The Vehicles of Calcium Hydroxide Pastes Interfere with Antimicrobial Effect, Biofilm Polysaccharidic Matrix, and Pastes’ Physicochemical Properties
Abstract
1. Introduction
2. Materials and Methods
- CH + propylene glycol (CHP), mixed in a ratio of 3:1 (powder weight/vehicle weight).
- UltraCal XS: CH + methylcellulose + barium sulfate (Ultradent Products Inc., South Jordan, UT, USA).
- Metapaste: CH + polypropylene glycol + barium sulfate (MetaBiomed Co., Ltd., Chungbuk, Korea).
- Metapex: CH + silicone oil + iodoform (MetaBiomed Co., Ltd., Chungbuk, Korea).
2.1. pH Analysis
2.2. Micro-Computed Tomographic Volumetric Analysis
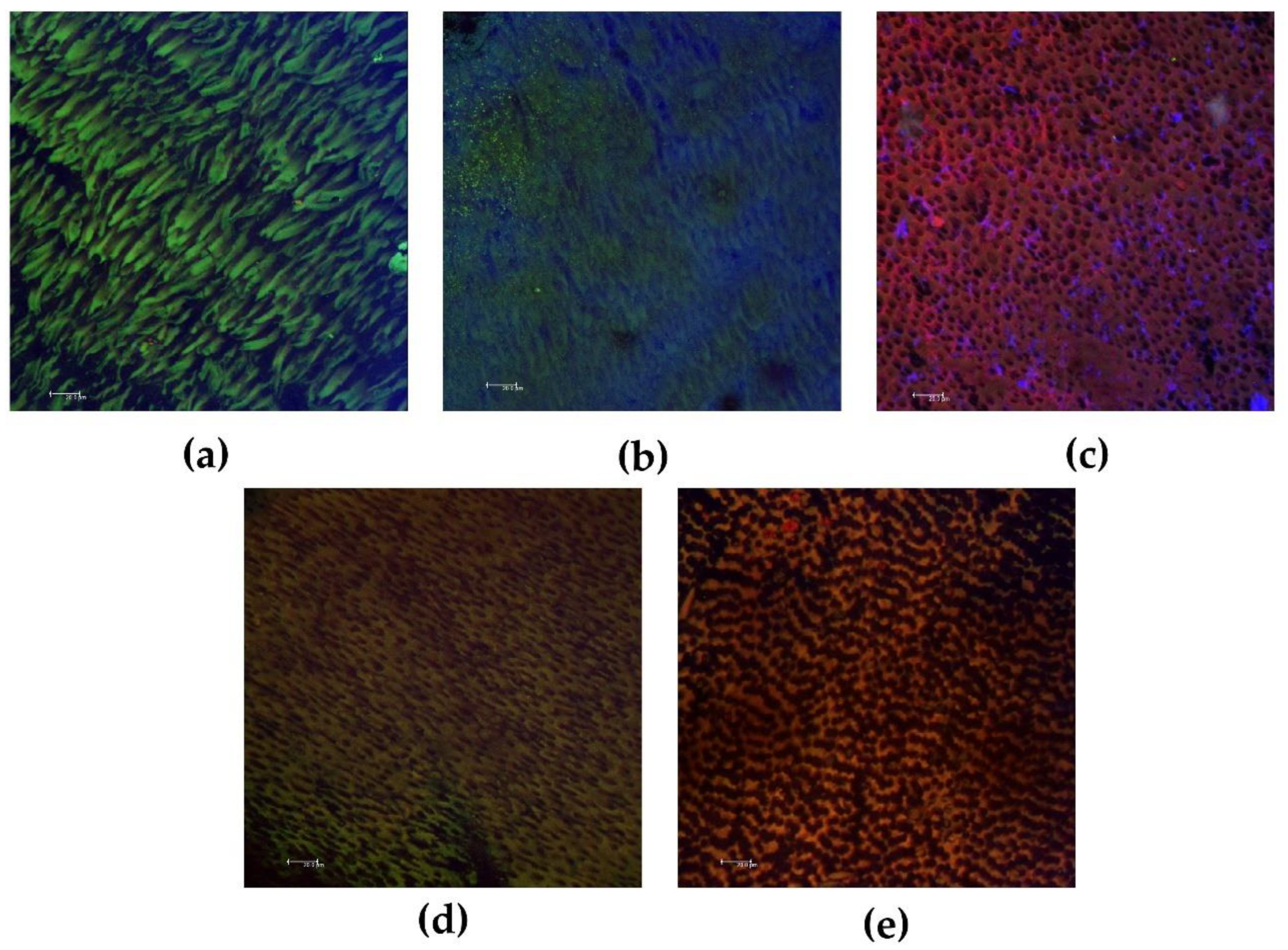
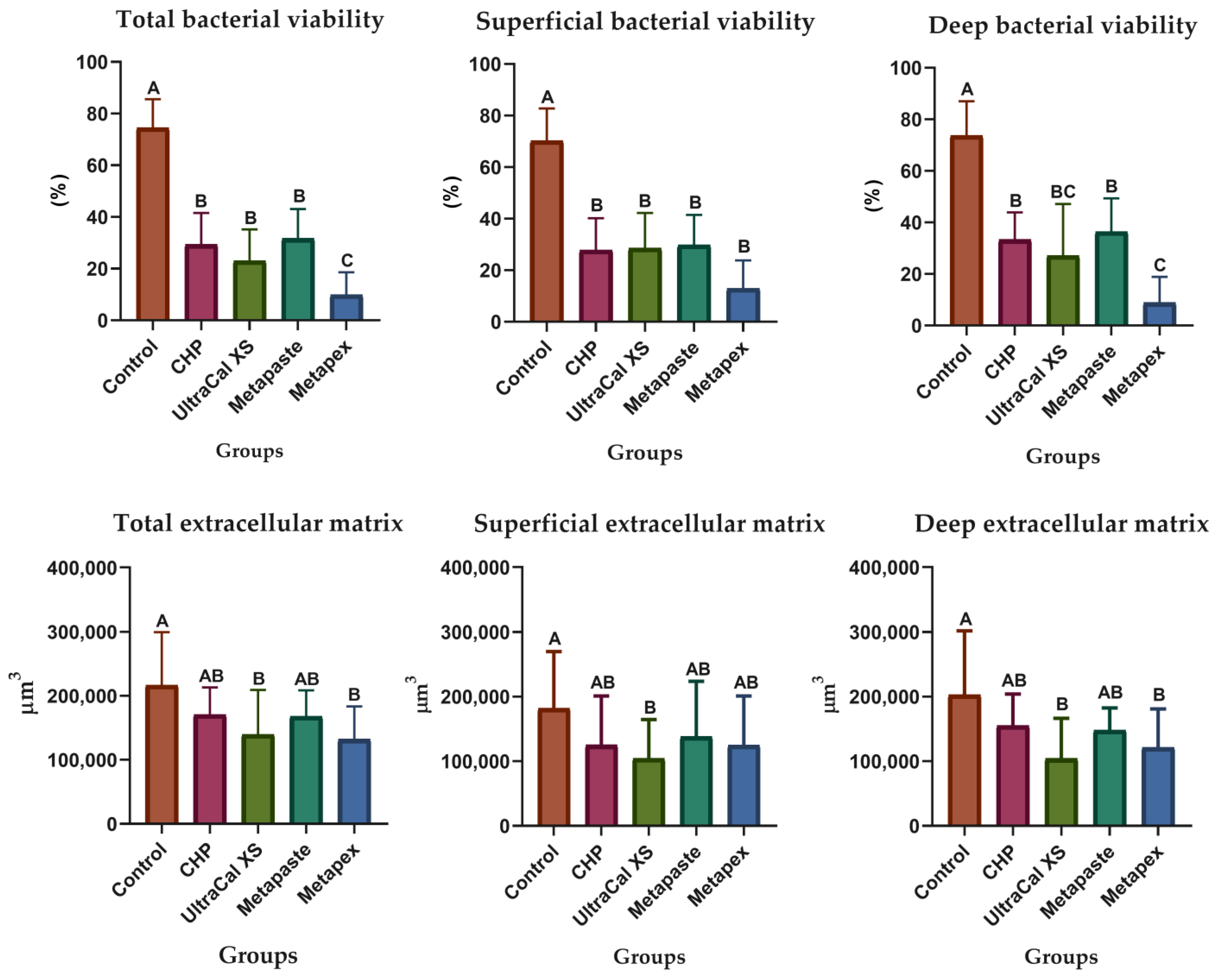
2.3. Antibiofilm Direct Contact Test
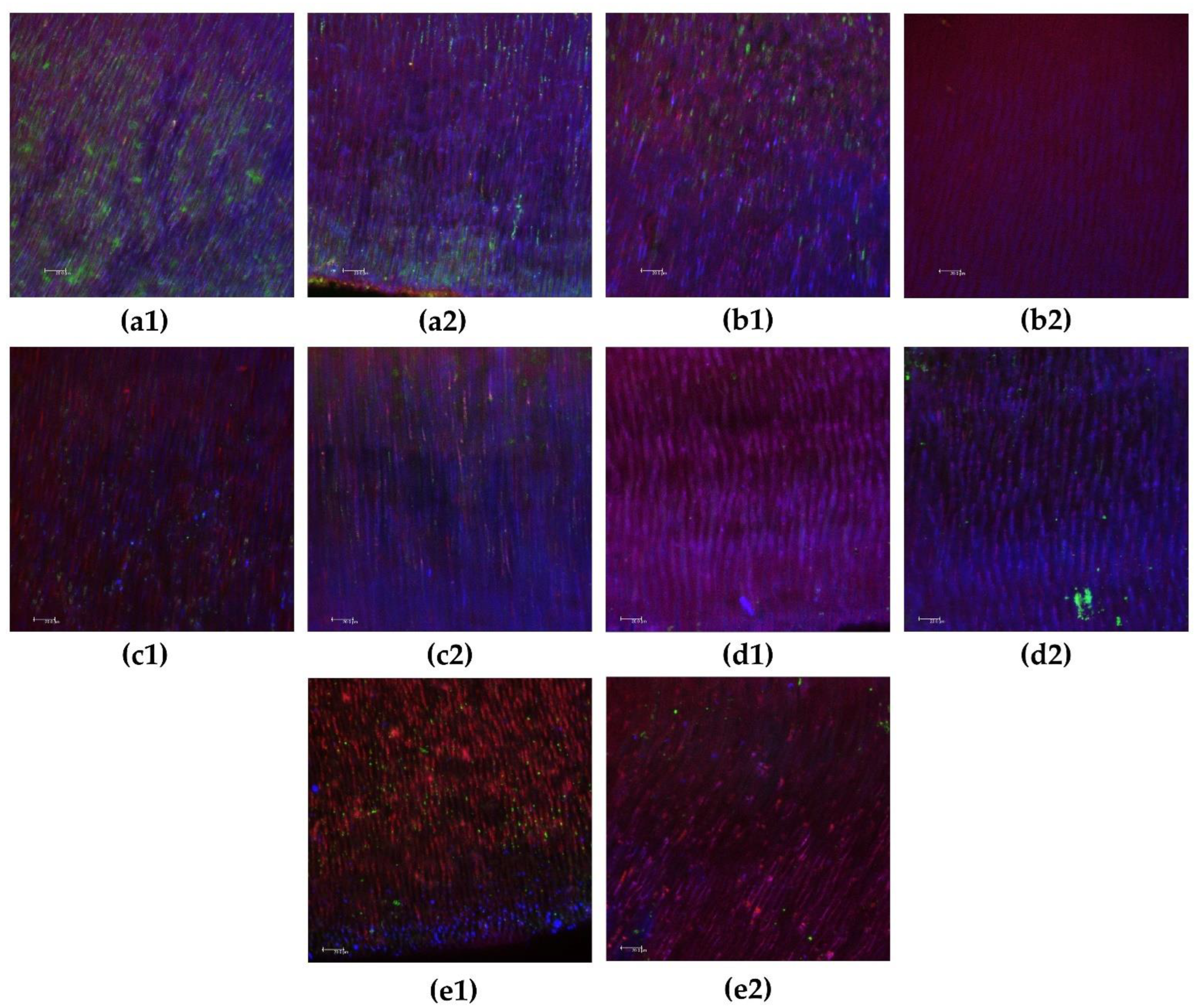
2.4. Antibiofilm Intratubular Test
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, T.C.; Vasconcelos, L.R.S.M.; Graeff, M.S.Z.; Ribeiro, M.C.M.; Duarte, M.A.H.; de Andrade, F.B. Intratubular decontamination ability and physicochemical properties of calcium hydroxide pastes. Clin. Oral Investig. 2019, 23, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Sirén, E.K.; Kerosuo, E.; Lavonius, E.; Meurman, J.H.; Haapasalo, M. Ca(OH)2 application modes: In vitro alkalinity and clinical effect on bacteria. Int. Endod. J. 2014, 47, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Zancan, R.F.; Vivan, R.R.; Lopes, M.R.M.; Weckwerth, P.H.; de Andrade, F.B.; Ponce, J.B.; Duarte, M.A. Antimicrobial Activity and Physicochemical Properties of Calcium Hydroxide Pastes Used as Intracanal Medication. J. Endod. 2016, 42, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.T.; Gomes, B.P.; Ferraz, C.C.; Sousa, E.L.; Teixeira, F.B.; Souza-Filho, F.J. Microorganisms from canals of root-filled teeth with periapical lesions. Int. Endod. J. 2003, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.B.; Torres, S.A.; Rosa, O.P.; Ferreira, C.M.; Garcia, R.B.; Marcucci, M.C.; Gomes, B.P. Antimicrobial effect of propolis and other substances against selected endodontic pathogens. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 709–716. [Google Scholar] [CrossRef]
