [Google Scholar] [CrossRef]
- Ryu, J.S.; Seo, S.Y.; Jeong, E.J.; Kim, J.Y.; Koh, Y.G.; Kim, Y.I.; Choo, Y.K. Ganglioside GM3 Up-Regulate Chondrogenic Differentiation by Transform Growth Factor Receptors. Int. J. Mol. Sci. 2020, 21, 1967. [Google Scholar] [CrossRef]
- Bieberich, E. Sphingolipids, and lipid rafts: Novel concepts and methods of analysis. Chem. Phys. Lipids 2018, 216, 114–131. [Google Scholar] [CrossRef]
- Kim, D.H.; Triet, H.M.; Ryu, S.H. Regulation of EGFR activation and signaling by lipids on the plasma membrane. Prog. Lipid. Res. 2021, 83, 101115. [Google Scholar] [CrossRef]
- Cumin, C.; Huang, Y.L.; Everest-Dass, A.; Jacob, F. Deciphering the Importance of Glycosphingolipids on Cellular and Molecular Mechanisms Associated with Epithelial-to-Mesenchymal Transition in Cancer. Biomolecules 2021, 11, 62. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Din, Y.; Levery, S.B.; Lobaton, M.; Handa, K.; Hakomori, S.I. Differential expression profiles of glycosphingolipids in human breast cancer stem cells vs. cancer non-stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 4968–4973. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Alam, S.; Konantz, M.; Liang, C.Y.; Kohler, R.S.; Everest-Dass, A.V.; Huang, Y.L.; Rimmer, N.; Fedier, A.; Schötzau, A.; et al. Transition of Mesenchymal and Epithelial Cancer Cells Depends on α1-4 Galactosyltransferase-Mediated Glycosphingolipids. Cancer Res. 2018, 78, 2952–2965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; van Die, I.; Tefsen, B.; van Vliet, S.J.; Laan, L.C.; Zhang, J.; Ten Dijke, P.; Wuhrer, M.; Belo, A.I. Differential O- and Glycosphingolipid Glycosylation in Human Pancreatic Adenocarcinoma Cells with Opposite Morphology and Metastatic Behavior. Front. Oncol. 2020, 10, 732. [Google Scholar] [CrossRef]
- Lee, S.H.; Ryu, J.S.; Lee, J.W.; Kwak, D.H.; Ko, K.; Choo, Y.K. Comparison of ganglioside expression between human adi-pose and dental pulp-derived stem cell differentiation into osteoblasts. Arch. Pharm. Res. 2010, 33, 585–591. [Google Scholar] [CrossRef]
- Porter, M.J.; Zhang, G.L.; Schnaar, R.L. Ganglioside Extraction, Purification and Profiling. J. Vis. Exp. 2021, 169, e62385. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, J.U.; Ryu, J.S.; Jin, J.W.; Yang, H.J.; Ko, K.; You, H.K.; Jung, K.Y.; Choo, Y.K. Effects of gangliosides on the differentiation of human mesenchymal stem cells into osteoblasts by modulating epidermal growth factor receptors. Biochem. Biophys. Res. Commun. 2008, 371, 866–871. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Ladisch, S. Exogenous ganglioside GD1a enhances epidermal growth factor receptor binding and dimerization. J. Biol. Chem. 2004, 279, 36481–36489. [Google Scholar] [CrossRef]
- Yu, R.K. Development regulation of ganglioside metabolism. Prog. Brain Res. 1994, 101, 31–44. [Google Scholar] [CrossRef]
- Inokuchi, J.; Nagafuku, M.; Ohno, I.; Suzuki, A. Heterogeneity of gangliosides among T cell subsets. Cell Mol. Life Sci. 2013, 70, 3067–3075. [Google Scholar] [CrossRef]
- Bergante, S.; Creo, P.; Piccoli, M.; Ghiroldi, A.; Menon, A.; Cirillo, F.; Rota, P.; Monasky, M.M.; Ciconte, G.; Pappone, C.; et al. GM1 Ganglioside Promotes Osteogenic Differentiation of Human Tendon Stem Cells. Stem Cells Int. 2018, 2018, 4706943. [Google Scholar] [CrossRef]
