Loss of the Synuclein Family Members Differentially Affects Baseline- and Apomorphine-Associated EEG Determinants in Single-, Double- and Triple-Knockout Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Implantation of Electrodes and EEG Recording
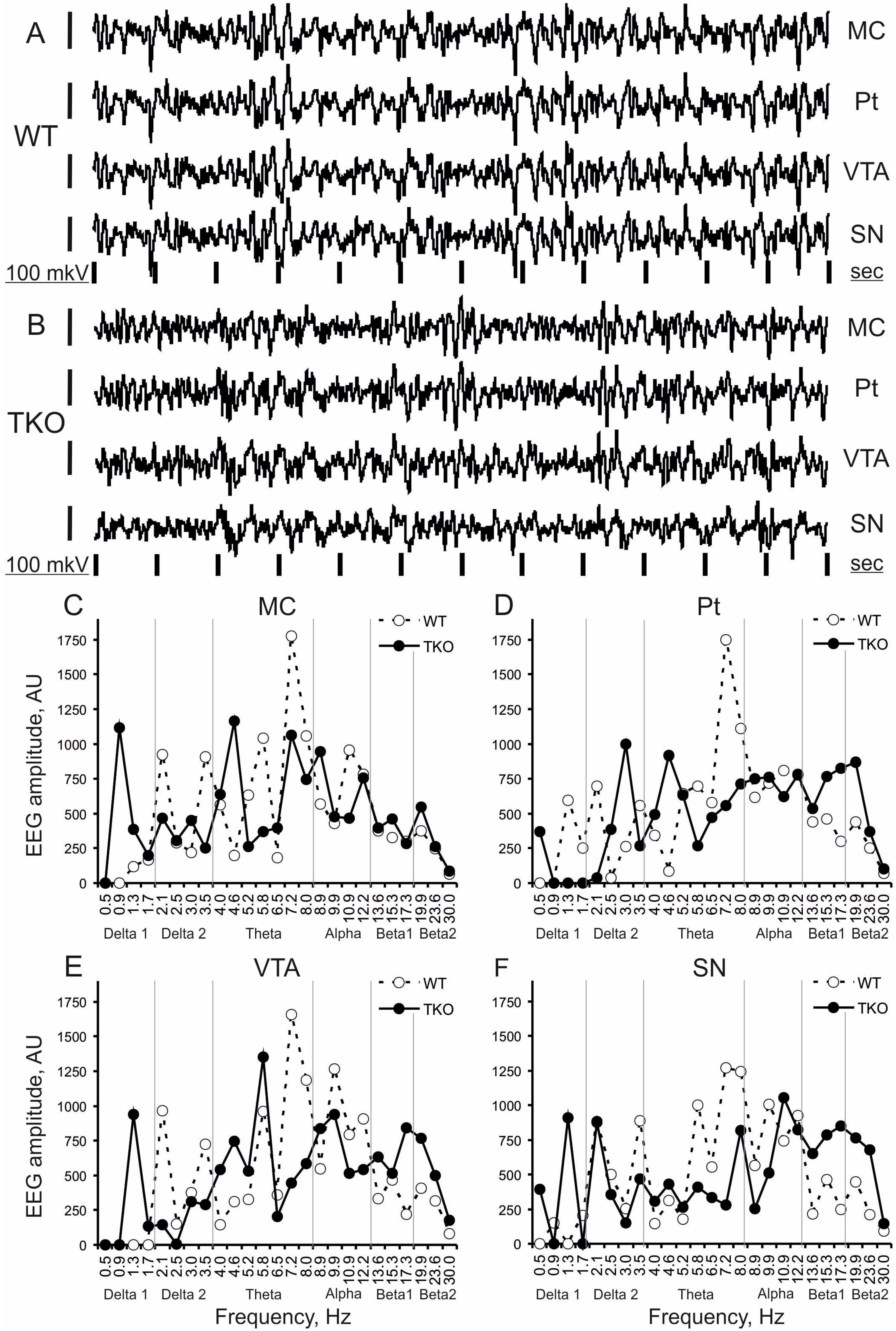
2.3. Computation of EEG Frequency Spectra
2.4. Statistics
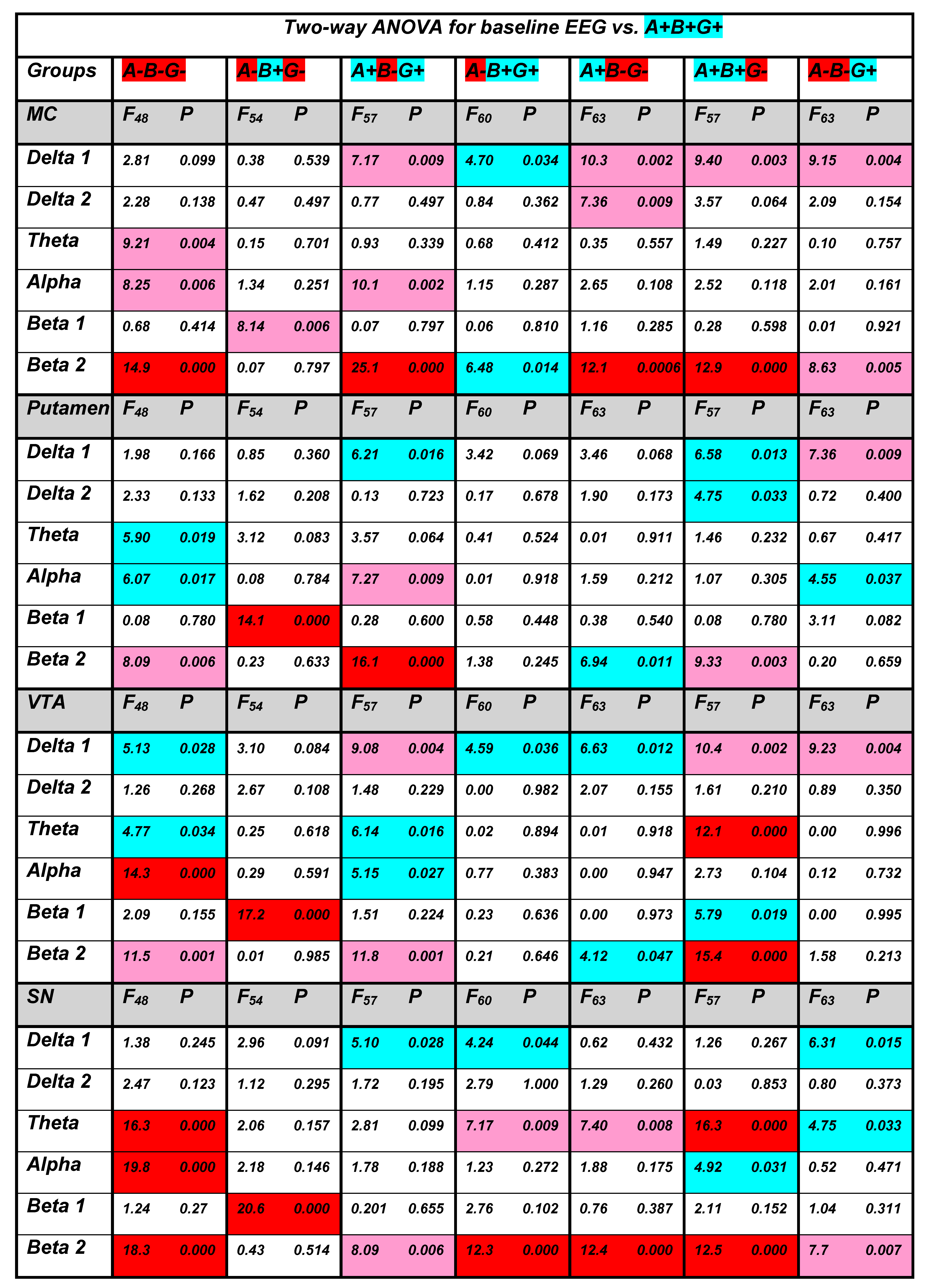
3. Results
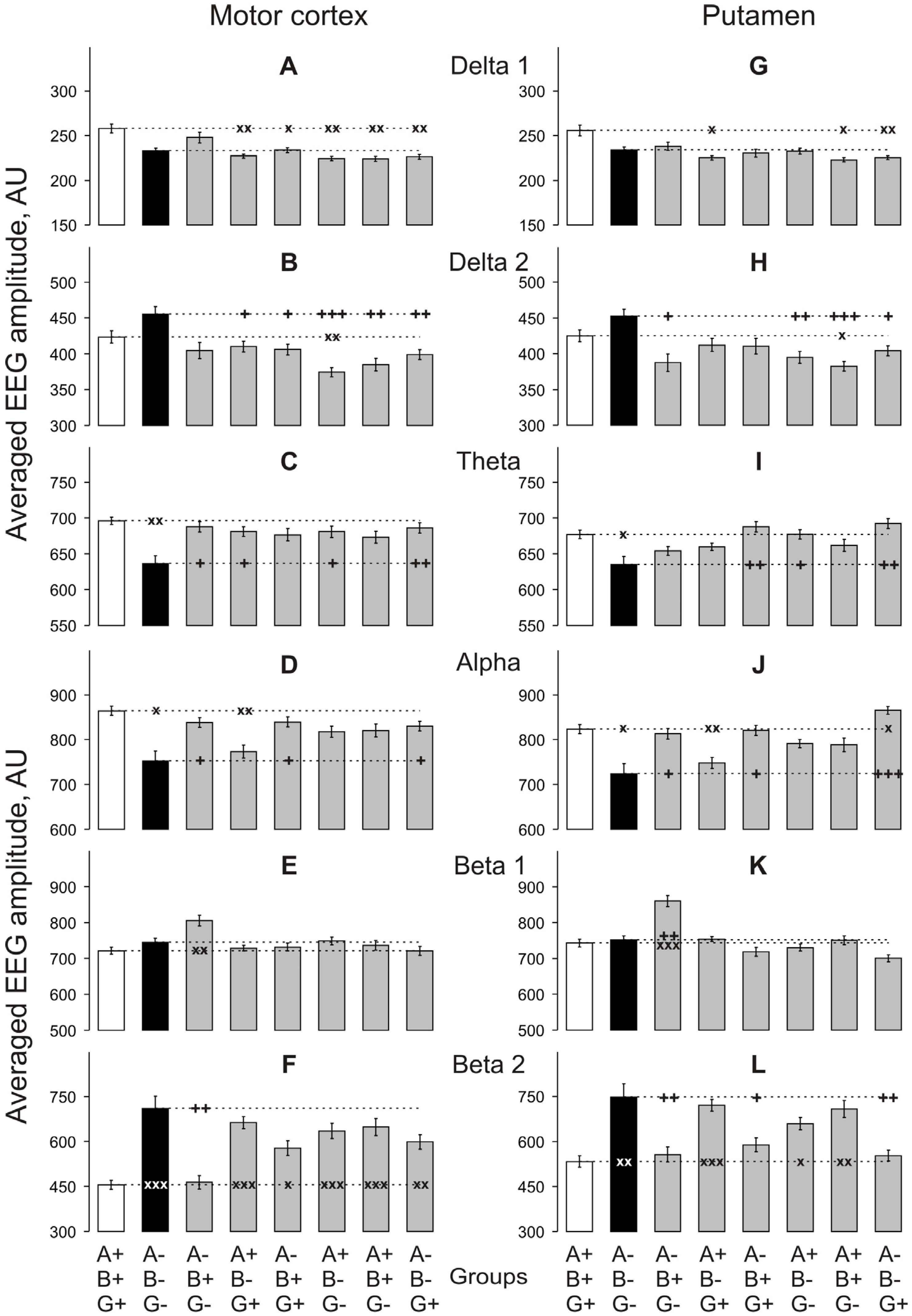
3.1. Baseline EEG
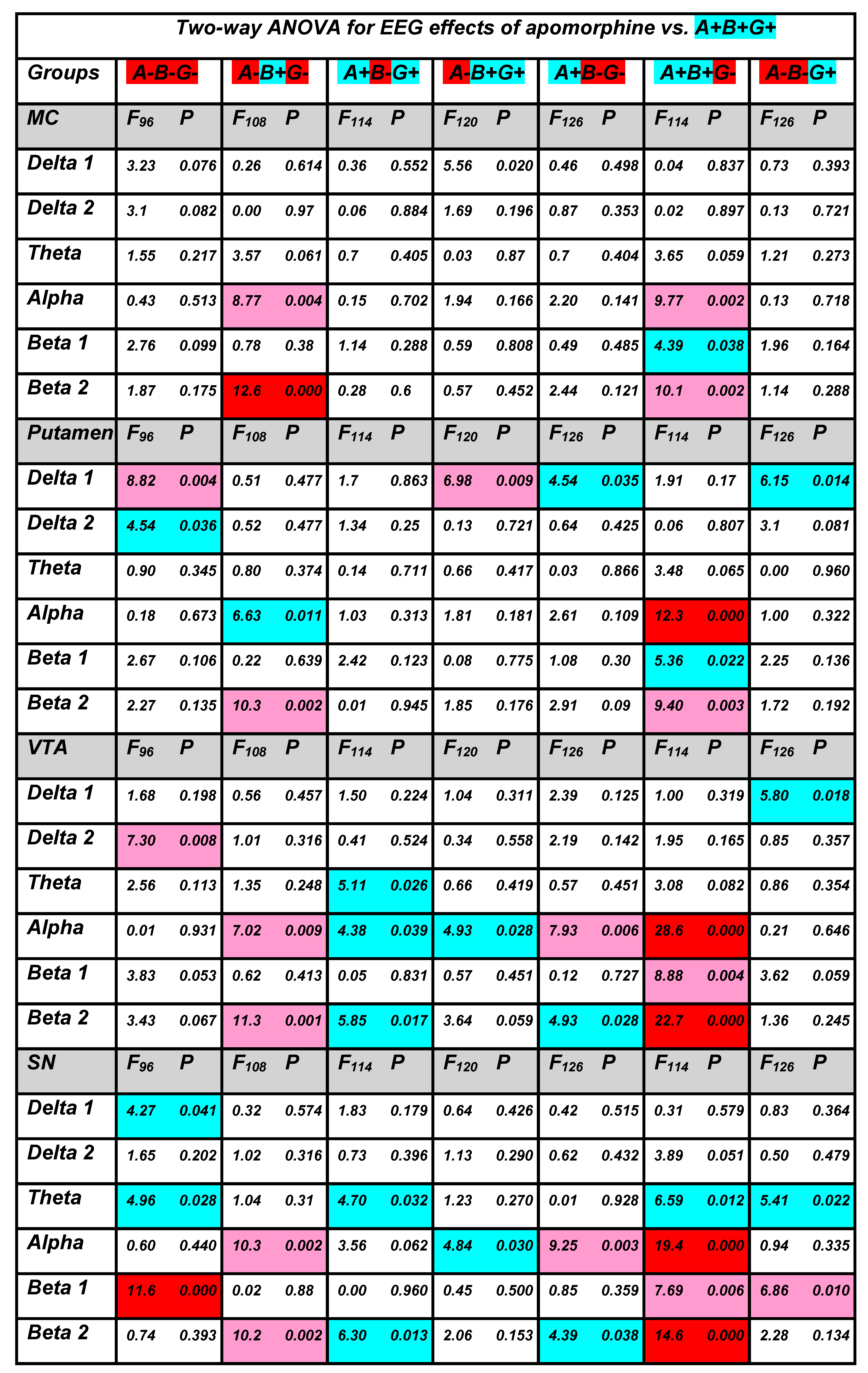
3.2. Apomorphine Effects
3.2.1. Apomorphine vs. Saline
3.2.2. APO Effects in Different Groups vs. Those in WT Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- George, J.M. The synucleins. Gen. Biol. 2001, 3, 3002.1–3002.6. [Google Scholar] [CrossRef] [PubMed]
- Burré, J. The Synaptic Function of α-Synuclein. J. Park. Dis. 2015, 5, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.W.P.; Buell, A.K.; Michaels, T.C.T.; Meisl, G.; Carozza, J.; Flagmeier, P.; Vendruscolo, M.; Knowles, T.P.J.; Dobson, C.M.; Galvagnion, C. β-Synuclein suppresses both the initiation and amplification steps of α-synuclein aggregation via competitive binding to surfaces. Sci. Rep. 2016, 6, 36010. [Google Scholar] [CrossRef] [PubMed]
- Venda, L.L.; Cragg, S.J.; Buchman, V.L.; Wade-Martins, R. α-Synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci. 2010, 33, 559–568. [Google Scholar] [CrossRef]
- Raina, A.; Leite, K.; Chakrabarti, K.S.; Guerin, S.; Mahajani, S.U.; Voll, D.; Becker, S.; Griesinger, C.; Bähr, M.; Kügler, S. Dopamine promotes the neurodegenerative potential of β-synuclein. J. Neurochem. 2021, 156, 674–691. [Google Scholar] [CrossRef]