- Ferreira, F.B.A.; Silva e Souza, P.A.; do Vale, M.S.; de Moraes, I.G.; Granjeiro, J.M. Evaluation of pH levels and calcium ion release in various calcium hydroxide endodontic dressings. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 388–392. [Google Scholar] [CrossRef]
- Oliveira, L.V.; da Silva, G.R.; Souza, G.L.; Magalhães, T.E.A.; Barbosa, G.L.R.; Turrioni, A.P.; Moura, C.C.G. A laboratory evaluation of cell viability, radiopacity and tooth discoloration induced by regenerative endodontic materials. Int. Endod. J. 2020, 53, 1140–1152. [Google Scholar] [CrossRef]
- Saoud, T.M.; Huang, G.T.; Gibbs, J.L.; Sigurdsson, A.; Lin, L.M. Management of Teeth with Persistent Apical Periodontitis after Root Canal Treatment Using Regenerative Endodontic Therapy. J. Endod. 2015, 41, 1743–1748. [Google Scholar] [CrossRef]
- Cwikla, S.J.; Bélanger, M.; Giguère, S.; Progulske-Fox, A.; Vertucci, F.J. Dentinal tubule disinfection using three calcium hydroxide formulations. J. Endod. 2005, 31, 50–52. [Google Scholar] [CrossRef]
- Morgental, R.D.; Vier-Pelisser, F.V.; Oliveira, S.D.; Antunes, F.C.; Cogo, D.M.; Kopper, P.M. Antibacterial activity of two MTA-based root canal sealers. Int. Endod. J. 2011, 44, 1128–1133. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Chiniforush, N.; Shahabi, S.; Palizvani, M.; Bahador, A. Antibacterial and Antibiofilm Efficacy of Antimicrobial Photodynamic Therapy Against Intracanal Enterococcus faecalis: An In Vitro Comparative Study with Traditional Endodontic Irrigation Solutions. J. Dent. 2018, 15, 197–204. [Google Scholar]
- Siqueira Jr, J.F.; Pérez, A.R.; Marceliano-Alves, M.F.; Provenzano, J.C.; Silva, S.G.; Pires, F.R.; Vieira, G.C.S.; Rôças, I.N.; Alves, F.R.F. What happens to unprepared root canal walls: A correlative analysis using micro-computed tomography and histology/scanning electron microscopy. Int. Endod. J. 2018, 51, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.C.; Dijkstra, R.J.B.; Petridis, X.; Sharma, P.K.; van de Meer, W.J.; van der Sluis, L.W.M.; de Andrade, F.B. Chemical and mechanical influence of root canal irrigation on biofilm removal from lateral