- Bergante, S.; Torretta, E.; Creo, P.; Sessarego, N.; Papini, N.; Piccoli, M.; Fania, C.; Cirillo, F.; Conforti, E.; Ghiroldi, A.; et al. Gangliosides as a potential new class of stem cell markers: The case of GD1a in human bone marrow mesenchymal stem cells. J. Lipid Res. 2014, 55, 549–560. [Google Scholar] [CrossRef]
- Martellucci, S.; Santacroce, C.; Santilli, F.; Piccoli, L.; Delle Monache, S.; Angelucci, A.; Misasi, R.; Sorice, M.; Mattei, V. Cellular and Molecular Mechanisms Mediated by recPrPC Involved in the Neuronal Differentiation Process of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 345. [Google Scholar] [CrossRef]
- Hohenwallner, K.; Troppmair, N.; Panzenboeck, L.; Kasper, C.; El Abiead, Y.; Koellensperger, G.; Lamp, L.M.; Hartler, J.; Egger, D.; Rampler, E. Decoding distinct ganglioside patterns of native and differentiated mesenchymal stem cells by a novel glycolipidomics profiling strategy. JACS Au 2022, 2, 2466–2480. [Google Scholar] [CrossRef]
- Kwak, D.H.; Yu, K.; Kim, S.M.; Lee, D.H.; Kim, S.M.; Jung, J.U.; Seo, J.W.; Kim, N.; Lee, S.; Jung, K.Y.; et al. Dynamic changes of gangliosides expression during the differentiation of embryonic and mesenchymal stem cells into neural cells. Exp. Mol. Med. 2006, 38, 668–676. [Google Scholar] [CrossRef]
- Fazzari, M.; Lunghi, G.; Chiricozzi, E.; Mauri, L.; Sonnino, S. Gangliosides, and the Treatment of Neurodegenerative Diseases: A Long Italian Tradition. Biomedicines 2022, 10, 363. [Google Scholar] [CrossRef]
- Cavallini, L.; Venerando, R.; Miotto, G.; Alexandre, A. Ganglioside GM1 protection from apoptosis of rat heart fibroblasts. Arch. Biochem. Biophys. 1999, 370, 156–162. [Google Scholar] [CrossRef]
- Tang, F.L.; Wang, J.; Itokazu, Y.; Yu, R.K. Ganglioside GD3 regulates dendritic growth in newborn neurons in adult mouse hippocampus via modulation of mitochondrial dynamics. J. Neurochem. 2021, 156, 819–833. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, A.; Wakade, C.; Yu, R.K. Ganglioside GD3 is required for neurogenesis and long-term maintenance of neural stem cells in the postnatal mouse brain. J. Neurosci. 2014, 34, 13790–13800. [Google Scholar] [CrossRef]
- Todeschini, A.R.; Dos Santos, J.N.; Handa, K.; Hakomori, S.I. Ganglioside GM2/GM3 complex affixed on silica nanospheres strongly inhibits cell motility through CD82/cMet-mediated pathway. Proc. Natl. Acad. Sci. USA 2008, 105, 1925–1930. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Lopez, P.H. Myelin-associated glycoprotein and its axonal receptors. J. Neurosci. Res. 2009, 87, 3267–3276. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.K.; Jaiswal, N.; Bruder, S.P.; Mbalaviele, G.; Marshak, D.R.; Pittenger, M.F. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J. Biol. Chem. 2000, 275, 9645–9652. [Google Scholar] [CrossRef] [PubMed]
- Itokazu, Y.; Wang, J.; Yu, R.K. Gangliosides in Nerve Cell Specification. Prog. Mol. Biol. Transl. Sci. 2018, 156, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Maric, D.; Maric, I.; Chang, Y.H.; Barker, J.L. Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self-renewal and differentiation. J. Neurosci. 2003, 23, 240–251. [Google Scholar] [CrossRef]
- Kwak, D.H.; Seo, B.B.; Chang, K.T.; Choo, Y.K. Roles of gangliosides in mouse embryogenesis and embryonic stem cell differentiation. Exp. Mol. Med. 2011, 43, 379–388. [Google Scholar] [CrossRef][Green Version]