- Ninkina, N.; Millership, S.J.; Peters, O.M.; Connor-Robson, N.; Chaprov, K.; Kopylov, A.T.; Montoya, A.; Kramer, H.; Withers, D.J.; Buchman, V.L. β-synuclein potentiates synaptic vesicle dopamine uptake and rescues dopaminergic neurons from MPTP-induced death in the absence of other synucleins. J. Biol. Chem. 2021, 297, 101375. [Google Scholar] [CrossRef]
- Dev, K.K.; Hofele, K.; Barbieri, S.; Buchman, V.L.; Van der Putten, H. Alpha-synuclein and its molecular pathophysiological role in neurodegenerative disease. Neuropharmacology 2003, 45, 14–44. [Google Scholar] [CrossRef]
- Carnazza, K.E.; Komer, L.E.; Xie, Y.X.; Pineda, A.; Briano, J.A.; Gao, V.; Na, Y.; Ramlall, T.; Buchman, V.L.; Eliezer, D.; et al. Synaptic vesicle binding of α-synuclein is modulated by β- and γ-synucleins. Cell Rep. 2022, 39, 110675. [Google Scholar] [CrossRef]
- Winham, C.L.; Le, T.; Jellison, E.R.; Silver, A.C.; Levesque, A.A.; Koob, A.O. γ-Synuclein induces human cortical astrocyte proliferation and subsequent BDNF expression and release. Neuroscience 2019, 410, 41–54. [Google Scholar] [CrossRef]
- Ducas, V.C.; Rhoades, E. Quantifying interactions of β-synuclein and γ-synuclein with model membranes. J. Mol. Biol. 2012, 423, 528–539. [Google Scholar] [CrossRef]
- Pavia-Collado, R.; Rodríguez-Aller, R.; Alarcón-Arís, D.; Miquel-Rio, L.; Ruiz-Bronchal, E.; Paz, V.; Campa, L.; Galofré, M.; Sgambato, V.; Bortolozzi, A. Up and Down γ-Synuclein Transcription in Dopamine Neurons Translates into Changes in Dopamine Neurotransmission and Behavioral Performance in Mice. Int. J. Mol. Sci. 2022, 23, 1807. [Google Scholar] [CrossRef] [PubMed]
- Visanji, N.P.; Brotchie, J.M.; Kalia, L.V.; Koprich, J.B.; Tandon, A.; Watts, J.C.; Lang, A.E. α-Synuclein-Based Animal Models of Parkinson’s Disease: Challenges and Opportunities in a New Era. Trends Neurosci. 2016, 39, 750–762. [Google Scholar] [CrossRef]
