Lipid Nanoparticles and Liposomes for Bone Diseases Treatment
Abstract
1. Introduction
2. Lipid Nanoparticles
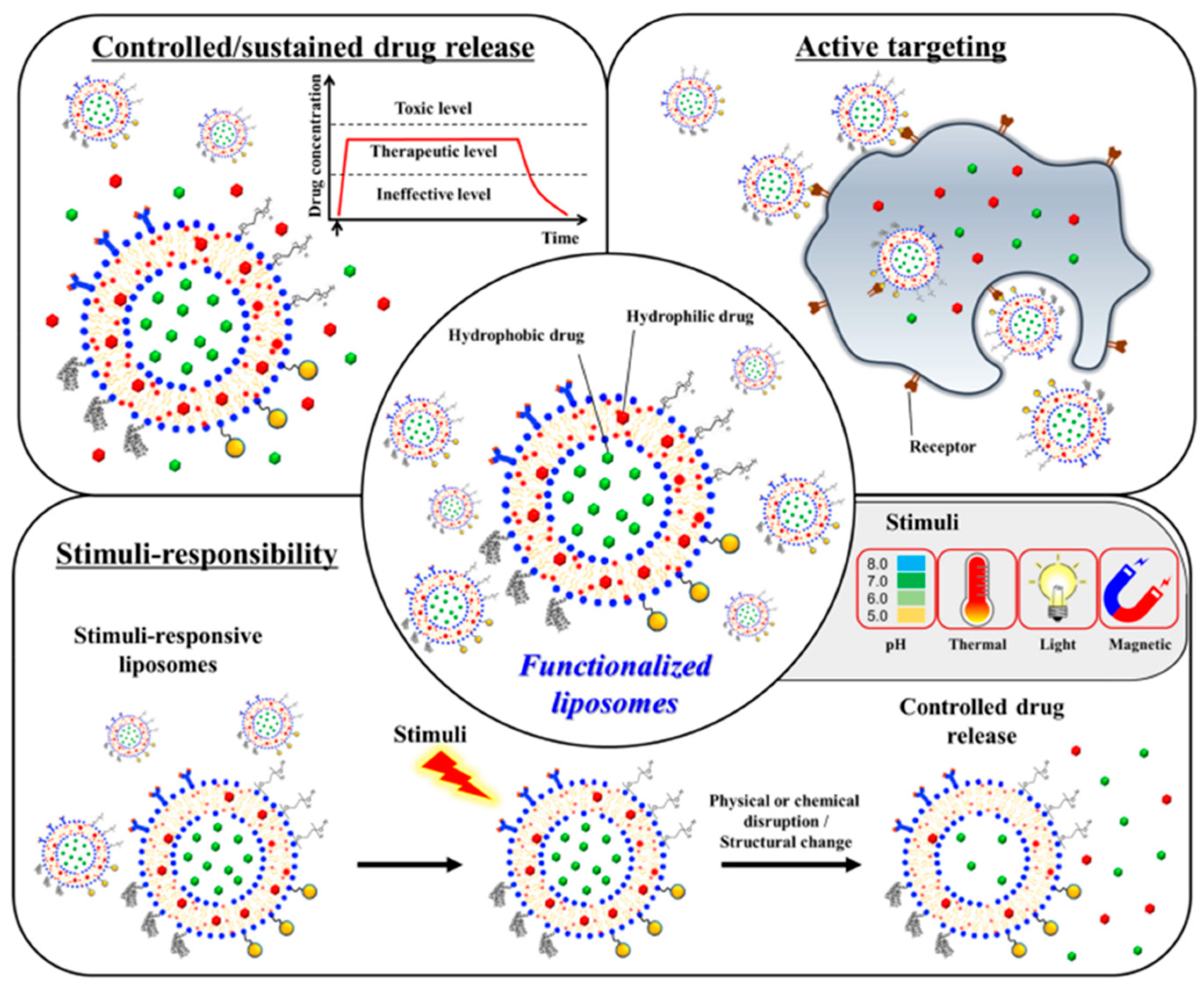
3. Liposomes
- passively—the active substance is encapsulated during liposome formation;
- actively—the active substance is encapsulated after the liposomes are formed [61].
