Inflammatory Cytokines and Radiotherapy in Pancreatic Ductal Adenocarcinoma
Abstract
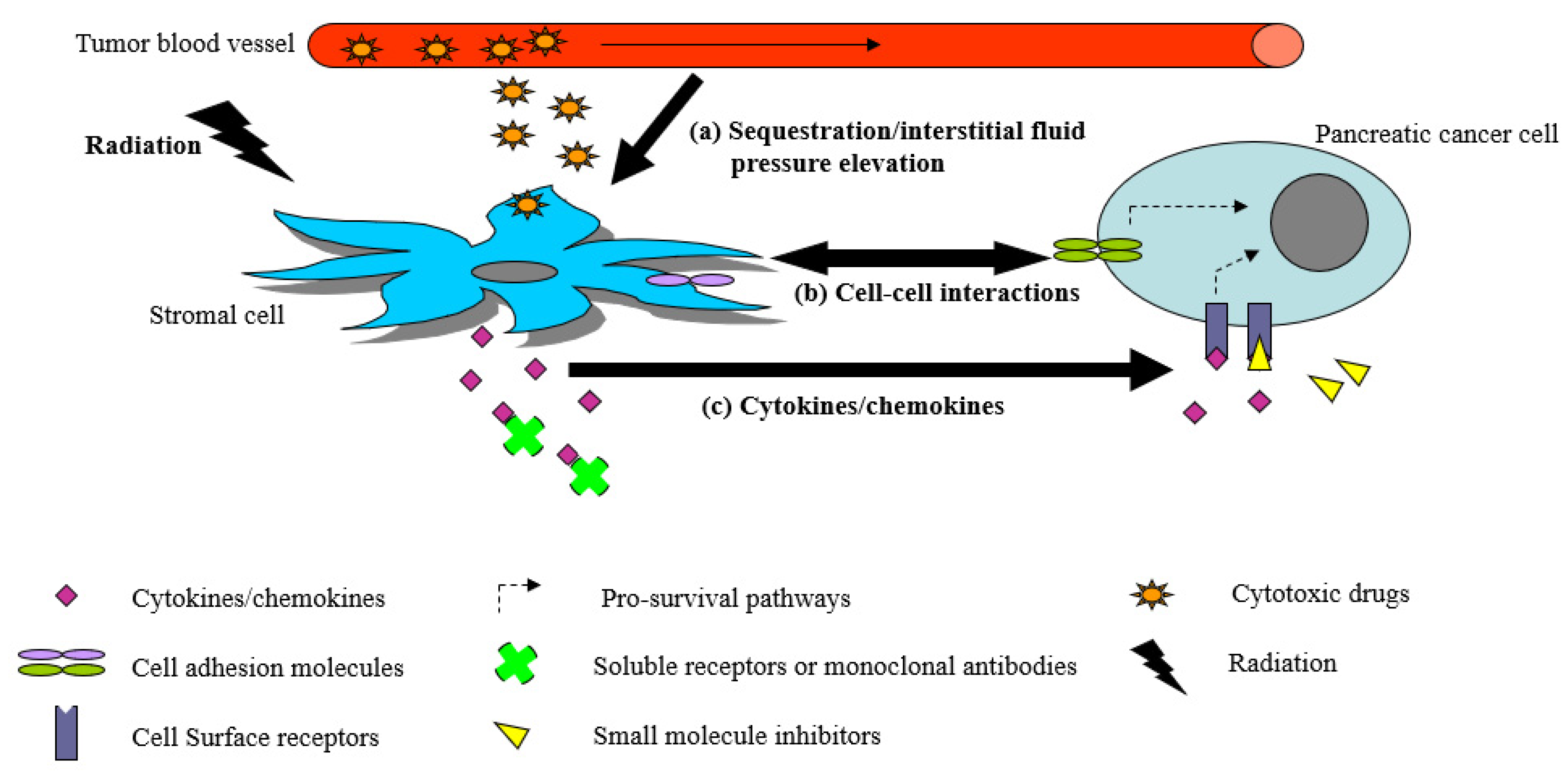
1. Introduction
2. Current Treatment Modalities for Pancreatic Cancer
Conventional Radiotherapy and Stereotactic Ablative Radiotherapy
3. Inflammatory Cytokines and Clinical Outcomes
3.1. Association of Various Inflammatory Cytokines with Prognosis
3.2. Interleukin-6
3.3. Effects of Radiotherapy on Circulating Cytokines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenberg, L. Treatment of Pancreatic Cancer. Promises and Problems of Tamoxifen, Somatostatin Analogs, and Gemcitabine. Int. J. Pancreatol. 1997, 22, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://cancer.ca/en/research/cancer-statistics (accessed on 1 December 2022).
