Comparing TIMP-1 and Hsp70 in Blood and Saliva as Potential Prognostic Markers in HNSCC
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. TIMP-1 and Hsp70 in Serum and Saliva
3.2. Comparing Subjects with HNSCC and Control Subjects with and without Infection
3.3. Marker Association with Clinical and Pathological Parameters
3.4. Prognostic Value of the Protein Markers
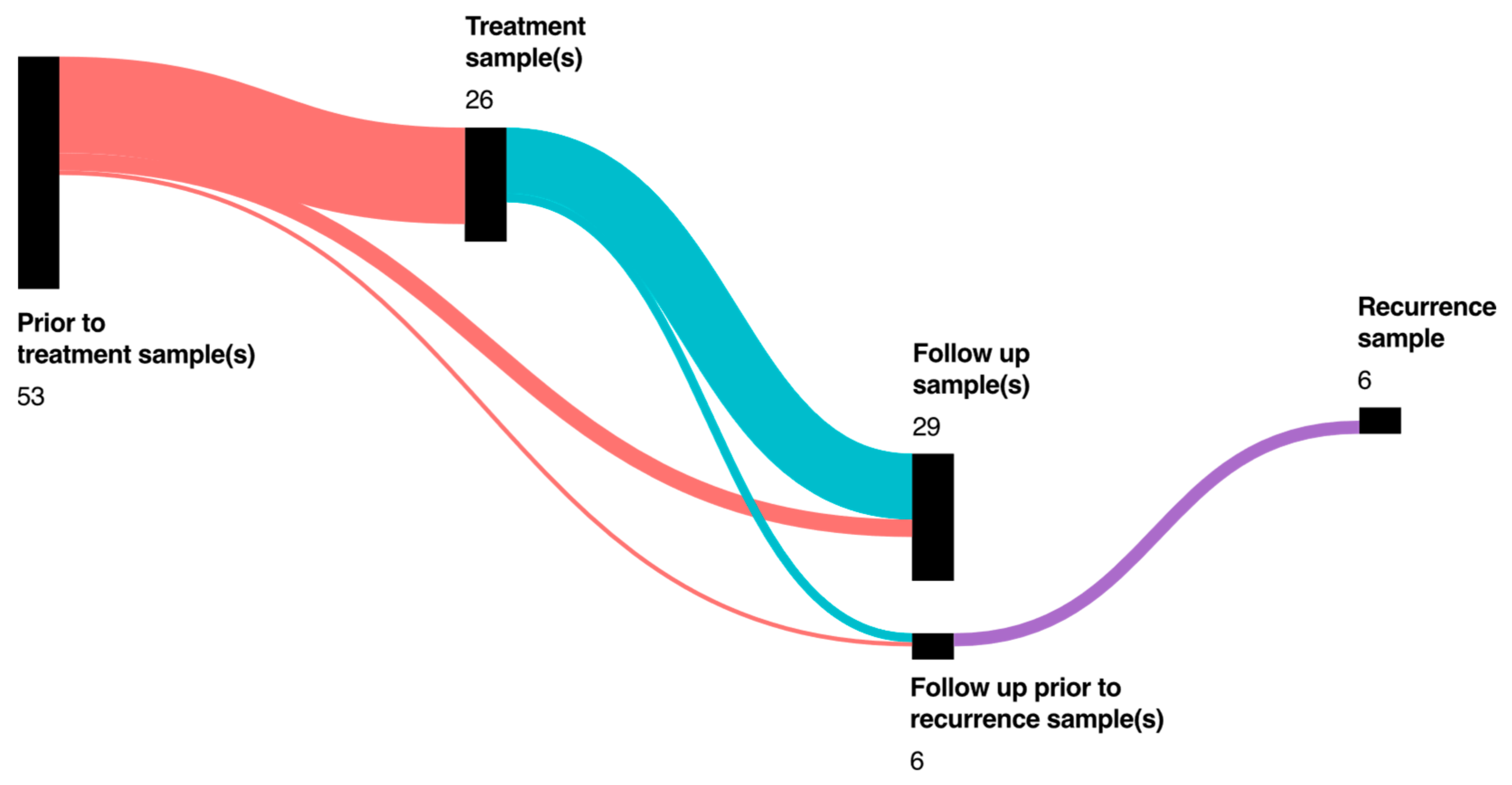
3.5. Follow-Up
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Institutional Epidemiologic Report: Krebs in Deutschland 2015/2016; Robert Koch Institut: Berlin, Germany; Gesellschaft der Epidemiologischen Krebsregister in Deutschland e.V.: Lübeck, Germany, 2019; Volume 12, pp. 24–59. ISBN 978-3-89606-298-7. Available online: https://edoc.rki.de/handle/176904/6012?locale-attribute=en (accessed on 23 April 2020).