3.1. Nanoliposomes
3.2. Tocosomes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sapino, S.; Chindamo, G.; Chirio, D.; Manzoli, M.; Peira, E.; Riganti, C.; Gallarate, M. Calcium Phosphate-Coated Lipid Nanoparticles as a Potential Tool in Bone Diseases Therapy. Nanomaterials 2021, 11, 2983. [Google Scholar] [CrossRef] [PubMed]
- Pu’ad, N.; Chuan, L.T.; Salman, N.S.; Selimin, M.A.; Latif, A.F.A.; Muhamad, M.S.; Abdullah, H.Z.; Idris, M.I.; Mustapha, S.N.H. Synthesis of bioactive calcium phosphate from cockle shell for biomedical applications. Biointerface Res. Appl. Chem. 2020, 10, 5787–5791. [Google Scholar]
- Ojo, O.A.; Olayide, I.I.; Akalabu, M.C.; Ajiboye, B.O.; Ojo, A.B.; Oyinloye, B.E.; Ramalingam, M. Nanoparticles and their biomedical applications. Biointerface Res. Appl. Chem 2021, 11, 8431–8445. [Google Scholar]
- Müller, R.H.; Alexiev, U.; Sinambela, P.; Keck, C.M. Nanostructured Lipid Carriers (NLC): The Second Generation of Solid Lipid Nanoparticles. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 161–185. [Google Scholar]
- Filipczak, N.; Yalamarty, S.S.K.; Li, X.; Khan, M.M.; Parveen, F.; Torchilin, V. Lipid-Based Drug Delivery Systems in Regenerative Medicine. Materials 2021, 14, 5371. [Google Scholar] [CrossRef]
- Shukla, T.; Upmanyu, N.; Prakash Pandey, S.; Gosh, D. Chapter 1—Lipid nanocarriers. In Lipid Nanocarriers for Drug Targeting; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 1–47. [Google Scholar]
- Grewal, I.K.; Singh, S.; Arora, S.; Sharma, N. Polymeric nanoparticles for breast cancer therapy: A comprehensive review. Biointerface Res. Appl. Chem. 2021, 11, 11151–11171. [Google Scholar]
- Akanda, M.; Getti, G.; Nandi, U.; Mithu, M.S.; Douroumis, D. Bioconjugated solid lipid nanoparticles (SLNs) for targeted prostate cancer therapy. Int. J. Pharm. 2021, 599, 120416. [Google Scholar] [CrossRef]
- Carrasco-Esteban, E.; Domínguez-Rullán, J.A.; Barrionuevo-Castillo, P.; Pelari-Mici, L.; Leaman, O.; Sastre-Gallego, S.; López-Campos, F. Current role of nanoparticles in the treatment of lung cancer. J. Clin. Transl. Res. 2021, 7, 140–155. [Google Scholar]
- Yang, Y.; Zhao, Z.; Xie, C.; Zhao, Y. Dual-targeting liposome modified by glutamic hexapeptide and folic acid for bone metastatic breast cancer. Chem. Phys. Lipids 2020, 228, 104882. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef]
- Liu, T.; Romanova, S.; Wang, S.; Hyun, M.A.; Zhang, C.; Cohen, S.M.; Singh, R.K.; Bronich, T.K. Alendronate-Modified Polymeric Micelles for the Treatment of Breast Cancer Bone Metastasis. Mol. Pharm. 2019, 16, 2872–2883. [Google Scholar] [CrossRef]
- Meng, F.; Xue, X.; Yin, Z.; Gao, F.; Wang, X.; Geng, Z. Research Progress of Exosomes in Bone Diseases: Mechanism, Diagnosis and Therapy. Front. Bioeng. Biotechnol. 2022, 10, 866627. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.J.; Dorkin, J.R.; Yang, J.H.; Heartlein, M.W.; DeRosa, F.; Mir, F.F.; Fenton, O.S.; Anderson, D.G. Optimization of Lipid Nanoparticle Formulations for mRNA Delivery In Vivo with Fractional Factorial and Definitive Screening Designs. Nano Lett. 2015, 15, 7300–7306. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.M.; Specht, D.; Brüßler, J. Influence of lipid matrix composition on biopharmaceutical properties of lipid nanoparticles. J. Control. Release 2021, 338, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.K.; Pal, H.; Puri, D.; Kumar, N.; Shanmugam, S.K. Formulation and development of hesperidin loaded solid lipid nanoparticles for diabetes. Biointerface Res. Appl. Chem. 2020, 10, 4728–4733. [Google Scholar]