morphological features of simulated root canals, dentine discs and dentinal tubules. Int. Endod. J. 2021, 54, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Busanello, F.H.; Petridis, X.; So, M.V.R.; Dijkstra, R.J.B.; Sharma, P.K.; van der Sluis, L.W.M. Chemical biofilm removal capacity of endodontic irrigants as a function of biofilm structure: Optical coherence tomography, confocal microscopy and viscoelasticity determination as integrated assessment tools. Int. Endod. J. 2019, 52, 461–474. [Google Scholar] [CrossRef]
- Pedrinha, V.F.; Cuellar, M.R.C.; Espedilla, E.G.V.; Duarte, M.A.H.; Andrade, F.B.; Rodrigues, P.A. Impact of irrigation protocols with some chelators and mechanical agitation on intratubular decontamination. Braz. Oral Res. 2021, 35, e127. [Google Scholar] [CrossRef]
- Duarte, M.A.; Midena, R.Z.; Zeferino, M.A.; Vivan, R.R.; Weckwerth, P.H.; Dos Santos, F.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Evaluation of pH and calcium ion release of calcium hydroxide pastes containing different substances. J. Endod. 2009, 35, 1274–1277. [Google Scholar] [CrossRef]
- Arias-Moliz, M.T.; Ordinola-Zapata, R.; Baca, P.; Ruiz-Linares, M.; García García, E.; Duarte, M.A.H.; Bramante, C.M.; Ferrer-Luque, C.M. Antimicrobial activity of Chlorhexidine, Peracetic acid and Sodium hypochlorite/etidronate irrigant solutions against Enterococcus faecalis biofilms. Int. Endod. J. 2015, 48, 1188–1193. [Google Scholar] [CrossRef]
- Méndez, D.A.C.; Cuéllar, M.R.C.; Pedrinha, V.F.; Espedilla, E.G.V.; de Andrade, F.B.; Rodrigues, P.A.; Cruvinel, T. Effects of curcumin-mediated antimicrobial photodynamic therapy associated to different chelators against Enterococcus faecalis biofilms. Photodiagnosis Photodyn. Ther. 2021, 35, 102464. [Google Scholar] [CrossRef] [PubMed]
- Cunha Neto, M.A.D.; Coêlho, J.A.; Pinto, K.P.; Cuellar, M.R.C.; Marcucci, M.C.; Silva, E.J.N.L.; Andrade, F.B.; Sassone, L.M. Antibacterial Efficacy of Triple Antibiotic Medication with Macrogol (3Mix-MP), Traditional Triple Antibiotic Paste, Calcium Hydroxide, and Ethanol Extract of Propolis: An Intratubular Dentin Ex Vivo Confocal Laser Scanning Microscopic Study. J. Endod. 2021, 47, 1609–1616. [Google Scholar] [CrossRef]