- Tsuji, S.; Arita, M.; Nagai, Y. GQ1b, a bioactive ganglioside that exhibits novel nerve growth factor (NGF)-like activities in the two neuroblastoma cell lines. J. Biochem. 1983, 94, 303–306. [Google Scholar] [CrossRef]
- Wolfe, M.; Pochampally, R.; Swaney, W.; Reger, R.L. Isolation and culture of bone marrow-derived human multipotent stromal cells (hMSCs). Methods Mol. Biol. 2008, 449, 3–25. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Oh, E.J.; Lee, H.W.; Kalimuthu, S.; Kim, T.J.; Kim, H.M.; Baek, S.H.; Zhu, L.; Oh, J.M.; Son, S.H.; Chung, H.Y.; et al. In vivo migration of mesenchymal stem cells to burn injury sites and their therapeutic effects in a living mouse model. J. Control. Release 2018, 279, 79–88. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, S.G.; Lee, S.W.; Oh, B.J.; Kim, J.H.; Kim, J.A.; Lee, G.; Jang, J.D.; Joe, Y.A. A Subset of Paracrine Factors as Efficient Biomarkers for Predicting Vascular Regenerative Efficacy of Mesenchymal Stromal/Stem Cells. Stem Cells 2019, 37, 77–88. [Google Scholar] [CrossRef]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Biol. 2016, 1416, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Lozano Navarro, L.V.; Chen, X.; Giratá Viviescas, L.T.; Ardila-Roa, A.K.; Luna-Gonzalez, M.L.; Sossa, C.L.; Arango-Rodríguez, M.L. Mesenchymal stem cells for critical limb ischemia: Their function, mechanism, and therapeutic potential. Stem Cell Res. Ther. 2022, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, P.; Chen, W.; Weir, M.D.; Bao, C.; Xu, H.H. Human embryonic stem cells and macroporous calcium phosphate construct for bone regeneration in cranial defects in rats. Acta Biomater. 2014, 10, 4484–4493. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, S.; Santacroce, C.; Manganelli, V.; Santilli, F.; Piccoli, L.; Cassetta, M.; Misasi, R.; Sorice, M.; Mattei, V. Isolation, Propagation, and Prion Protein Expression During Neuronal Differentiation of Human Dental Pulp Stem Cells. J. Vis. Exp. JoVE 2019, 145, e59282. [Google Scholar] [CrossRef]
- Mattei, V.; Martellucci, S.; Pulcini, F.; Santilli, F.; Sorice, M.; Delle Monache, S. Regenerative Potential of DPSCs and Revascularization: Direct, Paracrine or Autocrine Effect? Stem Cell Rev. Rep. 2021, 17, 1635–1646. [Google Scholar] [CrossRef]
- Delle Monache, S.; Martellucci, S.; Clementi, L.; Pulcini, F.; Santilli, F.; Mei, C.; Piccoli, L.; Angelucci, A.; Mattei, V. In Vitro Conditioning Determines the Capacity of Dental Pulp Stem Cells to Function as Pericyte-Like Cells. Stem Cells Dev. 2019, 28, 695–706. [Google Scholar] [CrossRef]
- Kobolak, J.; Dinnyes, A.; Memic, A.; Khademhosseini, A.; Mobasheri, A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties, and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 2016, 99, 62–68. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, J.; Wang, D.; Liu, J. The Use of Stem Cells in Neural Regeneration: A Review of Current Opinion. Curr. Stem Cell Res. Ther. 2018, 13, 608–617. [Google Scholar] [CrossRef]
- Can, A.; Karahuseyinoglu, S. Concise review: Human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells 2007, 25, 2886–2895. [Google Scholar] [CrossRef]