- Martins, S.; Sarmento, B.; Ferreira, D.C.; Souto, E.B. Lipid-based colloidal carriers for peptide and protein delivery—Liposomes versus lipid nanoparticles. Int. J. Nanomed. 2007, 2, 595–607. [Google Scholar]
- Heerklotz, H. Interactions of surfactants with lipid membranes. Q. Rev. Biophys. 2008, 41, 205–264. [Google Scholar] [CrossRef]
- Antila, H.S.; Wurl, A.; Ollila, O.H.S.; Miettinen, M.S.; Ferreira, T.M. Rotational decoupling between the hydrophilic and hydrophobic regions in lipid membranes. Biophys. J. 2022, 121, 68–78. [Google Scholar] [CrossRef]
- Pucek, A.; Tokarek, B.; Waglewska, E.; Bazylińska, U. Recent Advances in the Structural Design of Photosensitive Agent Formulations Using “Soft” Colloidal Nanocarriers. Pharmaceutics 2020, 12, 587. [Google Scholar] [CrossRef]
- Wei, B.; Wang, W.; Liu, X.; Xu, C.; Wang, Y.; Wang, Z.; Xu, J.; Guan, J.; Zhou, P.; Mao, Y. Gelatin methacrylate hydrogel scaffold carrying resveratrol-loaded solid lipid nanoparticles for enhancement of osteogenic differentiation of BMSCs and effective bone regeneration. Regen. Biomater. 2021, 8, rbab044. [Google Scholar] [CrossRef]
- Huynh, N.T.; Passirani, C.; Saulnier, P.; Benoit, J.P. Lipid nanocapsules: A new platform for nanomedicine. Int. J. Pharm. 2009, 379, 201–209. [Google Scholar] [CrossRef]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, F.; Kocyigit, A.; Onyuksel, H.; Dag, A.; Topcu, G. Cytotoxic, Apoptotic and Genotoxic Effects of Lipid-Based and Polymeric Nano Micelles, an In Vitro Evaluation. Toxics 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Bhattacharjee, H.; Mittal, N.; Sah, H.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Core-shell-type lipid-polymer hybrid nanoparticles as a drug delivery platform. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 474–491. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Esposito, E.; Cortesi, R. Lipid-Based Nanosystems as a Tool to Overcome Skin Barrier. Int. J. Mol. Sci. 2021, 22, 8319. [Google Scholar] [CrossRef]
- Monajjemi, M.; Naghsh, F.; Mollaamin, F. Bio-lipid nano capacitors: Resonance with helical myeline proteins. Biointerface Res. Appl. Chem. 2020, 10, 6695–6705. [Google Scholar] [CrossRef]
- Lasting impact of lipid nanoparticles. Nat. Rev. Mater. 2021, 6, 1071. [CrossRef]
- Mirchandani, Y.; Patravale, V.B.; Brijesh, S. Solid lipid nanoparticles for hydrophilic drugs. J. Control. Release 2021, 335, 457–464. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Nguyen, V.; Zhang, Y.; Gao, C.; Cao, X.; Tian, Y.; Carver, W.; Kiaris, H.; Cui, T.; Tan, W. The Spike Protein of SARS-CoV-2 Impairs Lipid Metabolism and Increases Susceptibility to Lipotoxicity: Implication for a Role of Nrf2. Cells 2022, 11, 1916. [Google Scholar] [CrossRef]
- Raoufi, E.; Bahramimeimandi, B.; Salehi-Shadkami, M.; Chaosri, P.; Mozafari, M.R. Methodical Design of Viral Vaccines Based on Avant-Garde Nanocarriers: A Multi-Domain Narrative Review. Biomedicines 2021, 9, 520. [Google Scholar] [CrossRef]
- Zylberberg, C.; Gaskill, K.; Pasley, S.; Matosevic, S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther. 2017, 24, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Saier, L.; Niazi, H.; Brizuela, L.; Levkau, B.; Peyruchaud, O. Bioactive lipids and cancer metastasis to bone. J. Cancer Metastasis Treat. 2021, 7, 43. [Google Scholar] [CrossRef]
- Duhan, N.; Barak, S.; Mudgil, D. Bioactive lipids: Chemistry & health benefits. Biointerface Res. Appl. Chem. 2020, 10, 6676–6687. [Google Scholar]