- Oda, D.F.; Duarte, M.A.H.; Andrade, F.B.; Moriyama, L.T.; Bagnato, V.S.; de Moraes, I.G. Antimicrobial action of photodynamic therapy in root canals using LED curing light, curcumin and carbopol gel. Int. Endod. J. 2019, 52, 1010–1019. [Google Scholar] [CrossRef]
- Haapasalo, M.; Orstavik, D. In vitro infection and disinfection of dentinal tubules. J Dent Res 1987, 66, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.B.; Arias, M.P.; Maliza, A.G.; Duarte, M.A.; Graeff, M.S.; Amoroso-Silva, P.A.; Midena, R.Z.; Moraes, I.G. A new improved protocol for in vitro intratubular dentinal bacterial contamination for antimicrobial endodontic tests: Standardization and validation by confocal laser scanning microscopy. J. Appl. Oral Sci. 2015, 23, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Z.; Shen, Y.; Haapasalo, M. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J. Endod. 2011, 37, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Giardino, L.; Del Fabbro, M.; Cesario, F.; Fernandes, F.S.; Andrade, F.B. Antimicrobial effectiveness of combinations of oxidant and chelating agents in infected dentine: An ex vivo confocal laser scanning microscopy study. Int. Endod. J. 2018, 51, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Sydney, G.B.; Pesce, H.F.; Felippe Júnior, O. Dentinal diffusion of hydroxyl ions of various calcium hydroxide pastes. Braz Dent. J. 1995, 6, 5–9. [Google Scholar] [PubMed]
- Simon, S.T.; Bhat, K.S.; Francis, R. Effect of four vehicles on the pH of calcium hydroxide and the release of calcium ion. Oral Surg Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 80, 459–464. [Google Scholar]
- Cruz Junior, J.A.; Coelho, M.S.; Kato, A.S.; Vivacqua-Gomes, N.; Fontana, C.E.; Rocha, D.G.; da Silveira Bueno, C.E. The Effect of Foraminal Enlargement of Necrotic Teeth with the Reciproc System on Postoperative Pain: A Prospective and Randomized Clinical Trial. J. Endod. 2016, 42, 8–11. [Google Scholar] [CrossRef]
- Estrela, C.; Pécora, J.D.; Souza-Neto, M.D.; Estrela, C.R.; Bammann, L.L. Effect of vehicle on antimicrobial properties of calcium hydroxide pastes. Braz. Dent. J. 1999, 10, 63–72. [Google Scholar]