- Xu, J.; Liao, W.; Gu, D.; Liang, L.; Liu, M.; Du, W.; Liu, P.; Zhang, L.; Lu, S.; Dong, C.; et al. Neural ganglioside GD2 identifies a subpopulation of mesenchymal stem cells in umbilical cord. Cell. Physiol. Biochem. 2009, 23, 415–424. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, R.; Jiang, J.; Peng, J.; Yang, C.; Zhang, W.; Wang, S.; Song, J. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res. Ther. 2020, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Filip, A.; Dabrowski, F.A.; Burdzinska, A.; Kulesza, A.; Sladowska, A.; Zolocinska, A.; Gala, K.; Paczek, L.; Wielgos, M. Comparison of the paracrine activity of mesenchymal stem cells derived from human umbilical cord, amniotic membrane, and adipose tissue. J. Obstet. Gynaecol. Res. 2017, 43, 1758–1768. [Google Scholar] [CrossRef]
- Ding, D.C.; Chang, Y.H.; Shyu, W.C.; Lin, S.Z. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Nam, H.Y.; Bae, Y.K.; Kim, S.Y.; Im, I.R.; Oh, W.; Yang, Y.S.; Choi, S.J.; Kim, S.W. GD2 expression is closely associated with neuronal differentiation of human umbilical cord blood-derived mesenchymal stem cells. Cell Mol. Life Sci. 2010, 67, 1845–1858. [Google Scholar] [CrossRef]
- Nan, C.; Shi, Y.; Zhao, Z.; Ma, S.; Liu, J.; Yan, D.; Song, G.; Liu, H. Monosialoteterahexosyl ganglioside induces the differentiation of human umbilical cord-derived mesenchymal stem cells into neuron-like cells. Int. J. Mol. Med. 2015, 36, 1057–1062. [Google Scholar] [CrossRef][Green Version]
- Brenner, M.; Messing, A. Regulation of GFAP Expression. ASN Neuro 2021, 13, 1759091420981206. [Google Scholar] [CrossRef]
- Xu, L.; Hanamatsu, H.; Homan, K.; Onodera, T.; Miyazaki, T.; Furukawa, J.I.; Hontani, K.; Tian, Y.; Baba, R.; Iwasaki, N. Alterations of Glycosphingolipid Glycans and Chondrogenic Markers during Differentiation of Human Induced Pluripotent Stem Cells into Chondrocytes. Biomolecules 2020, 10, 1622. [Google Scholar] [CrossRef]
- Jin, U.H.; Chung, T.W.; Song, K.H.; Kwak, C.H.; Choi, H.J.; Ha, K.T.; Chang, Y.C.; Lee, Y.C.; Kim, C.H. Ganglioside GM3 is required for caffeic acid phenethyl ester-induced megakaryocytic differentiation of human chronic myelogenous leukemia K562 cells. Biochem. Cell Biol. 2014, 92, 243–249. [Google Scholar] [CrossRef]
- Nagafuku, M.; Okuyama, K.; Onimaru, Y.; Suzuki, A.; Odagiri, Y.; Yamashita, T.; Iwasaki, K.; Fujiwara, M.; Takayanagi, M.; Ohno, I.; et al. CD4 and CD8 T cells require different membrane gangliosides for activation. Proc. Natl. Acad. Sci. USA 2012, 109, E336–E342. [Google Scholar] [CrossRef]
- Cho, J.H.; Ju, W.S.; Seo, S.Y.; Kim, B.H.; Kim, J.S.; Kim, J.G.; Park, S.J.; Choo, Y.K. The Potential Role of Human NME1 in Neuronal Differentiation of Porcine Mesenchymal Stem Cells: Application of NB-hNME1 as a Human NME1 Suppressor. Int. J. Mol. Sci. 2021, 22, 12194. [Google Scholar] [CrossRef]
- Nagafuku, M.; Sato, T.; Sato, S.; Shimizu, K.; Taira, T.; Inokuchi, J. Control of homeostatic and pathogenic balance in adipose tissue by ganglioside GM3. Glycobiology 2015, 25, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Rampler, E.; Egger, D.; Schoeny, H.; Rusz, M.; Pacheco, M.P.; Marino, G.; Kasper, C.; Naegele, T.; Koellensperger, G. The Power of LC-MS Based Multiomics: Exploring Adipogenic Differentiation of Human Mesenchymal Stem/Stromal Cells. Molecules 2019, 24, 3615. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.D.; Casali, K.R.; Ghem, C.; da Silva, M.K.; Sausen, G.; Palma, P.B.; Covas, D.T.; Kalil, R.A.K.; Schaan, B.D.; Nardi, N.B.; et al. Mesenchymal stem cells from sternum: The type of heart disease, ischemic or valvular, does not influence the cell culture establishment and growth kinetics. J. Transl. Med. 2017, 15, 161. [Google Scholar] [CrossRef] [PubMed]