- Kong, L.; Birkeland, A.C. Liquid Biopsies in Head and Neck Cancer: Current State and Future Challenges. Cancers 2021, 13, 1874. [Google Scholar] [CrossRef] [PubMed]
- Flach, S.; Howarth, K.; Hackinger, S.; Pipinikas, C.; Ellis, P.; McLay, K.; Marsico, G.; Forshew, T.; Walz, C.; Reichel, C.A.; et al. Liquid BIOpsy for MiNimal RESidual DiSease Detection in Head and Neck Squamous Cell Carcinoma (LIONESS)—A personalised circulating tumour DNA analysis in head and neck squamous cell carcinoma. Br. J. Cancer 2022, 126, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Ramírez, J.; Mir, M.; Samitier, J. Blood-Based Cancer Biomarkers in Liquid Biopsy: A Promising Non-Invasive Alternative to Tissue Biopsy. Int. J. Mol. Sci. 2018, 19, 2877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczor-Urbanowicz, K.E.; Wei, F.; Rao, S.L.; Kim, J.; Shin, H.; Cheng, J.; Tu, M.; Wong, D.T.W.; Kim, Y. Clinical validity of saliva and novel technology for cancer detection. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 49–59. [Google Scholar] [CrossRef]
- Hema Shree, K.; Ramani, P.; Sherlin, H.; Sukumaran, G.; Jeyaraj, G.; Don, K.R.; Santhanam, A.; Ramasubramanian, A.; Sundar, R. Saliva as a Diagnostic Tool in Oral Squamous Cell Carcinoma—A Systematic Review with Meta Analysis. Pathol. Oncol. Res. 2019, 25, 447–453. [Google Scholar] [CrossRef]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Fábián, T.K.; Fejérdy, P.; Nguyen, M.T.; Soti, C.; Csermely, P. Potential immunological functions of salivary Hsp70 in mucosal and periodontal defense mechanisms. Arch. Immunol. Exp. (Warsz) 2007, 55, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Holten-Andersen, L.; Jensen, S.B.; Bardow, A.; Harslund, J.; Thaysen-Andersen, M.; Lademann, U.; Usher, P.A.; Offenberg, H.; Højrup, P.; Reibel, J.; et al. Identifying sources and estimating glandular output of salivary TIMP-1. Scand. J. Clin. Lab. Investig. 2008, 68, 548–554. [Google Scholar] [CrossRef]
- Domeij, H.; Modéer, T.; Yucel-Lindberg, T. Matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 production in human gingival fibroblasts: The role of protein kinase C. J. Periodontal. Res. 2004, 39, 308–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, Y.Q.; Nakamura, H.; Tanaka, T.; Odani, T.; Perez, P.; Ji, Y.; French, B.N.; Pranzatelli, T.J.; Michael, D.G.; Yin, H.; et al. Lysosomal exocytosis of HSP70 stimulates monocytic BMP6 expression in Sjögren’s syndrome. J. Clin. Investig. 2022, 132, e152780. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.W.; Defamie, V.; Waterhouse, P.; Khokha, R. TIMPs: Versatile extracellular regulators in cancer. Nat. Rev. Cancer 2017, 17, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.K.; Liu, X.W.; Chirco, R.; Fridman, R.; Kim, H.R. Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J. 2006, 25, 3934–3942. [Google Scholar] [CrossRef] [Green Version]
- Braithwaite, J.; Wears, R.L.; Hollnagel, E. Resilient health care: Turning patient safety on its head. Int. J. Qual. Health Care 2015, 27, 418–420. [Google Scholar] [CrossRef]