- Fava, L.R.; Saunders, W.P. Calcium hydroxide pastes: Classification and clinical indications. Int. Endod. J. 1999, 32, 257–282. [Google Scholar] [CrossRef]
- Leonardo, M.R.; Silveira, F.F.; Silva, L.A.; Tanomaru Filho, M.; Utrilla, L.S. Calcium hydroxide root canal dressing. Histopathological evaluation of periapical repair at different time periods. Braz. Dent. J. 2002, 13, 17–22. [Google Scholar]
- Leonardo, M.R.; Hernandez, M.E.; Silva, L.A.; Tanomaru-Filho, M. Effect of a calcium hydroxide-based root canal dressing on periapical repair in dogs: A histological study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.N.; Park, J.S.; Jeon, S.Y.; Park, K.H. Carboxymethylcellulose (CMC) formed nanogels with branched poly(ethyleneimine) (bPEI) for inhibition of cytotoxicity in human MSCs as a gene delivery vehicles. Carbohydr. Polym. 2015, 20, 265–275. [Google Scholar] [CrossRef]
- Jacobs, J.C.; Troxel, A.; Ehrlich, Y.; Spolnik, K.; Bringas, J.S.; Gregory, R.L.; Yassen, G.H. Antibacterial Effects of Antimicrobials Used in Regenerative Endodontics against Biofilm Bacteria Obtained from Mature and Immature Teeth with Necrotic Pulps. J. Endod. 2017, 43, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Altomare, L.; Cochis, A.; Carletta, A.; Rimondini, L.; Farè, S. Thermo-responsive methylcellulose hydrogels as temporary substrate for cell sheet biofabrication. J. Mater. Sci. Mater. Med. 2016, 27, 95. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, R.C.; Ribeiro, M.S.; de Lima Machado, M.E. Determination of the minimum inhibitory concentration of four medicaments used as intracanal medication. Aust. Endod. J. 2007, 33, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.E.L.; Martins, G.H.R.; Carreira, K.; Peixoto, K.T.; Nabeshima, C.K.; Gales, A.C. Antimicrobial effect of two endodontic medicaments with diferente exposure times, and the morphologic alterations caused to Enterococcus faecalis. Rev. Odonto Ciênc. 2011, 26, 336–340. [Google Scholar] [CrossRef]