- Drela, K.; Stanaszek, L.; Snioch, K.; Kuczynska, Z.; Wrobel, M.; Sarzynska, S.; Legosz, P.; Maldyk, P.; Lukomska, B. Bone marrow-derived from the human femoral shaft as a new source of mesenchymal stem/stromal cells: An alternative cell material for banking and clinical transplantation. Stem Cell Res. Ther. 2020, 11, 262. [Google Scholar] [CrossRef]
- Fragkakis, E.M.; El-Jawhari, J.J.; Dunsmuir, R.A.; Millner, P.A.; Rao, A.S.; Henshaw, K.T.; Pountos, I.; Jones, E.; Giannoudis, P.V. Vertebral body versus iliac crest bone marrow as a source of multipotential stromal cells: Comparison of processing techniques, tri-lineage differentiation, and application on a scaffold for spine fusion. PLoS ONE 2018, 13, e0197969. [Google Scholar] [CrossRef]
- Liu, R.; Chang, W.; Wei, H.; Zhang, K. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from Bone Marrow and Skin. Stem Cells Int. 2016, 2016, 3658798. [Google Scholar] [CrossRef]
- Liu, C.; Tsai, A.L.; Li, P.C.; Huang, C.W.; Wu, C.C. Endothelial differentiation of bone marrow mesenchymal stem cells applicable to hypoxia and increased migration through Akt and NFκB signals. Stem Cell Res. Ther. 2017, 8, 29. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Wang, C.; Meng, H.; Wang, X.; Zhao, C.; Peng, J.; Wang, Y. Differentiation of Bone Marrow Mesenchymal Stem Cells in Osteoblasts and Adipocytes and its Role in Treatment of Osteoporosis. Med. Sci. Monit. 2016, 22, 226–233. [Google Scholar] [CrossRef]
- Sudo, K.; Kanno, M.; Miharada, K.; Ogawa, S.; Hiroyama, T.; Saijo, K.; Nakamura, Y. Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells 2007, 25, 1610–1617. [Google Scholar] [CrossRef]
- Moghadam, F.H.; Tayebi, T.; Dehghan, M.; Eslami, G.; Nadri, H.; Moradi, A.; Vahedian-Ardakani, H.; Barzegar, K. Differentiation of bone marrow mesenchymal stem cells into chondrocytes after short term culture in alkaline medium. Int. J. Hematol. Oncol. Stem Cell Res. 2014, 8, 12–19. [Google Scholar] [PubMed]
- Yang, H.; Xia, Y.; Lu, S.Q.; Soong, T.W.; Feng, Z.W. Basic fibroblast growth factor-induced neuronal differentiation of mouse bone marrow stromal cells requires FGFR-1, MAPK/ERK, and transcription factor AP-1. J. Biol. Chem. 2008, 283, 5287–5295. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.; Gronthos, S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int. J. Mol. Sci. 2020, 21, 9759. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Purwaningrum, M.; Jamilah, N.S.; Purbantoro, S.D.; Sawangmake, C.; Nantavisai, S. Comparative characteristic study from bone marrow-derived mesenchymal stem cells. J. Vet. Sci. 2021, 22, e74. [Google Scholar] [CrossRef] [PubMed]
- Funari, A.; Alimandi, M.; Pierelli, L.; Pino, V.; Gentileschi, S.; Sacchetti, B. Human Sinusoidal Subendothelial Cells Regulate Homing, and Invasion of Circulating Metastatic Prostate Cancer Cells to Bone Marrow. Cancers 2019, 11, 763. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lei, T.; Liu, Y.; Yang, Y.; Bi, W.; Du, H. The potential therapy with dental tissue-derived mesenchymal stem cells in Parkinson’s disease. Stem Cell Res. Ther. 2021, 12, 5. [Google Scholar] [CrossRef]