- Buchman, V.L.; Ninkina, N. Modulation of α-synuclein expression in transgenic animals for modelling synucleinopathies—Is the juice worth the squeeze? Neurotox. Res. 2008, 14, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.C.; Schmidt, O.; Ninkina, N.; Jones, P.A.; Sharkey, J.; Buchman, V.L. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J. Neurochem. 2004, 89, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Maroteaux, L.; Campanelli, J.T.; Scheller, R.H. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988, 8, 2804–2815. [Google Scholar] [CrossRef]
- Greten-Harrison, B.; Polydoro, M.; Morimoto-Tomita, M.; Diao, L.; Williams, A.M.; Nie, E.H.; Makani, S.; Tian, N.; Castillo, P.E.; Buchman, V.L.; et al. αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc. Natl. Acad. Sci. USA 2010, 107, 19573–19578. [Google Scholar] [CrossRef] [PubMed]
- Ninkina, N.; Tarasova, T.V.; Chaprov, K.D.; Roman, A.Y.; Kukharsky, M.S.; Kolik, L.G.; Ovchinnikov, R.; Ustyugov, A.A.; Durnev, A.D.; Buchman, V.L. Alterations in the nigrostriatal system following conditional inactivation of α-synuclein in neurons of adult and aging mice. Neurobiol. Aging 2020, 91, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Connor-Robson, N.; Peters, O.M.; Millership, S.; Ninkina, N.; Buchman, V.L. Combinational losses of synucleins reveal their differential requirements for compensating age-dependent alterations in motor behavior and dopamine metabolism. Neurobiol. Aging 2016, 46, 107–112. [Google Scholar] [CrossRef]
- Chandra, S.; Fornai, F.; Kwon, H.-B.; Yazdani, U.; Atasoy, D.; Liu, X.; Hammer, R.E.; Battaglia, G.; German, D.C.; Castillo, P.E.; et al. Double-knockout mice for alpha- and beta-synucleins: Effect on synaptic functions. Proc. Natl. Acad. Sci. USA 2004, 101, 14966–14971. [Google Scholar] [CrossRef]