- Sirén, E.K.; Haapasalo, M.P.; Waltimo, T.M.; Ørstavik, D. In vitro antibacterial effect of calcium hydroxide combined with chlorhexidine or iodine potassium iodide on Enterococcus faecalis. Eur. J. Oral Sci. 2004, 112, 326–331. [Google Scholar] [CrossRef]
- Ferrer-Luque, C.M.; Bejarano, I.; Ruiz-Linares, M.; Baca, P. Reduction in Enteroccocus faecalis counts: A comparison between rotary and reciprocating systems. Int. Endod. J. 2014, 47, 380–386. [Google Scholar] [CrossRef]
- Pacios, M.G.; de la Casa, M.L.; de Bulacio, M.l.; López, M.E. Influence of different vehicles on the pH of calcium hydroxide pastes. J. Oral Sci. 2004, 46, 107–111. [Google Scholar] [CrossRef]
- AlShwaimi, E.; Bogari, D.; Ajaj, R.; Al-Shahrani, S.; Almas, K.; Majeed, A. In Vitro Antimicrobial Effectiveness of Root Canal Sealers against Enterococcus faecalis: A Systematic Review. J. Endod. 2016, 42, 1588–1597. [Google Scholar] [CrossRef]
- Gomes, B.P.; Ferraz, C.C.; Vianna, M.E.; Rosalen, P.L.; Zaia, A.A.; Teixeira, F.B.; Souza-Filho, F.J. In vitro antimicrobial activity of calcium hydroxide pastes and their vehicles against selected microorganisms. Braz. Dent. J. 2002, 13, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Tawakoli, P.N.; Ragnarsson, K.T.; Rechenberg, D.K.; Mohn, D.; Zehnder, M. Effect of endodontic irrigants on biofilm matrix polysaccharides. Int. Endod. J. 2017, 50, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, T.; Takenaka, S.; Wakamatsu, R.; Sakaue, Y.; Narisawa, N.; Senpuku, H.; Ohshima, H.; Terao, Y.; Okiji, T. Residual structure of Streptococcus mutans biofilm following complete disinfection favors secondary bacterial adhesion and biofilm re-development. PLoS ONE 2015, 10, e0116647. [Google Scholar] [CrossRef] [PubMed]
| Groups | 7 Days | 15 Days | 30 Days |
|---|---|---|---|
| Distilled water | 7.03 | 7.03 | 7.03 |
| CHP | 10.5 Aa (9.80–10.8) | 7.6 Ba (7.3–7.8) | 7.5 Ba (7.5–7.6) |
| UltraCal XS | 10.3 Aa (9.6–10.8) | 7.5 Ba (7.4–8.2) | 7.7 Ba (7.4–8.0) |
| Metapaste | 10.6 Aa (10.2–10.7) | 8.9 Bb (8.0–9.4) | 8.3 Ba (7.5–8.9) |
| Metapex | 9.40 Ab (8.90–10.5) | 7.5 Ba (7.3–9.0) | 6.3 Bb (5.7–6.6) |
| Groups | Initial Volume | Final Volume | Volumetric Alteration |
|---|---|---|---|