- Delle Monache, S.; Pulcini, F.; Santilli, F.; Martellucci, S.; Santacroce, C.; Fabrizi, J.; Angelucci, A.; Sorice, M.; Mattei, V. Hypoxia Induces DPSC Differentiation versus a Neurogenic Phenotype by the Paracrine Mechanism. Biomedicines 2022, 10, 1056. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Nguyen, A.L.; Yu, W.H.; Le, A.D. Human oral mucosa and gingiva: A unique reservoir for mesenchymal stem cells. J. Dent. Res. 2012, 91, 1011–1018. [Google Scholar] [CrossRef]
- Gronthos, S.; Arthur, A.; Bartold, P.M.; Shi, S. A method to isolate and culture expand human dental pulp stem cells. Methods Mol. Biol. 2011, 698, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Al Madhoun, A.; Sindhu, S.; Haddad, D.; Atari, M.; Ahmad, R.; Al-Mulla, F. Dental Pulp Stem Cells Derived from Adult Human Third Molar Tooth: A Brief Review. Front. Cell Dev. Biol. 2021, 9, 717624. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Han, X.; Kato, H.; Sato, H.; Zhang, Y.; Sun, X.; Hirofuji, Y.; Yamaza, H.; Yamada, A.; Fukumoto, S. Dental Pulp-Derived Mesenchymal Stem Cells for Modeling Genetic Disorders. Int. J. Mol. Sci. 2021, 22, 2269. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, J.; Polo, Y.; Pastor-Alonso, O.; Pardo-Rodríguez, B.; Larrañaga, A.; Unda, F.; Sarasua, J.R.; Pineda, J.R.; Ibarretxe, G. Advances and Perspectives in Dental Pulp Stem Cell Based Neuroregeneration Therapies. Int. J. Mol. Sci. 2021, 22, 3546. [Google Scholar] [CrossRef] [PubMed]
- Tirino, V.; Paino, F.; De Rosa, A.; Papaccio, G. Identification, isolation, characterization, and banking of human dental pulp stem cells. Methods Mol. Biol. 2012, 879, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Rodas-Junco, B.A.; Villicaña, C. Dental Pulp Stem Cells: Current Advances in Isolation, Expansion and Preservation. Tissue Eng. Regen. Med. 2017, 14, 333–347. [Google Scholar] [CrossRef]
- Aghajani, F.; Hooshmand, T.; Khanmohammadi, M.; Khanjani, S.; Edalatkhah, H.; Zarnani, A.H.; Kazemnejad, S. Comparative Immunophenotypic Characteristics, Proliferative Features, and Osteogenic Differentiation of Stem Cells Isolated from Human Permanent and Deciduous Teeth with Bone Marrow. Mol. Biotechnol. 2016, 58, 415–427. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura-Yamada, S.; Kusano, K.; Baba, S. Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. Int. J. Mol. Sci. 2019, 20, 1132. [Google Scholar] [CrossRef]
- Delle Monache, S.; Pulcini, F.; Frosini, R.; Mattei, V.; Talesa, V.N.; Antognelli, C. Methylglyoxal-Dependent Glycative Stress Is Prevented by the Natural Antioxidant Oleuropein in Human Dental Pulp Stem Cells through Nrf2/Glo1 Pathway. Antioxidants 2021, 10, 716. [Google Scholar] [CrossRef]