- Anwar, S.; Peters, O.; Millership, S.; Ninkina, N.; Doig, N.; Connor-Robson, N.; Threlfell, S.; Kooner, G.; Deacon, R.M.; Bannerman, D.M.; et al. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J. Neurosci. 2011, 31, 7264–7274. [Google Scholar] [CrossRef]
- Saleh, H.; Saleh, A.; Yao, H.; Cui, J.; Shen, Y.; Li, R. Mini review: Linkage between alpha-Synuclein protein and cognition. Transl. Neurodegener. 2015, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Kokhan, V.S.; Afanasyeva, M.A.; Van’kin, G.I. α-Synuclein knockout mice have cognitive impairments. Behav. Brain Res. 2012, 231, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Kokhan, V.S.; Bolkunov, A.V.; Ustiugov, A.A.; Van’kin, G.I.; Shelkovnikova, T.A.; Redkozubova, O.M.; Strekalova, T.V.; Bukhman, V.L.; Ninkina, N.N.; Bachurin, S.O. Targeted inactivation of gamma-synuclein gene affects anxiety and exploratory behaviour of mice. Zhurnal Vyss. Nervn. Deiatelnosti Im. IP Pavlov. 2011, 61, 85–93. [Google Scholar]
- Kokhan, V.S.; Van’kin, G.I.; Bachurin, S.O.; Shamakina, I.Y. Differential involvement of the gamma-synuclein in cognitive abilities on the model of knockout mice. BMC Neurosci. 2013, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Oswal, A.; Brown, P.; Litvak, V. Synchronized neural oscillations and the pathophysiology of Parkinson’s disease. Curr. Opin. Neurol. 2013, 26, 662–670. [Google Scholar] [CrossRef]
- Nimmrich, V.; Draguhn, A.; Axmacher, N. Neuronal Network Oscillations in Neurodegenerative Diseases. Neuromolecular Med. 2015, 17, 270–284. [Google Scholar] [CrossRef]