- Gudjonsson, B. Pancreatic Cancer: 80 Years of Surgery-Percentage and Repetitions. HPB Surg. 2016, 2016, 6839687. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.F.; Smalley, S.R.; Jewell, W.; Paradelo, J.C.; Reymond, R.D.; Hassanein, R.E.; Evans, R.G. Patterns of Failure after Curative Resection of Pancreatic Carcinoma. Cancer 1990, 66, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Nitecki, S.S.; Sarr, M.G.; Colby, T.V.; van Heerden, J.A. Long-Term Survival after Resection for Ductal Adenocarcinoma of the Pancreas. Is It Really Improving? Ann. Surg. 1995, 221, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Tepper, J.; Nardi, G.; Sutt, H. Carcinoma of the Pancreas: Review of MGH Experience from 1963 to 1973. Analysis of Surgical Failure and Implications for Radiation Therapy. Cancer 1976, 37, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, S.; Pestalozzi, B.C.; Schäfer, M.; Weber, A.; Bauerfeind, P.; Knuth, A.; Clavien, P.-A. Prospective Phase II Trial of Neoadjuvant Chemotherapy with Gemcitabine and Cisplatin for Resectable Adenocarcinoma of the Pancreatic Head. J. Clin. Oncol. 2008, 26, 2526–2531. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with Nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Available online: https://www.nccn.org/guidelines/category_1 (accessed on 1 December 2022).
- Iacobuzio-Donahue, C.A.; Fu, B.; Yachida, S.; Luo, M.; Abe, H.; Henderson, C.M.; Vilardell, F.; Wang, Z.; Keller, J.W.; Banerjee, P.; et al. DPC4 Gene Status of the Primary Carcinoma Correlates with Patterns of Failure in Patients with Pancreatic Cancer. J. Clin. Oncol. 2009, 27, 1806–1813. [Google Scholar] [CrossRef]
- Moertel, C.G.; Frytak, S.; Hahn, R.G.; O’Connell, M.J.; Reitemeier, R.J.; Rubin, J.; Schutt, A.J.; Weiland, L.H.; Childs, D.S.; Holbrook, M.A.; et al. Therapy of Locally Unresectable Pancreatic Carcinoma: A Randomized Comparison of High Dose (6000 Rads) Radiation Alone, Moderate Dose Radiation (4000 Rads + 5-Fluorouracil), and High Dose Radiation + 5-Fluorouracil: The Gastrointestinal Tumor Study Group. Cancer 1981, 48, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Chauffert, B.; Mornex, F.; Bonnetain, F.; Rougier, P.; Mariette, C.; Bouché, O.; Bosset, J.F.; Aparicio, T.; Mineur, L.; Azzedine, A.; et al. Phase III Trial Comparing Intensive Induction Chemoradiotherapy (60 Gy, Infusional 5-FU and Intermittent Cisplatin) Followed by Maintenance Gemcitabine with Gemcitabine Alone for Locally Advanced Unresectable Pancreatic Cancer. Definitive Results of the 2000-01 FFCD/SFRO Study. Ann. Oncol. 2008, 19, 1592–1599. [Google Scholar] [PubMed]
- Loehrer, P.J., Sr.; Feng, Y.; Cardenes, H.; Wagner, L.; Brell, J.M.; Cella, D.; Flynn, P.; Ramanathan, R.K.; Crane, C.H.; Alberts, S.R.; et al. Gemcitabine Alone versus Gemcitabine plus Radiotherapy in Patients with Locally Advanced Pancreatic Cancer: An Eastern Cooperative Oncology Group Trial. J. Clin. Oncol. 2011, 29, 4105–4112. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Rana, V.; Janjan, N.A.; Varadhachary, G.R.; Abbruzzese, J.L.; Das, P.; Delclos, M.E.; Gould, M.S.; Evans, D.B.; Wolff, R.A.; et al. Induction Chemotherapy Selects Patients with Locally Advanced, Unresectable Pancreatic Cancer for Optimal Benefit from Consolidative Chemoradiation Therapy. Cancer 2007, 110, 47–55. [Google Scholar] [CrossRef]
- Hammel, P.; Huguet, F.; van Laethem, J.-L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouché, O.; Shannon, J.; André, T.; et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef]