- Mortada, I.; Mortada, R.; Al Bazzal, M. Dental Pulp Stem Cells and Neurogenesis. Adv. Exp. Med. Biol. 2018, 1083, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Ching, H.S.; Luddin, N.; Rahman, I.A.; Ponnuraj, K.T. Expression of Odontogenic and Osteogenic Markers in DPSCs and SHED: A Review. Curr. Stem Cell Res. Ther. 2017, 12, 71–79. [Google Scholar] [CrossRef]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental stem cell and dental tissue regeneration. Front. Med. 2019, 13, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Cubedo, J. Adipose tissue depots and inflammation: Effects on plasticity and resident mesenchymal stem cell function. Cardiovasc. Res. 2017, 113, 1064–1073. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.I.; Kim, K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef]
- Minteer, D.; Marra, K.G.; Rubin, J.P. Adipose-derived mesenchymal stem cells: Biology and potential applications. Adv. Biochem. Eng. Biotechnol. 2013, 129, 59–71. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- Wilson, A.; Chee, M.; Butler, P.; Boyd, A.S. Isolation and Characterisation of Human Adipose-Derived Stem Cells. Methods Mol. Biol. 2019, 1899, 3–13. [Google Scholar] [CrossRef]
- Delle Monache, S.; Calgani, A.; Sanità, P.; Zazzeroni, F.; Gentile Warschauer, E.; Giuliani, A.; Amicucci, G.; Angelucci, A. Adipose-derived stem cells sustain prolonged angiogenesis through leptin secretion. Growth Factors 2016, 34, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells-a review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Futerman, A.H. Determination of the localization of gangliosides using anti-ganglioside antibodies: Comparison of fixation methods. J. Histochem. Cytochem. 1997, 45, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Kannagi, R.; Cai, B.H.; Huang, H.C.; Chao, C.C.; Sakuma, K. Gangliosides and Tumors. Methods Mol. Biol. 2018, 1804, 143–171. [Google Scholar] [CrossRef]
- Ngamukote, S.; Yanagisawa, M.; Ariga, T.; Ando, S.; Yu, R.K. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J. Neurochem. 2007, 103, 2327–2341. [Google Scholar] [CrossRef]
- Xu, J.; Fan, W.; Tu, X.X.; Zhang, T.; Hou, Z.J.; Guo, T.; Shu, X.; Luo, X.; Liu, Y.; Peng, F.; et al. Neural ganglioside GD2(+) cells define a subpopulation of mesenchymal stem cells in adult murine bone marrow. Cell. Physiol. Biochem. 2013, 32, 889–898. [Google Scholar] [CrossRef]
- Wang, Q.; Song, Y.H.; Tang, Z.; Wang, Z.P.; Xu, Q.; Bao, N. Effects of ganglioside GM1 and neural growth factor on neural stem cell proliferation and differentiation. Genet. Mol. Res. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Rossdam, C.; Konze, S.A.; Oberbeck, A.; Rapp, E.; Gerardy-Schahn, R.; von Itzstein, M.; Buettner, F.F.R. Approach for Profiling of Glycosphingolipid Glycosylation by Multiplexed Capillary Gel Electrophoresis Coupled to Laser-Induced Fluorescence Detection to Identify Cell-Surface Markers of Human Pluripotent Stem Cells and Derived Cardiomyocytes. Anal. Chem. 2019, 91, 6413–6418. [Google Scholar] [CrossRef]
- Battula, V.L.; Evans, K.W.; Hollier, B.G.; Shi, Y.; Marini, F.C.; Ayyanan, A.; Wang, R.Y.; Brisken, C.; Guerra, R.; Andreeff, M.; et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells 2010, 28, 1435–1445. [Google Scholar] [CrossRef]