- Carpen, T.; Sorsa, T.; Jouhi, L.; Tervahartiala, T.; Haglund, C.; Syrjanen, S.; Tarkkanen, J.; Mohamed, H.; Makitie, A.; Hagstrom, J.; et al. High levels of tissue inhibitor of metalloproteinase-1 (TIMP-1) in the serum are associated with poor prognosis in HPV-negative squamous cell oropharyngeal cancer. Cancer Immunol. Immunother. 2019, 68, 1263–1272. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Wang, J.; Fan, W.; Pu, X.; Zhang, D.; Fan, C.; Xiong, L.; Zhu, H.; Xu, N.; Chen, R.; et al. Upregulated TIMP-1 correlates with poor prognosis of laryngeal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 246–254. [Google Scholar]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef]
- Multhoff, G. Heat shock protein 70 (Hsp70): Membrane location, export and immunological relevance. Methods 2007, 43, 229–237. [Google Scholar] [CrossRef]
- Stangl, S.; Tontcheva, N.; Sievert, W.; Shevtsov, M.; Niu, M.; Schmid, T.E.; Pigorsch, S.; Combs, S.E.; Haller, B.; Balermpas, P.; et al. Heat shock protein 70 and tumor-infiltrating NK cells as prognostic indicators for patients with squamous cell carcinoma of the head and neck after radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int. J. Cancer 2018, 142, 1911–1925. [Google Scholar] [CrossRef] [Green Version]
- Gehrmann, M.; Specht, H.M.; Bayer, C.; Brandstetter, M.; Chizzali, B.; Duma, M.; Breuninger, S.; Hube, K.; Lehnerer, S.; van Phi, V.; et al. Hsp70--a biomarker for tumor detection and monitoring of outcome of radiation therapy in patients with squamous cell carcinoma of the head and neck. Radiat. Oncol. 2014, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Springer, S.; Mulvey, C.L.; Silliman, N.; Schaefer, J.; Sausen, M.; James, N.; Rettig, E.M.; Guo, T.; Pickering, C.R.; et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci. Transl. Med. 2015, 7, 293ra104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Ruokolainen, H.; Pääkkö, P.; Turpeenniemi-Hujanen, T. Tissue inhibitor of matrix metalloproteinase-1 is prognostic in head and neck squamous cell carcinoma: Comparison of the circulating and tissue immunoreactive protein. Clin. Cancer Res. 2005, 11, 3257–3264. [Google Scholar] [CrossRef] [Green Version]
- Najy, A.J.; Jung, Y.S.; Kim, S.; Fridman, R.; Kim, H.C. Regulation of Tumor Metabolism and Extracellular Acidosis by the TIMP-10-CD63 Axis in Breast Carcinoma. Cells 2021, 10, 2721. [Google Scholar] [CrossRef]
- Bodnar, M.; Szylberg, Ł.; Kazmierczak, W.; Marszalek, A. Tumor progression driven by pathways activating matrix metalloproteinases and their inhibitors. J. Oral. Pathol. Med. 2015, 44, 437–443. [Google Scholar] [CrossRef]
- Pradhan-Palikhe, P.; Vesterinen, T.; Tarkkanen, J.; Leivo, I.; Sorsa, T.; Salo, T.; Mattila, P.S. Plasma level of tissue inhibitor of matrix metalloproteinase-1 but not that of matrix metalloproteinase-8 predicts survival in head and neck squamous cell cancer. Oral Oncol. 2010, 46, 514–518. [Google Scholar] [CrossRef]
- Holten-Andersen, L.; Thaysen-Andersen, M.; Jensen, S.B.; Buchwald, C.; Højrup, P.; Offenberg, H.; Nielsen, H.J.; Brünner, N.; Nauntofte, B.; Reibel, J. Salivary tissue inhibitor of metalloproteinases-1 localization and glycosylation profile analysis. Apmis 2011, 119, 741–749. [Google Scholar] [CrossRef]