- Koay, E.J.; Hanania, A.N.; Hall, W.A.; Taniguchi, C.M.; Rebueno, N.; Myrehaug, S.; Aitken, K.L.; Dawson, L.A.; Crane, C.H.; Herman, J.M.; et al. Dose-Escalated Radiation Therapy for Pancreatic Cancer: A Simultaneous Integrated Boost Approach. Pract. Radiat. Oncol. 2020, 10, e495–e507. [Google Scholar] [CrossRef]
- Rosati, L.M.; Kumar, R.; Herman, J.M. Integration of Stereotactic Body Radiation Therapy into the Multidisciplinary Management of Pancreatic Cancer. Semin. Radiat. Oncol. 2017, 27, 256–267. [Google Scholar] [CrossRef]
- Moningi, S.; Raman, S.P.; Dholakia, A.S.; Hacker-Prietz, A.; Pawlik, T.M.; Zheng, L.; Weiss, M.; Laheru, D.A.; Wolfgang, C.L.; Herman, J.M. Stereotactic Body Radiation Therapy for Pancreatic Cancer: Single Institutional Experience. J. Clin. Oncol. 2014, 32, 328. [Google Scholar] [CrossRef]
- Pollom, E.L.; Alagappan, M.; Chan, C.; Shultz, D.; Kunz, P.L.; Koong, A.; Chang, D.T. Outcomes and Toxicity of SBRT for Patients with Unresectable Pancreatic Adenocarcinoma. J. Clin. Oncol. 2014, 32, 317. [Google Scholar] [CrossRef]
- Herman, J.M.; Chang, D.T.; Goodman, K.A.; Dholakia, A.S.; Raman, S.P.; Hacker-Prietz, A.; Iacobuzio-Donahue, C.A.; Griffith, M.E.; Pawlik, T.M.; Pai, J.S.; et al. Phase 2 Multi-Institutional Trial Evaluating Gemcitabine and Stereotactic Body Radiotherapy for Patients with Locally Advanced Unresectable Pancreatic Adenocarcinoma. Cancer 2015, 121, 1128–1137. [Google Scholar] [CrossRef]
- Mahadevan, A.; Miksad, R.; Goldstein, M.; Sullivan, R.; Bullock, A.; Buchbinder, E.; Pleskow, D.; Sawhney, M.; Kent, T.; Vollmer, C.; et al. Induction Gemcitabine and Stereotactic Body Radiotherapy for Locally Advanced Nonmetastatic Pancreas Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e615–e622. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Comito, T.; Ghidini, A.; Torri, V.; Scorsetti, M.; Barni, S. Stereotactic Body Radiation Therapy for Locally Advanced Pancreatic Cancer: A Systematic Review and Pooled Analysis of 19 Trials. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Moningi, S.; Dholakia, A.S.; Raman, S.P.; Blackford, A.; Cameron, J.L.; Le, D.T.; De Jesus-Acosta, A.M.C.; Hacker-Prietz, A.; Rosati, L.M.; Assadi, R.K.; et al. The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience. Ann. Surg. Oncol. 2015, 22, 2352–2358. [Google Scholar] [CrossRef]
- Palta, M.; Godfrey, D.; Goodman, K.A.; Hoffe, S.; Dawson, L.A.; Dessert, D.; Hall, W.A.; Herman, J.M.; Khorana, A.A.; Merchant, N.; et al. Radiation Therapy for Pancreatic Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2019, 9, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Caravatta, L.; Cellini, F.; Simoni, N.; Rosa, C.; Niespolo, R.M.; Lupattelli, M.; Picardi, V.; Macchia, G.; Sainato, A.; Mantello, G.; et al. Magnetic Resonance Imaging (MRI) Compared with Computed Tomography (CT) for Interobserver Agreement of Gross Tumor Volume Delineation in Pancreatic Cancer: A Multi-Institutional Contouring Study on Behalf of the AIRO Group for Gastrointestinal Cancers. Acta Oncol. 2019, 58, 439–447. [Google Scholar] [CrossRef]
- Chuong, M.D.; Bryant, J.; Mittauer, K.E.; Hall, M.; Kotecha, R.; Alvarez, D.; Romaguera, T.; Rubens, M.; Adamson, S.; Godley, A.; et al. Ablative 5-Fraction Stereotactic Magnetic Resonance-Guided Radiation Therapy With On-Table Adaptive Replanning and Elective Nodal Irradiation for Inoperable Pancreas Cancer. Pract. Radiat. Oncol. 2021, 11, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Chuong, M.D.; Herrera, R.; Kaiser, A.; Rubens, M.; Romaguera, T.; Alvarez, D.; Kotecha, R.; Hall, M.D.; McCulloch, J.; Ucar, A.; et al. Induction Chemotherapy and Ablative Stereotactic Magnetic Resonance Image-Guided Adaptive Radiation Therapy for Inoperable Pancreas Cancer. Front. Oncol. 2022, 12, 888462. [Google Scholar] [CrossRef]