- Battula, V.L.; Shi, Y.; Evans, K.W.; Wang, R.Y.; Spaeth, E.L.; Jacamo, R.O.; Guerra, R.; Sahin, A.A.; Marini, F.C.; Hortobagyi, G.; et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J. Clin. Investig. 2012, 122, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Ogden, A.T.; Waziri, A.E.; Lochhead, R.A.; Fusco, D.; Lopez, K.; Ellis, J.A.; Kang, J.; Assanah, M.; McKhann, G.M.; Sisti, M.B.; et al. Identification of A2B5+CD133- tumor- initiating cells in adult human gliomas. Neurosurgery 2008, 62, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Tchoghandjian, A.; Baeza, N.; Colin, C.; Cayre, M.; Metellus, P.; Beclin, C.; Ouafik, L.; Figarella-Branger, D. A2B5 cells from human glioblastoma have cancer stem cell properties. Brain Pathol. 2010, 20, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K.; Tsai, Y.T.; Ariga, T.; Yanagisawa, M. Structures, biosynthesis, and functions of gangliosides an overview. J. Oleo Sci. 2011, 60, 537–544. [Google Scholar] [CrossRef]
- Shao, C.; Anand, V.; Andreeff, M.; Battula, V.L. Ganglioside GD2: A novel therapeutic target in triple-negative breast cancer. Ann. N. Y. Acad. Sci. 2022, 1508, 35–53. [Google Scholar] [CrossRef]
- Priester, C.; MacDonald, A.; Dhar, M.; Bow, A. Examining the Characteristics and Applications of Mesenchymal, Induced Pluripotent, and Embryonic Stem Cells for Tissue Engineering Approaches across the Germ Layers. Pharmaceuticals 2020, 13, 344. [Google Scholar] [CrossRef]
| Gangliosides | Role in Stem Cells | Ref |
|---|---|---|
| GM3 |
| [47,55] |
| GM2 |
| [4,5,29] |
| GM1 |
| [56,57] |
| GD3 |
| [29,55,58,59] |
| GD2 |
| [26,55,60] |
| GD1a |
| [28,29,47,61,62] |
| GD1b |
| [63] |
| GT3, GT1c, GQ1c |
| [64] |
| GT1b |
| [55,65] |
| GQ1b |
| [65,66] |
| Gangliosides Expression | ||||||
|---|---|---|---|---|---|---|
| Undifferentiated | Osteogenic Differentiation | Chondrogenic Differentiation | Neurogenic Differentiation | Adipogenic Differentiation | Ref | |
| UC-MSCs | GM3 GM1 GD2 | n.i. | n.i. | n.i. n.i. GD2 | n.i. | [80,84,85] |
| BM-MSCs | GM3 GM2 GM1 GD3 GD2 GD1a | GM3 ↓ GM2 ↑ GM1 ↑ GD3 ↓ n.e. GD1a ↑↑ | GM3 ↑ ↓ n.e. GM1 ↑ GD3 ↑ n.i. GD1a ↑ | GM3 n.e. GM1 n.i. n.e. n.i. | n.i. | [26,28,29,35,37,47,52,80,84,85,87,88,89] |
| DPSCs | GM3 GM2 n.e. GD1a | GM3 ↑ GM2 ↑ n.e. GD1a ↑↑ | n.i. | GM3 GM2 ↓ GD3 ↑ GD1a ↑ | n.i. | [4,5,28,29,35,36,45,47] |
| ADSCs | GM3 GM2 GM1 GD3 GD2 GD1a | GM3 ↑ GM2 ↑ GM1 ↑ GD3 ↑↑ n.e. GD1a ↑ | GM3 ↑ GM2 ↑ GM1 ↓ GD3 ↓ n.e. n.e. | n.i. n.i. n.i. GD3 n.i. n.i. | GM3 ↑↑ GM2 ↑↑ GM1 ↑ GD3 ↑ GD2 ↓ GD1a ↑ | [26,36,45,47,52,54,90,91,92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santilli, F.; Fabrizi, J.; Pulcini, F.; Santacroce, C.; Sorice, M.; Delle Monache, S.; Mattei, V. Gangliosides and Their Role in Multilineage Differentiation of Mesenchymal Stem Cells. Biomedicines 2022, 10, 3112. https://doi.org/10.3390/biomedicines10123112
Santilli F, Fabrizi J, Pulcini F, Santacroce C, Sorice M, Delle Monache S, Mattei V. Gangliosides and Their Role in Multilineage Differentiation of Mesenchymal Stem Cells. Biomedicines. 2022; 10(12):3112. https://doi.org/10.3390/biomedicines10123112
Chicago/Turabian StyleSantilli, Francesca, Jessica Fabrizi, Fanny Pulcini, Costantino Santacroce, Maurizio Sorice, Simona Delle Monache, and Vincenzo Mattei. 2022. "Gangliosides and Their Role in Multilineage Differentiation of Mesenchymal Stem Cells" Biomedicines 10, no. 12: 3112. https://doi.org/10.3390/biomedicines10123112
APA StyleSantilli, F., Fabrizi, J., Pulcini, F., Santacroce, C., Sorice, M., Delle Monache, S., & Mattei, V. (2022). Gangliosides and Their Role in Multilineage Differentiation of Mesenchymal Stem Cells. Biomedicines, 10(12), 3112. https://doi.org/10.3390/biomedicines10123112