| CHP | 2.48 Aa (1.02) | 2.17 Ab (1.12) | 17.66 A (10.54) |
| UltraCal XS | 2.26 Aa (0.84) | 1.71 Ab (0.64) | 19.46 A (12.03) |
| Metapaste | 2.66 Aa (0.86) | 1.40 Ab (0.52) | 37.11 A (24.48) |
| Metapex | 2.91 Aa (0.84) | 1.69 Ab (0.56) | 37.07 A (18.53) |
| Groups | Bacterial Viability | Extracellular Matrix |
|---|---|---|
| Control | 77.9 a (48.5–96.4) | 720,259 a (611,556–1,367,897) |
| CHP | 24.4 ab (5.24–61.2) | 339,421 ab (120,756–1,167,868) |
| UltraCal XS | 7.74 b (1.29–26.0) | 264,119 b (8116–782,927) |
| Metapaste | 4.33 bc (0.41–28.9) | 580,443 a (88,202–1,682,094) |
| Metapex | 1.72 c (0.03–10.3) | 145,552 b (478–1,529,176) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrinha, V.F.; Cuellar, M.R.C.; de Barros, M.C.; Titato, P.C.G.; Shahbazi, M.-A.; Sharma, P.K.; de Andrade, F.B. The Vehicles of Calcium Hydroxide Pastes Interfere with Antimicrobial Effect, Biofilm Polysaccharidic Matrix, and Pastes’ Physicochemical Properties. Biomedicines 2022, 10, 3123. https://doi.org/10.3390/biomedicines10123123
Pedrinha VF, Cuellar MRC, de Barros MC, Titato PCG, Shahbazi M-A, Sharma PK, de Andrade FB. The Vehicles of Calcium Hydroxide Pastes Interfere with Antimicrobial Effect, Biofilm Polysaccharidic Matrix, and Pastes’ Physicochemical Properties. Biomedicines. 2022; 10(12):3123. https://doi.org/10.3390/biomedicines10123123
Chicago/Turabian StylePedrinha, Victor Feliz, Maricel Rosario Cardenas Cuellar, Mirela Cesar de Barros, Pedro César Gomes Titato, Mohammad-Ali Shahbazi, Prashant Kumar Sharma, and Flaviana Bombarda de Andrade. 2022. "The Vehicles of Calcium Hydroxide Pastes Interfere with Antimicrobial Effect, Biofilm Polysaccharidic Matrix, and Pastes’ Physicochemical Properties" Biomedicines 10, no. 12: 3123. https://doi.org/10.3390/biomedicines10123123
APA StylePedrinha, V. F., Cuellar, M. R. C., de Barros, M. C., Titato, P. C. G., Shahbazi, M.-A., Sharma, P. K., & de Andrade, F. B. (2022). The Vehicles of Calcium Hydroxide Pastes Interfere with Antimicrobial Effect, Biofilm Polysaccharidic Matrix, and Pastes’ Physicochemical Properties. Biomedicines, 10(12), 3123. https://doi.org/10.3390/biomedicines10123123