- Reyngold, M.; O’Reilly, E.M.; Varghese, A.M.; Fiasconaro, M.; Zinovoy, M.; Romesser, P.B.; Wu, A.; Hajj, C.; Cuaron, J.J.; Tuli, R.; et al. Association of Ablative Radiation Therapy With Survival Among Patients With Inoperable Pancreatic Cancer. JAMA Oncol. 2021, 7, 735–738. [Google Scholar] [CrossRef]
- Hammer, L.; Hausner, D.; Ben-Ayun, M.; Shacham-Shmueli, E.; Morag, O.; Margalit, O.; Boursi, B.; Yarom, N.; Jacobson, G.; Katzman, T.; et al. Single-Fraction Celiac Plexus Radiosurgery: A Preliminary Proof-of-Concept Phase 2 Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 588–593. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Tucker, S.L.; Li, D.; Abbruzzese, J.L.; Kurzrock, R. Cytokines in Pancreatic Carcinoma: Correlation with Phenotypic Characteristics and Prognosis. Cancer 2004, 101, 2727–2736. [Google Scholar] [CrossRef]
- Babic, A.; Schnure, N.; Neupane, N.P.; Zaman, M.M.; Rifai, N.; Welch, M.W.; Brais, L.K.; Rubinson, D.A.; Morales-Oyarvide, V.; Yuan, C.; et al. Plasma Inflammatory Cytokines and Survival of Pancreatic Cancer Patients. Clin. Transl. Gastroenterol. 2018, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Bellone, G.; Smirne, C.; Mauri, F.A.; Tonel, E.; Carbone, A.; Buffolino, A.; Dughera, L.; Robecchi, A.; Pirisi, M.; Emanuelli, G. Cytokine Expression Profile in Human Pancreatic Carcinoma Cells and in Surgical Specimens: Implications for Survival. Cancer Immunol. Immunother. 2006, 55, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Falconer, J.S.; Fearon, K.C.; Plester, C.E.; Ross, J.A.; Carter, D.C. Cytokines, the Acute-Phase Response, and Resting Energy Expenditure in Cachectic Patients with Pancreatic Cancer. Ann. Surg. 1994, 219, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Okusaka, T.; Ishii, H.; Kyogoku, A.; Yoshimori, M.; Kajimura, N.; Yamaguchi, K.; Kakizoe, T. Elevated Serum Interleukin-6 Levels in Patients with Pancreatic Cancer. Jpn. J. Clin. Oncol. 1998, 28, 12–15. [Google Scholar] [CrossRef]
- Dima, S.O.; Tanase, C.; Albulescu, R.; Herlea, V.; Chivu-Economescu, M.; Purnichescu-Purtan, R.; Dumitrascu, T.; Duda, D.G.; Popescu, I. An Exploratory Study of Inflammatory Cytokines as Prognostic Biomarkers in Patients with Ductal Pancreatic Adenocarcinoma. Pancreas 2012, 41, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Lippitz, B.E. Cytokine Patterns in Patients with Cancer: A Systematic Review. Lancet Oncol. 2013, 14, e218–e228. [Google Scholar] [CrossRef] [PubMed]
- Roshani, R.; McCarthy, F.; Hagemann, T. Inflammatory Cytokines in Human Pancreatic Cancer. Cancer Lett. 2014, 345, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Radisky, D. Putting Tumours in Context. Nat. Rev. Cancer 2001, 1, 46–54. [Google Scholar] [CrossRef]
- Mueller, M.M.; Fusenig, N.E. Friends or Foes—Bipolar Effects of the Tumour Stroma in Cancer. Nat. Rev. Cancer 2004, 4, 839–849. [Google Scholar] [CrossRef]
- Micke, P.; Ostman, A. Tumour-Stroma Interaction: Cancer-Associated Fibroblasts as Novel Targets in Anti-Cancer Therapy? Lung Cancer 2004, 45 (Suppl. 2), S163–S175. [Google Scholar] [CrossRef]
- Hojilla, C.V.; Mohammed, F.F.; Khokha, R. Matrix Metalloproteinases and Their Tissue Inhibitors Direct Cell Fate during Cancer Development. Br. J. Cancer 2003, 89, 1817–1821. [Google Scholar] [CrossRef] [PubMed]