- Womelsdorf, T.; Schoffelen, J.M.; Oostenveld, R.; Singer, W.; Desimone, R.; Engel, A.K.; Fries, P. Modulation of neuronal interactions through neuronal synchronization. Science 2007, 316, 1609–1612. [Google Scholar] [CrossRef]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012, 13, 407–420. [Google Scholar] [CrossRef]
- Brown, P. Oscillatory nature of human basal ganglia activity: Relationship to the pathophysiology of Parkinson’s disease. Mov. Disord. 2003, 18, 357–363. [Google Scholar] [CrossRef]
- Bousleiman, H.; Zimmermann, R.; Ahmed, S.; Hardmeier, M.; Hatz, F.; Schindler, C.; Roth, V.; Gschwandtner, U.; Fuhr, P. Power spectra for screening parkinsonian patients for mild cognitive impairment. Ann. Clin. Transl. Neurol. 2014, 1, 884–890. [Google Scholar] [CrossRef]
- Vorobyov, V.; Sengpiel, F. Apomorphine-induced differences in cortical and striatal EEG and their glutamatergic mediation in 6-hydroxydopamine-treated rats. Exp. Brain Res. 2008, 191, 277–287. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Xie, J.; Chen, D.; Geng, X.; Sun, S.; Liu, B.; Wang, M. Beta band modulation by dopamine D2 receptors in the primary motor cortex and pedunculopontine nucleus in a rat model of Parkinson’s disease. Brain Res. Bull. 2022, 181, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Brittain, J.S.; Brown, P. Oscillations and the basal ganglia: Motor control and beyond. Neuroimage 2014, 85 Pt 2, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Oscillatory activity in the cortico-basal ganglia-thalamic neural circuits in Parkinson’s disease. Eur. J. Neurosci. 2018, 48, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- Sharott, A.; Magill, P.J.; Harnack, D.; Kupsch, A.; Meissner, W.; Brown, P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur. J. Neurosci. 2005, 21, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Prosperetti, C.; Di Giovanni, G.; Stefani, A.; Möller, J.C.; Galati, S. Acute nigro-striatal blockade alters cortico-striatal encoding: An in vivo electrophysiological study. Exp. Neurol. 2013, 247, 730–736. [Google Scholar] [CrossRef]