- Wang, L.; Yang, P. Nanostructured Scaffold and Its Bioactive Potentials in Bone Tissue Engineering. In Nanobiomaterials in Hard Tissue Engineering; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 241–270. [Google Scholar]
- Nagi, S.; Al-Namnam, N.; Shaghayegh, G. Osteoprotegerin (OPG) pathways in bone diseases and its application in therapeutic perspectives. Biointerface Res. Appl. Chem. 2021, 10, 5193–5200. [Google Scholar] [CrossRef]
- Yamamoto, N.; Tsuchiya, H. Chemotherapy for osteosarcoma—Where does it come from? What is it? Where is it going? Expert Opin. Pharm. 2013, 14, 2183–2193. [Google Scholar] [CrossRef]
- Shukla, T.; Upmanyu, N.; Pandey, S.P.; Sudheesh, M.S. Chapter 14—Site-specific drug delivery, targeting, and gene therapy. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 473–505. [Google Scholar]
- González-Fernández, Y.; Zalacain, M.; Imbuluzqueta, E.; Sierrasesumaga, L.; Patiño-García, A.; Blanco-Prieto, M.J. Lipid nanoparticles enhance the efficacy of chemotherapy in primary and metastatic human osteosarcoma cells. J. Drug Deliv. Sci. Technol. 2015, 30, 435–442. [Google Scholar] [CrossRef]
- Khalifehzadeh, R.; Arami, H. Biodegradable calcium phosphate nanoparticles for cancer therapy. Adv. Colloid Interface Sci. 2020, 279, 102157. [Google Scholar] [CrossRef]
- Chaiin, P.; Yostaworakul, J.; Rungnim, C.; Khemthong, P.; Yata, T.; Boonrungsiman, S. Self-calcifying lipid nanocarrier for bone tissue engineering. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130047. [Google Scholar] [CrossRef]
- Bozorgi, A.; Khazaei, M.; Soleimani, M.; Jamalpoor, Z. Application of nanoparticles in bone tissue engineering; a review on the molecular mechanisms driving osteogenesis. Biomater. Sci. 2021, 9, 4541–4567. [Google Scholar] [CrossRef]
- Wong, R.H.; Thaung Zaw, J.J.; Xian, C.J.; Howe, P.R. Regular Supplementation with Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2020, 35, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Sosa, B. Vanadium Compounds Modulate Osteoblast Proliferation and Function. Master’s Thesis, Secton Hall University, South Orange, NJ, USA, 2019. [Google Scholar]
- León, I.E.; Ruiz, M.C.; Franca, C.A.; Parajón-Costa, B.S.; Baran, E.J. Metvan, bis(4,7-Dimethyl-1,10-phenanthroline) sulfatooxidovanadium (IV): DFT and spectroscopic study—Antitumor action on human bone and colorectal cancer cell lines. Biol. Trace Elem. Res. 2019, 191, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, P.K.; Malviya, R. Stimuli-responsive supramolecules for bone tissue engineering. Biointerface Res. Appl. Chem. 2020, 10, 5122–5127. [Google Scholar]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Mariani, P.; Cortesi, R.; Huang, N.; Simelière, F.; Marchetti, N.; Drechsler, M.; Ruzgas, T.; Esposito, E. Design and characterization of ethosomes for transdermal delivery of caffeic acid. Pharmaceutics 2020, 12, 740. [Google Scholar] [CrossRef]
- Hallan, S.S.; Amirian, J.; Brangule, A.; Bandere, D. Lipid-Based Nano-Sized Cargos as a Promising Strategy in Bone Complications: A Review. Nanomaterials 2022, 12, 1146. [Google Scholar] [CrossRef]
- Ahmad, N.; Banala, V.T.; Kushwaha, P.; Karvande, A.; Sharma, S.; Tripathi, A.K.; Verma, A.; Trivedi, R.; Mishra, P.R. Quercetin-loaded solid lipid nanoparticles improve osteoprotective activity in an ovariectomized rat model: A preventive strategy for post-menopausal osteoporosis. RSC Adv. 2016, 6, 97613–97628. [Google Scholar] [CrossRef]