- Vacchelli, E.; Galluzzi, L.; Eggermont, A.; Galon, J.; Tartour, E.; Zitvogel, L.; Kroemer, G. Trial Watch: Immunostimulatory Cytokines. Oncoimmunology 2012, 1, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Dendorfer, U. Molecular Biology of Cytokines. Artif. Organs 1996, 20, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Lierova, A.; Jelicova, M.; Nemcova, M.; Proksova, M.; Pejchal, J.; Zarybnicka, L.; Sinkorova, Z. Cytokines and Radiation-Induced Pulmonary Injuries. J. Radiat. Res. 2018, 59, 709–753. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Linares, A.; Alejandre, M.J.; Palomino-Morales, R.J.; Caba, O.; Prados, J.; Aránega, A.; Delgado, J.R.; Irigoyen, A.; Martínez-Galán, J.; et al. Prognosis Relevance of Serum Cytokines in Pancreatic Cancer. Biomed Res. Int. 2015, 2015, 518284. [Google Scholar] [CrossRef]
- van der Sijde, F.; Mustafa, D.A.M.; Vietsch, E.E.; Katsikis, P.D.; van Eijck, C.H.J. Circulating Immunological Biomarkers: Prognosis of Pancreatic Cancer Patients Reflected by the Immune System. Pancreas 2021, 50, 933–941. [Google Scholar] [CrossRef]
- Ng, S.S.W.; Zhang, H.; Wang, L.; Citrin, D.; Dawson, L.A. Association of pro-Inflammatory Soluble Cytokine Receptors Early during Hepatocellular Carcinoma Stereotactic Radiotherapy with Liver Toxicity. NPJ Precis. Oncol. 2020, 4, 17. [Google Scholar] [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Bardeesy, N.; DePinho, R.A. Pancreatic Cancer Biology and Genetics. Nat. Rev. Cancer 2002, 2, 897–909. [Google Scholar] [CrossRef]
- Protti, M.P.; De Monte, L. Immune Infiltrates as Predictive Markers of Survival in Pancreatic Cancer Patients. Front. Physiol. 2013, 4, 210. [Google Scholar] [CrossRef] [PubMed]
- Neesse, A.; Bauer, C.A.; Öhlund, D.; Lauth, M.; Buchholz, M.; Michl, P.; Tuveson, D.A.; Gress, T.M. Stromal Biology and Therapy in Pancreatic Cancer: Ready for Clinical Translation? Gut 2019, 68, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Neesse, A.; Algül, H.; Tuveson, D.A.; Gress, T.M. Stromal Biology and Therapy in Pancreatic Cancer: A Changing Paradigm. Gut 2015, 64, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.C.; Kimmelman, A.C.; Hezel, A.F.; DePinho, R.A. Stromal Biology of Pancreatic Cancer. J. Cell. Biochem. 2007, 101, 887–907. [Google Scholar] [CrossRef]
- Mahadevan, D.; Von Hoff, D.D. Tumor-Stroma Interactions in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2007, 6, 1186–1197. [Google Scholar] [CrossRef]
- Ren, C.; Chen, Y.; Han, C.; Fu, D.; Chen, H. Plasma Interleukin-11 (IL-11) Levels Have Diagnostic and Prognostic Roles in Patients with Pancreatic Cancer. Tumour Biol. 2014, 35, 11467–11472. [Google Scholar] [CrossRef]
- Mitsunaga, S.; Ikeda, M.; Shimizu, S.; Ohno, I.; Furuse, J.; Inagaki, M.; Higashi, S.; Kato, H.; Terao, K.; Ochiai, A. Serum Levels of IL-6 and IL-1β Can Predict the Efficacy of Gemcitabine in Patients with Advanced Pancreatic Cancer. Br. J. Cancer 2013, 108, 2063–2069. [Google Scholar] [CrossRef]
- van der Sijde, F.; Dik, W.A.; Mustafa, D.A.M.; Vietsch, E.E.; Besselink, M.G.; Debets, R.; Koerkamp, B.G.; Haberkorn, B.C.M.; Homs, M.Y.V.; Janssen, Q.P.; et al. Serum Cytokine Levels Are Associated with Tumor Progression during FOLFIRINOX Chemotherapy and Overall Survival in Pancreatic Cancer Patients. Front. Immunol. 2022, 13, 898498. [Google Scholar] [CrossRef]
- Nishimoto, N.; Kishimoto, T. Interleukin 6: From Bench to Bedside. Nat. Clin. Pract. Rheumatol. 2006, 2, 619–626. [Google Scholar] [CrossRef]
- Taga, T. IL6 Signalling through IL6 Receptor and Receptor-Associated Signal Transducer, gp130. Res. Immunol. 1992, 143, 737–739. [Google Scholar] [CrossRef]