- McDowell, K.A.; Shin, D.; Roos, K.P.; Chesselet, M.F. Sleep dysfunction and EEG alterations in mice overexpressing alpha-synuclein. J. Park. Dis. 2014, 4, 531–539. [Google Scholar] [CrossRef]
- Caviness, J.N.; Lue, L.F.; Hentz, J.G.; Schmitz, C.T.; Adler, C.H.; Shill, H.A.; Sabbagh, M.N.; Beach, T.G.; Walker, D.G. Cortical phosphorylated α-Synuclein levels correlate with brain wave spectra in Parkinson’s disease. Mov. Disord. 2016, 31, 1012–1019. [Google Scholar] [CrossRef]
- Ninkina, N.; Papachroni, K.; Robertson, D.C.; Schmidt, O.; Delaney, L.; O’Neill, F.; Court, F.; Rosenthal, A.; Fleetwood-Walker, S.M.; Davies, A.M.; et al. Neurons expressing the highest levels of γ-synuclein are unaffected by targeted inactivation of the gene. Mol. Cell. Biol. 2003, 23, 8233–8245. [Google Scholar] [CrossRef]
- Ninkina, N.; Connor-Robson, N.; Ustyugov, A.A.; Tarasova, T.V.; Shelkovnikova, T.A.; Buchman, V.L. A novel resource for studying function and dysfunction of α-synuclein: Mouse lines for modulation of endogenous Snca gene expression. Sci. Rep. 2015, 5, 16615. [Google Scholar] [CrossRef]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Al-Wandi, A.; Ninkina, N.; Millership, S.; Williamson, S.J.; Jones, P.A.; Buchman, V.L. Absence of alpha-synuclein affects dopamine metabolism and synaptic markers in the striatum of aging mice. Neurobiol. Aging 2010, 31, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Goloborshcheva, V.V.; Chaprov, K.D.; Teterina, E.V.; Ovchinnikov, R.; Buchman, V.L. Reduced complement of