- Sriwidodo; Umar, A.K.; Wathoni, N.; Zothantluanga, J.H.; Das, S.; Luckanagul, J.A. Liposome-polymer complex for drug delivery system and vaccine stabilization. Heliyon 2022, 8, e08934. [Google Scholar] [CrossRef]
- Sun, X.; Wei, J.; Lyu, J.; Bian, T.; Liu, Z.; Huang, J.; Pi, F.; Li, C.; Zhong, Z. Bone-targeting drug delivery system of biomineral-binding liposomes loaded with icariin enhances the treatment for osteoporosis. J. Nanobiotechnol. 2019, 17, 10. [Google Scholar] [CrossRef]
- de la Fuente-Herreruela, D.; Monnappa, A.K.; Muñoz-Úbeda, M.; Morallón-Piña, A.; Enciso, E.; Sánchez, L.; Giusti, F.; Natale, P.; López-Montero, I. Lipid-peptide bioconjugation through pyridyl disulfide reaction chemistry and its application in cell targeting and drug delivery. J. Nanobiotechnol. 2019, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Qadir, A.; Gao, Y.; Suryaji, P.; Tian, Y.; Lin, X.; Dang, K.; Jiang, S.; Li, Y.; Miao, Z.; Qian, A. Non-Viral Delivery System and Targeted Bone Disease Therapy. Int. J. Mol. Sci. 2019, 20, 565. [Google Scholar] [CrossRef] [PubMed]
- Roohani-Esfahani, S.I.; Zreiqat, H. Nanoparticles: A promising new therapeutic platform for bone regeneration? Nanomedicine 2017, 12, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xiang, Y.; Wang, Z.; Yang, X.; Yu, X.; Lu, Y.; Deng, L.; Cui, W. Adhesive liposomes loaded onto an injectable, self-healing and antibacterial hydrogel for promoting bone reconstruction. NPG Asia Mater. 2019, 11, 81. [Google Scholar] [CrossRef]
- Cattalini, J.P.; Boccaccini, A.R.; Lucangioli, S.; Mouriño, V. Bisphosphonate-based strategies for bone tissue engineering and orthopedic implants. Tissue Eng. Part B Rev. 2012, 18, 323–340. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Z.; Akhter, M.P.; Gao, X.; Wang, X.; Wang, X.; Zhao, G.; Wei, X.; Zhou, Y.; Wang, X.; et al. Bone-targeting liposome formulation of Salvianic acid A accelerates the healing of delayed fracture Union in Mice. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2271–2282. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, H.; Gao, X.; Zheng, X.; Chen, H.; Li, H.; Peng, J.; Liang, W.; Wang, W.; Qiu, Z.; et al. Bone-Targeting Liposome-Encapsulated Salvianic Acid A Improves Nonunion Healing through the Regulation of HDAC3-Mediated Endochondral Ossification. Drug Des. Dev. 2020, 14, 3519–3533. [Google Scholar] [CrossRef]
- Axmann, M.; Strobl, W.M.; Plochberger, B.; Stangl, H. Cholesterol transfer at the plasma membrane. Atherosclerosis 2019, 290, 111–117. [Google Scholar] [CrossRef]
- Fu, Q.; Zhao, Y.; Yang, Z.; Yue, Q.; Xiao, W.; Chen, Y.; Yang, Y.; Guo, L.; Wu, Y. Liposomes actively recognizing the glucose transporter GLUT(1) and integrin α(v) β(3) for dual-targeting of glioma. Arch. Pharm. 2019, 352, e1800219. [Google Scholar] [CrossRef]
- Yang, T.; Cui, F.D.; Choi, M.K.; Lin, H.; Chung, S.J.; Shim, C.K.; Kim, D.D. Liposome formulation of paclitaxel with enhanced solubility and stability. Drug Deliv. 2007, 14, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Chen, W.; Wu, J.; Li, H. Folic acid-coupled nano-paclitaxel liposome reverses drug resistance in SKOV3/TAX ovarian cancer cells. Anti-Cancer Drugs 2014, 25, 244–254. [Google Scholar] [CrossRef]
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef]
- He, J.; Yang, X.; Liu, F.; Li, D.; Zheng, B.; Abdullah, A.O.; Liu, Y. The Impact of Curcumin on Bone Osteogenic Promotion of MC3T3 Cells under High Glucose Conditions and Enhanced Bone Formation in Diabetic Mice. Coatings 2020, 10, 258. [Google Scholar] [CrossRef]