- Chalaris, A.; Garbers, C.; Rabe, B.; Rose-John, S.; Scheller, J. The Soluble Interleukin 6 Receptor: Generation and Role in Inflammation and Cancer. Eur. J. Cell Biol. 2011, 90, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S.; Waetzig, G.H.; Scheller, J.; Grötzinger, J.; Seegert, D. The IL-6/sIL-6R Complex as a Novel Target for Therapeutic Approaches. Expert Opin. Ther. Targets 2007, 11, 613–624. [Google Scholar] [CrossRef]
- Nakahara, H.; Song, J.; Sugimoto, M.; Hagihara, K.; Kishimoto, T.; Yoshizaki, K.; Nishimoto, N. Anti-Interleukin-6 Receptor Antibody Therapy Reduces Vascular Endothelial Growth Factor Production in Rheumatoid Arthritis. Arthritis Rheum. 2003, 48, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Puthier, D.; Derenne, S.; Barillé, S.; Moreau, P.; Harousseau, J.L.; Bataille, R.; Amiot, M. Mcl-1 and Bcl-xL Are Co-Regulated by IL-6 in Human Myeloma Cells. Br. J. Haematol. 1999, 107, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Spets, H.; Strömberg, T.; Georgii-Hemming, P.; Siljason, J.; Nilsson, K.; Jernberg-Wiklund, H. Expression of the Bcl-2 Family of pro- and Anti-Apoptotic Genes in Multiple Myeloma and Normal Plasma Cells: Regulation during Interleukin-6(IL-6)-Induced Growth and Survival. Eur. J. Haematol. 2002, 69, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Hodge, D.R.; Hurt, E.M.; Farrar, W.L. The Role of IL-6 and STAT3 in Inflammation and Cancer. Eur. J. Cancer 2005, 41, 2502–2512. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Lin, H.-K.; Kan, P.-Y.; Xie, S.; Tsai, M.-Y.; Wang, P.-H.; Chen, Y.-T.; Chang, C. Interleukin-6 Differentially Regulates Androgen Receptor Transactivation via PI3K-Akt, STAT3, and MAPK, Three Distinct Signal Pathways in Prostate Cancer Cells. Biochem. Biophys. Res. Commun. 2003, 305, 462–469. [Google Scholar] [CrossRef]
- Nakanishi, H.; Yoshioka, K.; Joyama, S.; Araki, N.; Myoui, A.; Ishiguro, S.; Ueda, T.; Yoshikawa, H.; Itoh, K. Interleukin-6/soluble Interleukin-6 Receptor Signaling Attenuates Proliferation and Invasion, and Induces Morphological Changes of a Newly Established Pleomorphic Malignant Fibrous Histiocytoma Cell Line. Am. J. Pathol. 2004, 165, 471–480. [Google Scholar] [CrossRef]
- Müllberg, J.; Dittrich, E.; Graeve, L.; Gerhartz, C.; Yasukawa, K.; Taga, T.; Kishimoto, T.; Heinrich, P.C.; Rose-John, S. Differential Shedding of the Two Subunits of the Interleukin-6 Receptor. FEBS Lett. 1993, 332, 174–178. [Google Scholar] [CrossRef]
- Müllberg, J.; Schooltink, H.; Stoyan, T.; Günther, M.; Graeve, L.; Buse, G.; Mackiewicz, A.; Heinrich, P.C.; Rose-John, S. The Soluble Interleukin-6 Receptor Is Generated by Shedding. Eur. J. Immunol. 1993, 23, 473–480. [Google Scholar] [CrossRef]
- Matthews, V.; Schuster, B.; Schütze, S.; Bussmeyer, I.; Ludwig, A.; Hundhausen, C.; Sadowski, T.; Saftig, P.; Hartmann, D.; Kallen, K.-J.; et al. Cellular Cholesterol Depletion Triggers Shedding of the Human Interleukin-6 Receptor by ADAM10 and ADAM17 (TACE). J. Biol. Chem. 2003, 278, 38829–38839. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Koyanagi, Y.; Zhou, Y.; Miyamoto, H.; Tanaka, Y.; Waki, M.; Matsumoto, A.; Yamamoto, M.; Yamamoto, N. Soluble Interleukin-6 Receptors Released from T Cell or Granulocyte/macrophage Cell Lines and Human Peripheral Blood Mononuclear Cells Are Generated through an Alternative Splicing Mechanism. Eur. J. Immunol. 1994, 24, 1945–1948. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Ohnesorge, N.; Rose-John, S. Interleukin-6 Trans-Signalling in Chronic Inflammation and Cancer. Scand. J. Immunol. 2006, 63, 321–329. [Google Scholar] [CrossRef]