dopaminergic neurons in the substantia nigra pars compacta of mice with a constitutive “low footprint” genetic knockout of alpha-synuclein. Mol. Brain 2020, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Hökfelt, T.; Martensson, R.; Björklund, A.; Kheinau, S.; Goldstein, M. Handbook of Chemical Neuroanatomy; Björklund, A., Hökfelt, T., Eds.; Elsevier Science B.V: Amsterdam, The Netherlands, 1984; Volume 2, pp. 277–379. [Google Scholar]
- Gal’chenko, A.A.; Vorobyov, V.V. Analysis of electroencephalograms using a modified amplitude-interval algorithm. Neurosci. Behav. Physiol. 1999, 29, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Eusebio, A.; Brown, P. Synchronisation in the beta frequency-band—the bad boy of parkinsonism or an innocent bystander? Exp. Neurol. 2009, 217, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, L.; Fink, G.R. Pathological network activity in Parkinson’s disease: From neural activity and connectivity to causality? Brain 2011, 134, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Haumesser, J.K.; Beck, M.H.; Pellegrini, F.; Kühn, J.; Neumann, W.J.; Altschüler, J.; Harnack, D.; Kupsch, A.; Nikulin, V.V.; Kühn, A.A.; et al. Subthalamic beta oscillations correlate with dopaminergic degeneration in experimental parkinsonism. Exp. Neurol. 2021, 335, 113513. [Google Scholar] [CrossRef]
- Doyle, L.M.; Kuhn, A.A.; Hariz, M.; Kupsch, A.; Schneider, G.H.; Brown, P. Levodopa-induced modulation of subthalamic beta oscillations during self-paced movements in patients with Parkinson’s disease. Eur. J. Neurosci. 2005, 21, 1403–1412. [Google Scholar] [CrossRef]
- Kühn, A.A.; Kempf, F.; Brucke, C.; Gaynor Doyle, L.; Martinez-Torres, I.; Pogosyan, A.; Trottenberg, T.; Kupsch, A.; Schneider, G.-H.; Hariz, M.I.; et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J. Neurosci. 2008, 28, 6165–6173. [Google Scholar]