- Feng, S.; Wu, Z.-X.; Zhao, Z.; Liu, J.; Sun, K.; Guo, C.; Wang, H.; Wu, Z. Engineering of Bone- and CD44-Dual-Targeting Redox-Sensitive Liposomes for the Treatment of Orthotopic Osteosarcoma. ACS Appl. Mater. Interfaces 2019, 11, 7357–7368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef]
- Aljawadi, A.; Naylor, T.; Islam, A.; Madhi, I.; Niazi, N.; Elmajee, M.; Pillai, A. Radiological Analysis of Gentamicin Eluting Synthetic Bone Graft Substitute Used in the Management of Patients with Traumatic Bone Voids. Cureus 2022, 14, e20969. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Y.; Pan, J.; Wang, H.; Li, H.; Xu, X.; Fu, X.; Shi, R.; Luo, Z.; Li, Y.; et al. A hybrid 3D-printed aspirin-laden liposome composite scaffold for bone tissue engineering. J. Mater. Chem. B 2019, 7, 619–629. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, M.; Dehiya, B.S.; Sindhu, A.; Kumar, V.; Kumar, R.; Lamberti, L.; Pruncu, C.I.; Thakur, R. Comprehensive Survey on Nanobiomaterials for Bone Tissue Engineering Applications. Nanomaterials 2020, 10, 2019. [Google Scholar] [CrossRef]
- Akoury, E.; Ahangar, P.; Nour, A.; Lapointe, J.; Guérard, K.P.; Haglund, L.; Rosenzweig, D.H.; Weber, M.H. Low-dose zoledronate for the treatment of bone metastasis secondary to prostate cancer. Cancer Cell Int. 2019, 19, 28. [Google Scholar] [CrossRef]
- Kang, M.; Lee, C.S.; Lee, M. Bioactive Scaffolds Integrated with Liposomal or Extracellular Vesicles for Bone Regeneration. Bioengineering 2021, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, J.; Lee, C.S.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Apatite-binding nanoparticulate agonist of hedgehog signaling for bone repair. Adv. Funct. Mater. 2020, 30, 1909218. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Hsu, G.C.-Y.; Sono, T.; Lee, M.; James, A.W. Development of a biomaterial scaffold integrated with osteoinductive oxysterol liposomes to enhance hedgehog signaling and bone repair. Mol. Pharm. 2021, 18, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Cottrill, E.; Lazzari, J.; Pennington, Z.; Ehresman, J.; Schilling, A.; Dirckx, N.; Theodore, N.; Sciubba, D.; Witham, T. Oxysterols as promising small molecules for bone tissue engineering: Systematic review. World J. Orthop. 2020, 11, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Kim, S.; Fan, J.; Hwang, H.S.; Aghaloo, T.; Lee, M. Smoothened agonist sterosome immobilized hybrid scaffold for bone regeneration. Sci. Adv. 2020, 6, eaaz7822. [Google Scholar] [CrossRef]
- Zamani, P.; Momtazi-Borojeni, A.A.; Nik, M.E.; Oskuee, R.K.; Sahebkar, A. Nanoliposomes as the adjuvant delivery systems in cancer immunotherapy. J. Cell. Physiol. 2018, 233, 5189–5199. [Google Scholar] [CrossRef]
- Mozafari, M.R. Nanoliposomes: Preparation and analysis. In Liposomes; Springer: Berlin/Heidelberg, Germany, 2010; pp. 29–50. [Google Scholar]
- Lin, X.; Wang, Q.; Gu, C.; Li, M.; Chen, K.; Chen, P.; Tang, Z.; Liu, X.; Pan, H.; Liu, Z.; et al. Smart Nanosacrificial Layer on the Bone Surface Prevents Osteoporosis through Acid–Base Neutralization Regulated Biocascade Effects. J. Am. Chem. Soc. 2020, 142, 17543–17556. [Google Scholar] [CrossRef]
- Kleinerman, E.S. Biologic therapy for osteosarcoma using liposome-encapsulated muramyl tripeptide. Hematol./Oncol. Clin. N. Am. 1995, 9, 927–938. [Google Scholar] [CrossRef]
- Tan, W.; Li, Y.; Chen, M.; Wang, Y. Berberine hydrochloride: Anticancer activity and nanoparticulate delivery system. Int. J. Nanomed. 2011, 6, 1773–1777. [Google Scholar] [CrossRef]