- Hayakawa, H.; Yamashita, K.; Ohwaki, K.; Sawa, M.; Noguchi, T.; Iwata, K.; Hayakawa, T. Collagenase activity and tissue inhibitor of metalloproteinases-1 (TIMP-1) content in human whole saliva from clinically healthy and periodontally diseased subjects. J. Periodontal Res. 1994, 29, 305–308. [Google Scholar] [CrossRef]
- Fábián, T.K.; Gáspár, J.; Fejérdy, L.; Kaán, B.; Bálint, M.; Csermely, P.; Fejérdy, P. Hsp70 is present in human saliva. Med. Sci. Monit. 2003, 9, Br62–Br65. [Google Scholar]
- Lancaster, G.I.; Febbraio, M.A. Exosome-dependent trafficking of HSP70: A novel secretory pathway for cellular stress proteins. J. Biol. Chem. 2005, 280, 23349–23355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agaimy, A.; Weichert, W.; Haller, F.; Hartmann, A. Diagnostic and predictive molecular pathology of head and neck neoplasms. Pathologe 2018, 39, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary defense proteins: Their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, J.G.; Kramer, J.M.; Visser, M.B. Danger signals in oral cavity-related diseases. J. Leukoc. Biol. 2019, 106, 193–200. [Google Scholar] [CrossRef]
- Ju, H.; Hu, Z.; Lu, Y.; Wu, Y.; Zhang, L.; Wei, D.; Guo, W.; Xia, W.; Liu, S.; Ren, G.; et al. TLR4 activation leads to anti-EGFR therapy resistance in head and neck squamous cell carcinoma. Am. J. Cancer Res. 2020, 10, 454–472. [Google Scholar]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Tait, S.W.G. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef]
- Pouwels, S.D.; Hesse, L.; Faiz, A.; Lubbers, J.; Bodha, P.K.; Ten Hacken, N.H.; van Oosterhout, A.J.; Nawijn, M.C.; Heijink, I.H. Susceptibility for cigarette smoke-induced DAMP release and DAMP-induced inflammation in COPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L881–L892. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; DeBusk, W.T.; Stepanov, I.; Gomez, A.; Khariwala, S.S. Oral Microbiome Profiling in Smokers with and without Head and Neck Cancer Reveals Variations Between Health and Disease. Cancer Prev. Res. (Phila) 2020, 13, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; You, Y.; Huang, J.; Wang, X.; Zhu, H.; Wang, Z. LAMP3 and TP53 overexpression predicts poor outcome in laryngeal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 5519–5527. [Google Scholar]
- Liao, C.; An, J.; Tan, Z.; Xu, F.; Liu, J.; Wang, Q. Changes in Protein Glycosylation in Head and Neck Squamous Cell Carcinoma. J. Cancer 2021, 12, 1455–1466. [Google Scholar] [CrossRef] [PubMed]



| (a) HNSCC Patients Characteristics | (b) TNM Staging | (c) Control Subjects Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| sample before therapy | sample before therapy | Head/Neck infection | |||||||||
| Characteristic | Overall, | no, | yes, | Characteristic | Overall, | no, | yes, | Characteristic | Overall, | no, | yes, |
| n= 73 1 | n= 20 1 | n= 53 1 | n = 73 1 | n = 20 1 | n = 53 1 | n = 40 1 | n = 25 1 | n = 15 1 | |||