- Peters, M.; Müller, A.M.; Rose-John, S. Interleukin-6 and Soluble Interleukin-6 Receptor: Direct Stimulation of gp130 and Hematopoiesis. Blood 1998, 92, 3495–3504. [Google Scholar] [CrossRef] [PubMed]
- Jostock, T.; Müllberg, J.; Ozbek, S.; Atreya, R.; Blinn, G.; Voltz, N.; Fischer, M.; Neurath, M.F.; Rose-John, S. Soluble gp130 Is the Natural Inhibitor of Soluble Interleukin-6 Receptor Transsignaling Responses. Eur. J. Biochem. 2001, 268, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Narazaki, M.; Yasukawa, K.; Saito, T.; Ohsugi, Y.; Fukui, H.; Koishihara, Y.; Yancopoulos, G.D.; Taga, T.; Kishimoto, T. Soluble Forms of the Interleukin-6 Signal-Transducing Receptor Component gp130 in Human Serum Possessing a Potential to Inhibit Signals through Membrane-Anchored gp130. Blood 1993, 82, 1120–1126. [Google Scholar] [CrossRef]
- Becker, C.; Fantini, M.C.; Wirtz, S.; Nikolaev, A.; Lehr, H.A.; Galle, P.R.; Rose-John, S.; Neurath, M.F. IL-6 Signaling Promotes Tumor Growth in Colorectal Cancer. Cell Cycle 2005, 4, 217–220. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Rose-John, S. The Soluble Interleukin 6 Receptor: Advanced Therapeutic Options in Inflammation. Clin. Pharmacol. Ther. 2017, 102, 591–598. [Google Scholar] [CrossRef]
- Domingo-Domenech, J.; Oliva, C.; Rovira, A.; Codony-Servat, J.; Bosch, M.; Filella, X.; Montagut, C.; Tapia, M.; Campás, C.; Dang, L.; et al. Interleukin 6, a Nuclear Factor-kappaB Target, Predicts Resistance to Docetaxel in Hormone-Independent Prostate Cancer and Nuclear Factor-kappaB Inhibition by PS-1145 Enhances Docetaxel Antitumor Activity. Clin. Cancer Res. 2006, 12, 5578–5586. [Google Scholar] [CrossRef]
- Duan, Z.; Foster, R.; Bell, D.A.; Mahoney, J.; Wolak, K.; Vaidya, A.; Hampel, C.; Lee, H.; Seiden, M.V. Signal Transducers and Activators of Transcription 3 Pathway Activation in Drug-Resistant Ovarian Cancer. Clin. Cancer Res. 2006, 12, 5055–5063. [Google Scholar] [CrossRef]
- Ikuta, K.; Takemura, K.; Kihara, M.; Nishimura, M.; Ueda, N.; Naito, S.; Lee, E.; Shimizu, E.; Yamauchi, A. Overexpression of Constitutive Signal Transducer and Activator of Transcription 3 mRNA in Cisplatin-Resistant Human Non-Small Cell Lung Cancer Cells. Oncol. Rep. 2005, 13, 217–222. [Google Scholar] [PubMed]
- Miyamoto, Y.; Hosotani, R.; Doi, R.; Wada, M.; Ida, J.; Tsuji, S.; Kawaguchi, M.; Nakajima, S.; Kobayashi, H.; Masui, T.; et al. Interleukin-6 Inhibits Radiation Induced Apoptosis in Pancreatic Cancer Cells. Anticancer Res. 2001, 21, 2449–2456. [Google Scholar] [PubMed]
- Sahu, R.P.; Srivastava, S.K. The Role of STAT-3 in the Induction of Apoptosis in Pancreatic Cancer Cells by Benzyl Isothiocyanate. J. Natl. Cancer Inst. 2009, 101, 176–193. [Google Scholar] [CrossRef]
- Feurino, L.W.; Zhang, Y.; Bharadwaj, U.; Zhang, R.; Li, F.; Fisher, W.E.; Brunicardi, F.C.; Chen, C.; Yao, Q.; Min, L. IL-6 Stimulates Th2 Type Cytokine Secretion and Upregulates VEGF and NRP-1 Expression in Pancreatic Cancer Cells. Cancer Biol. Ther. 2007, 6, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, H.S.; Cho, Y.; Lee, I.J.; Kim, H.J.; Lee, D.E.; Kang, H.W.; Park, J.S. Intraoperative Radiation Therapy Induces Immune Response Activity after Pancreatic Surgery. BMC Cancer 2021, 21, 1097. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Kim, M.-S.; Cho, L.C.; Dusenbery, K.; Sperduto, P.W. Radiobiological Basis of SBRT and SRS. Int. J. Clin. Oncol. 2014, 19, 570–578. [Google Scholar] [CrossRef]
- Garcia-Barros, M. Tumor Response to Radiotherapy Regulated by Endothelial Cell Apoptosis. Science 2003, 300, 1155–1159. [Google Scholar] [CrossRef]