- Calvo, A.; Moreno, E.; Larrea, E.; Sanmartín, C.; Irache, J.M.; Espuelas, S. Berberine-Loaded Liposomes for the Treatment of Leishmania infantum-Infected BALB/c Mice. Pharmaceutics 2020, 12, 858. [Google Scholar] [CrossRef]
- Haghir Alsadat, B.F.; Amirpour, Z.; Nafisi, B. Development of Slow Release Berberine-Containing Nanoliposome for Delivery to Bone Cancer Cells Saos2. Iran. J. Pediatr. Hematol. Oncol. 2020, 10, 221–229. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Xia, H.; Cao, J.; Wang, Z.; Yang, Y.; Lin, Y. MicroRNA-21 Inhibits the Apoptosis of Osteosarcoma Cell Line SAOS-2 via Targeting Caspase 8. Oncol. Res. 2017, 25, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Barzegari Firouzabadi, F.; Oryan, S.H.; Sheikhha, M.H.; Kalantar, S.M.; Javed, A. Preparation and Evaluation of a Novel Liposomal Nano-Formulation in Metastatic Cancer Treatment Studies. Cell J. 2019, 21, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Sultana, Y.; Aqil, M. Liposomal drug delivery systems: An update review. Curr. Drug Deliv. 2007, 4, 297–305. [Google Scholar] [CrossRef]
- Zarrabi, A.; Alipoor Amro Abadi, M.; Khorasani, S.; Mohammadabadi, M.R.; Jamshidi, A.; Torkaman, S.; Taghavi, E.; Mozafari, M.R.; Rasti, B. Nanoliposomes and Tocosomes as Multifunctional Nanocarriers for the Encapsulation of Nutraceutical and Dietary Molecules. Molecules 2020, 25, 638. [Google Scholar] [CrossRef]
- Ogru, E.; Libinaki, R.; Gianello, R.; West, S.; Munteanu, A.; Zingg, J.M.; Azzi, A. Modulation of cell proliferation and gene expression by alpha-tocopheryl phosphates: Relevance to atherosclerosis and inflammation. Ann. N. Y. Acad. Sci. 2004, 1031, 405–411. [Google Scholar] [CrossRef]
- Libinaki, R.; Tesanovic, S.; Heal, A.; Nikolovski, B.; Vinh, A.; Widdop, R.E.; Gaspari, T.A.; Devaraj, S.; Ogru, E. Effect of tocopheryl phosphate on key biomarkers of inflammation: Implication in the reduction of atherosclerosis progression in a hypercholesterolaemic rabbit model. Clin. Exp. Pharmacol. Physiol. 2010, 37, 587–592. [Google Scholar] [CrossRef]
- Nishio, K.; Ishida, N.; Saito, Y.; Ogawa-Akazawa, Y.; Shichiri, M.; Yoshida, Y.; Hagihara, Y.; Noguchi, N.; Chirico, J.; Atkinson, J.; et al. α-Tocopheryl phosphate: Uptake, hydrolysis, and antioxidant action in cultured cells and mouse. Free Radic. Biol. Med. 2011, 50, 1794–1800. [Google Scholar] [CrossRef]
- Saitoh, Y.; Yumoto, A.; Miwa, N. alpha-tocopheryl phosphate suppresses tumor invasion concurrently with dynamic morphological changes and delocalization of cortactin from invadopodia. Int. J. Oncol. 2009, 35, 1277–1288. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Javanmard, R.; Raji, M. Tocosome: Novel drug delivery system containing phospholipids and tocopheryl phosphates. Int. J. Pharm. 2017, 528, 381–382. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burdușel, A.-C.; Andronescu, E. Lipid Nanoparticles and Liposomes for Bone Diseases Treatment. Biomedicines 2022, 10, 3158. https://doi.org/10.3390/biomedicines10123158
Burdușel A-C, Andronescu E. Lipid Nanoparticles and Liposomes for Bone Diseases Treatment. Biomedicines. 2022; 10(12):3158. https://doi.org/10.3390/biomedicines10123158
Chicago/Turabian StyleBurdușel, Alexandra-Cristina, and Ecaterina Andronescu. 2022. "Lipid Nanoparticles and Liposomes for Bone Diseases Treatment" Biomedicines 10, no. 12: 3158. https://doi.org/10.3390/biomedicines10123158
APA StyleBurdușel, A.-C., & Andronescu, E. (2022). Lipid Nanoparticles and Liposomes for Bone Diseases Treatment. Biomedicines, 10(12), 3158. https://doi.org/10.3390/biomedicines10123158