| Age at inclusion | 63 (56, 72) | 64 (58, 70) | 63 (55, 72) | Staging | Age at inclusion | 60 (54, 67) | 61 (55, 67) | 59 (52, 66) | |||
| Gender | I | 14 (19%) | 6 (30%) | 8 (15%) | Gender | ||||||
| Female | 18 (25%) | 5 (25%) | 13 (25%) | II | 21 (29%) | 4 (20%) | 17 (32%) | Female | 15 (38%) | 7 (28%) | 8 (53%) |
| Male | 55 (75%) | 15 (75%) | 40 (75%) | III | 18 (25%) | 3 (15%) | 15 (28%) | Male | 25 (62%) | 18 (72%) | 7 (47%) |
| Localization | IV | 19 (26%) | 7 (35%) | 12 (23%) | Smoking | ||||||
| CUP | 4 (5.5%) | 0 (0%) | 4 (7.5%) | Tis | 1 (1.4%) | 0 (0%) | 1 (1.9%) | Non-smoker | 11 (28%) | 5 (20%) | 6 (40%) |
| Hypopharynx | 6 (8.2%) | 1 (5.0%) | 5 (9.4%) | Tumor | Ex-smoker | 5 (12%) | 5 (20%) | 0 (0%) | |||
| Larynx | 8 (11%) | 5 (25%) | 3 (5.7%) | T0 | 5 (6.8%) | 0 (0%) | 5 (9.4%) | Smoker | 15 (38%) | 11 (44%) | 4 (27%) |
| Nasopharynx | 2 (2.7%) | 1 (5.0%) | 1 (1.9%) | T1 | 12 (16%) | 3 (15%) | 9 (17%) | Unknown | 9 (22%) | 4 (16%) | 5 (33%) |
| Oral Cavity | 19 (26%) | 5 (25%) | 14 (26%) | T2 | 21 (29%) | 8 (40%) | 13 (25%) | Alcohol | |||
| Oropharynx | 34 (47%) | 8 (40%) | 26 (49%) | T3 | 19 (26%) | 3 (15%) | 16 (30%) | Not regularly | 19 (48%) | 11 (44%) | 8 (53%) |
| p16 IHC | T4 | 16 (22%) | 6 (30%) | 10 (19%) | Regularly | 12 (30%) | 10 (40%) | 2 (13%) | |||
| No p16 IHC | 13 (18%) | 2 (10%) | 11 (21%) | Nodus | Unknown | 9 (22%) | 4 (16%) | 5 (33%) | |||
| p16- | 32 (44%) | 9 (45%) | 23 (43%) | N0 | 25 (34%) | 10 (50%) | 15 (28%) | 1 Median (IQR); n (%) | |||
| p16+ | 28 (38%) | 9 (45%) | 19 (36%) | N1 | 21 (29%) | 5 (25%) | 16 (30%) | ||||
| Smoking | N2 | 24 (33%) | 4 (20%) | 20 (38%) | |||||||
| Non-smoker | 19 (26%) | 3 (15%) | 16 (30%) | N3 | 2 (2.7%) | 0 (0%) | 2 (3.8%) | ||||
| Ex-smoker | 7 (9.6%) | 3 (15%) | 4 (7.5%) | Nx | 1 (1.4%) | 1 (5.0%) | 0 (0%) | ||||
| Smoker | 42 (58%) | 14 (70%) | 28 (53%) | Metastasis | |||||||
| Unknown | 5 (6.8%) | 0 (0%) | 5 (9.4%) | M0 | 65 (89%) | 14 (70%) | 51 (96%) | ||||
| Alcohol | Mx | 1 (1.4%) | 0 (0%) | 1 (1.9%) | |||||||
| Not regularly | 21 (29%) | 7 (35%) | 14 (26%) | M1 | 7 (9.6%) | 6 (30%) | 1 (1.9%) | ||||
| Ex-regularly | 1 (1.4%) | 0 (0%) | 1 (1.9%) | 1n (%) | |||||||
| Regularly | 35 (48%) | 8 (40%) | 27 (51%) | ||||||||
| Unknown | 16 (22%) | 5 (25%) | 11 (21%) | ||||||||
| 1 Median (IQR); n (%) | |||||||||||
| Marker | Hsp70 Serum | Hsp70 Saliva | TIMP-1 Serum |
|---|---|---|---|
| Hsp70 serum | |||
| Hsp70 saliva | 0.01 | ||
| TIMP-1 serum | −0.12 | 0.27 1 | |
| TIMP-1 saliva | −0.03 | 0.47 1 | 0.30 1 |
| Staging Parameters | ||||||
|---|---|---|---|---|---|---|
| Staging | Tumor (T) | |||||
| Concentration (ng/mL) | ≤II, n = 26 1 | >II, n = 27 1 | p-value 2 | ≤II, n = 27 1 | >II, n = 26 1 | p-value 2 |
| TIMP-1 serum | 237 (213, 274) | 274 (237, 321) | 0.047 | 263 (212, 282) | 270 (233, 314) | 0.2 |
| TIMP-1 saliva | 55 (37, 78) | 68 (45, 107) | 0.3 | 55 (39, 84) | 76 (44, 107) | 0.6 |
| Hsp70 serum | 0.05 (0.05, 0.31) | 0.05 (0.05, 0.45) | 0.9 | 0.05 (0.05, 0.41) | 0.05 (0.05, 0.36) | 0.6 |
| Hsp70 saliva | 8 (3, 18) | 8 (3, 22) | 0.8 | 9 (3, 22) | 7 (3, 21) | >0.9 |
| Nodus (N) | Metastasis (M) | |||||