- Eusebio, A.; Chen, C.C.; Lu, C.S.; Lee, S.T.; Tsai, C.H.; Limousin, P.; Hariz, M.; Brown, P. Effects of low-frequency stimulation of the subthalamic nucleus on movement in Parkinson’s disease. Exp. Neurol. 2008, 209, 125–130. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorobyov, V.; Deev, A.; Sukhanova, I.; Morozova, O.; Oganesyan, Z.; Chaprov, K.; Buchman, V.L. Loss of the Synuclein Family Members Differentially Affects Baseline- and Apomorphine-Associated EEG Determinants in Single-, Double- and Triple-Knockout Mice. Biomedicines 2022, 10, 3128. https://doi.org/10.3390/biomedicines10123128
Vorobyov V, Deev A, Sukhanova I, Morozova O, Oganesyan Z, Chaprov K, Buchman VL. Loss of the Synuclein Family Members Differentially Affects Baseline- and Apomorphine-Associated EEG Determinants in Single-, Double- and Triple-Knockout Mice. Biomedicines. 2022; 10(12):3128. https://doi.org/10.3390/biomedicines10123128
Chicago/Turabian StyleVorobyov, Vasily, Alexander Deev, Iuliia Sukhanova, Olga Morozova, Zoya Oganesyan, Kirill Chaprov, and Vladimir L. Buchman. 2022. "Loss of the Synuclein Family Members Differentially Affects Baseline- and Apomorphine-Associated EEG Determinants in Single-, Double- and Triple-Knockout Mice" Biomedicines 10, no. 12: 3128. https://doi.org/10.3390/biomedicines10123128
APA StyleVorobyov, V., Deev, A., Sukhanova, I., Morozova, O., Oganesyan, Z., Chaprov, K., & Buchman, V. L. (2022). Loss of the Synuclein Family Members Differentially Affects Baseline- and Apomorphine-Associated EEG Determinants in Single-, Double- and Triple-Knockout Mice. Biomedicines, 10(12), 3128. https://doi.org/10.3390/biomedicines10123128