- Park, H.J.; Griffin, R.J.; Hui, S.; Levitt, S.H.; Song, C.W. Radiation-Induced Vascular Damage in Tumors: Implications of Vascular Damage in Ablative Hypofractionated Radiotherapy (SBRT and SRS). Radiat. Res. 2012, 177, 311–327. [Google Scholar] [CrossRef]
- Finkelstein, S.E.; Timmerman, R.; McBride, W.H.; Schaue, D.; Hoffe, S.E.; Mantz, C.A.; Wilson, G.D. The Confluence of Stereotactic Ablative Radiotherapy and Tumor Immunology. Clin. Dev. Immunol. 2011, 2011, 439752. [Google Scholar] [CrossRef]
- Wild, A.T.; Herman, J.M.; Dholakia, A.S.; Moningi, S.; Lu, Y.; Rosati, L.M.; Hacker-Prietz, A.; Assadi, R.K.; Saeed, A.M.; Pawlik, T.M.; et al. Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy in Patients With Unresectable Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Baine, M.J.; Zhao, N.; Li, S.; Li, X.; Lin, C. Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy Compared to Conventional Fractionated Radiation Therapy in Patients with Locally Advanced Pancreatic Cancer. BMC Cancer 2019, 19, 977. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, S.G. Field Size Effects on the Risk and Severity of Treatment-Induced Lymphopenia in Patients Undergoing Radiation Therapy for Solid Tumors. Adv. Radiat. Oncol. 2018, 3, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Demaria, S. Combining Radiotherapy and Cancer Immunotherapy: A Paradigm Shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef]
- Grassberger, C.; Ellsworth, S.G.; Wilks, M.Q.; Keane, F.K.; Loeffler, J.S. Assessing the Interactions between Radiotherapy and Antitumour Immunity. Nat. Rev. Clin. Oncol. 2019, 16, 729–745. [Google Scholar] [CrossRef]
- Luke, J.J.; Lemons, J.M.; Karrison, T.G.; Pitroda, S.P.; Melotek, J.M.; Zha, Y.; Al-Hallaq, H.A.; Arina, A.; Khodarev, N.N.; Janisch, L.; et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2018, 36, 1611–1618. [Google Scholar] [CrossRef]
- Bahig, H.; Aubin, F.; Stagg, J.; Gologan, O.; Ballivy, O.; Bissada, E.; Nguyen-Tan, F.-P.; Soulières, D.; Guertin, L.; Filion, E.; et al. Phase I/II Trial of Durvalumab plus Tremelimumab and Stereotactic Body Radiotherapy for Metastatic Head and Neck Carcinoma. BMC Cancer 2019, 19, 68. [Google Scholar] [CrossRef]
- Tubin, S.; Yan, W.; Mourad, W.F.; Fossati, P.; Khan, M.K. The Future of Radiation-Induced Abscopal Response: Beyond Conventional Radiotherapy Approaches. Future Oncol. 2020, 16, 1137–1151. [Google Scholar] [CrossRef]
- Mima, T.; Nishimoto, N. Clinical Value of Blocking IL-6 Receptor. Curr. Opin. Rheumatol. 2009, 21, 224–230. [Google Scholar] [CrossRef]
- Le, R.Q.; Li, L.; Yuan, W.; Shord, S.S.; Nie, L.; Habtemariam, B.A.; Przepiorka, D.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist 2018, 23, 943–947. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, S.S.W.; Dawson, L.A. Inflammatory Cytokines and Radiotherapy in Pancreatic Ductal Adenocarcinoma. Biomedicines 2022, 10, 3215. https://doi.org/10.3390/biomedicines10123215
Ng SSW, Dawson LA. Inflammatory Cytokines and Radiotherapy in Pancreatic Ductal Adenocarcinoma. Biomedicines. 2022; 10(12):3215. https://doi.org/10.3390/biomedicines10123215
Chicago/Turabian StyleNg, Sylvia S. W., and Laura A. Dawson. 2022. "Inflammatory Cytokines and Radiotherapy in Pancreatic Ductal Adenocarcinoma" Biomedicines 10, no. 12: 3215. https://doi.org/10.3390/biomedicines10123215
APA StyleNg, S. S. W., & Dawson, L. A. (2022). Inflammatory Cytokines and Radiotherapy in Pancreatic Ductal Adenocarcinoma. Biomedicines, 10(12), 3215. https://doi.org/10.3390/biomedicines10123215