| Concentration (ng/mL) | =0, n = 15 1 | ≥I, n = 38 1 | p-value 2 | 0, n = 51 1 | X or I, n = 2 1 | p-value 3 |
| TIMP-1 serum | 259 (222, 285) | 266 (221, 295) | 0.8 | 267 (220, 295) | 239 (232, 246) | 0.5 |
| TIMP-1 saliva | 54 (42, 85) | 67 (39, 104) | 0.6 | 62 (41, 100) | 23 (23, 23) | 0.3 |
| Hsp70 serum | 0.13 (0.05, 0.23) | 0.05 (0.05, 0.60) | 0.8 | 0.05 (0.05, 0.37) | 0.43 (0.33, 0.53) | 0.2 |
| Hsp70 saliva | 11 (5, 24) | 7 (3, 19) | 0.2 | 8 (3, 22) | 5 (5, 5) | 0.8 |
| Pathological and Cliniacal Parameters | ||||
| p16 IHC | ||||
| Concentration (ng/mL) | no p16 IHC, n = 11 1 | p16-, n = 231 | p16+, n = 19 1 | p-value 2 |
| TIMP-1 serum | 274 (264, 285) | 267 (219, 367) | 236 (218, 277) | 0.2 |
| TIMP-1 saliva | 90 (46, 113) | 51 (44, 81) | 67 (37, 89) | 0.6 |
| Hsp70 serum | 0.05 (0.05, 0.13) | 0.13 (0.05, 0.67) | 0.05 (0.05, 0.48) | 0.4 |
| Hsp70 saliva | 8 (3, 26) | 5 (2, 15) | 11 (5, 24) | 0.2 |
| Smoking | ||||
| Concentration (ng/mL) | non-smoker, n = 16 1 | ex-smoker, n = 4 1 | smoker, n = 28 1 | p-value 2 |
| TIMP-1 serum | 236 (223, 288) | 232 (215, 246) | 271 (220, 305) | 0.3 |
| TIMP-1 saliva | 67 (32, 103) | 46 (37, 58) | 62 (46, 104) | 0.5 |
| Hsp70 serum | 0.05 (0.05, 0.19) | 0.14 (0.05, 0.87) | 0.05 (0.05,0.43) | 0.9 |
| Hsp70 saliva | 17 (6, 26) | 5 (5, 7) | 5 (3, 16) | 0.11 |
| Alcohol | ||||
| Concentration (ng/mL) | not regularly, n = 14 1 | ex-regularly, n = 1 1 | regularly, n = 27 1 | p-value 2 |
| TIMP-1 serum | 250 (214, 273) | 267 (267, 267) | 274 (224, 331) | 0.3 |
| TIMP-1 saliva | 52 (40, 106) | 62 (62, 62) | 76 (48, 100) | 0.8 |
| Hsp70 serum | 0.13 (0.05, 0.30) | 1.15 (1.15, 1.15) | 0.05 (0.05, 0.45) | 0.2 |
| Hsp70 saliva | 16 (5, 26) | 9 (9, 9) | 6 (3, 11) | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinecker, J.; Roesch, R.; Krippgans, S.; Nieberler, M.; Stark, L.; Stangl, S.; Haller, B.; Fritsche, K.; Multhoff, G.; Knopf, A.; et al. Comparing TIMP-1 and Hsp70 in Blood and Saliva as Potential Prognostic Markers in HNSCC. Biomedicines 2022, 10, 3225. https://doi.org/10.3390/biomedicines10123225
Rinecker J, Roesch R, Krippgans S, Nieberler M, Stark L, Stangl S, Haller B, Fritsche K, Multhoff G, Knopf A, et al. Comparing TIMP-1 and Hsp70 in Blood and Saliva as Potential Prognostic Markers in HNSCC. Biomedicines. 2022; 10(12):3225. https://doi.org/10.3390/biomedicines10123225
Chicago/Turabian StyleRinecker, Jakob, Romina Roesch, Sara Krippgans, Markus Nieberler, Leonhard Stark, Stefan Stangl, Bernhard Haller, Kristin Fritsche, Gabriele Multhoff, Andreas Knopf, and et al. 2022. "Comparing TIMP-1 and Hsp70 in Blood and Saliva as Potential Prognostic Markers in HNSCC" Biomedicines 10, no. 12: 3225. https://doi.org/10.3390/biomedicines10123225
APA StyleRinecker, J., Roesch, R., Krippgans, S., Nieberler, M., Stark, L., Stangl, S., Haller, B., Fritsche, K., Multhoff, G., Knopf, A., Winter, C., Wollenberg, B., & Wirth, M. (2022). Comparing TIMP-1 and Hsp70 in Blood and Saliva as Potential Prognostic Markers in HNSCC. Biomedicines, 10(12), 3225. https://doi.org/10.3390/biomedicines10123225







