Opportunities and Challenges of Human IPSC Technology in Kidney Disease Research
Abstract
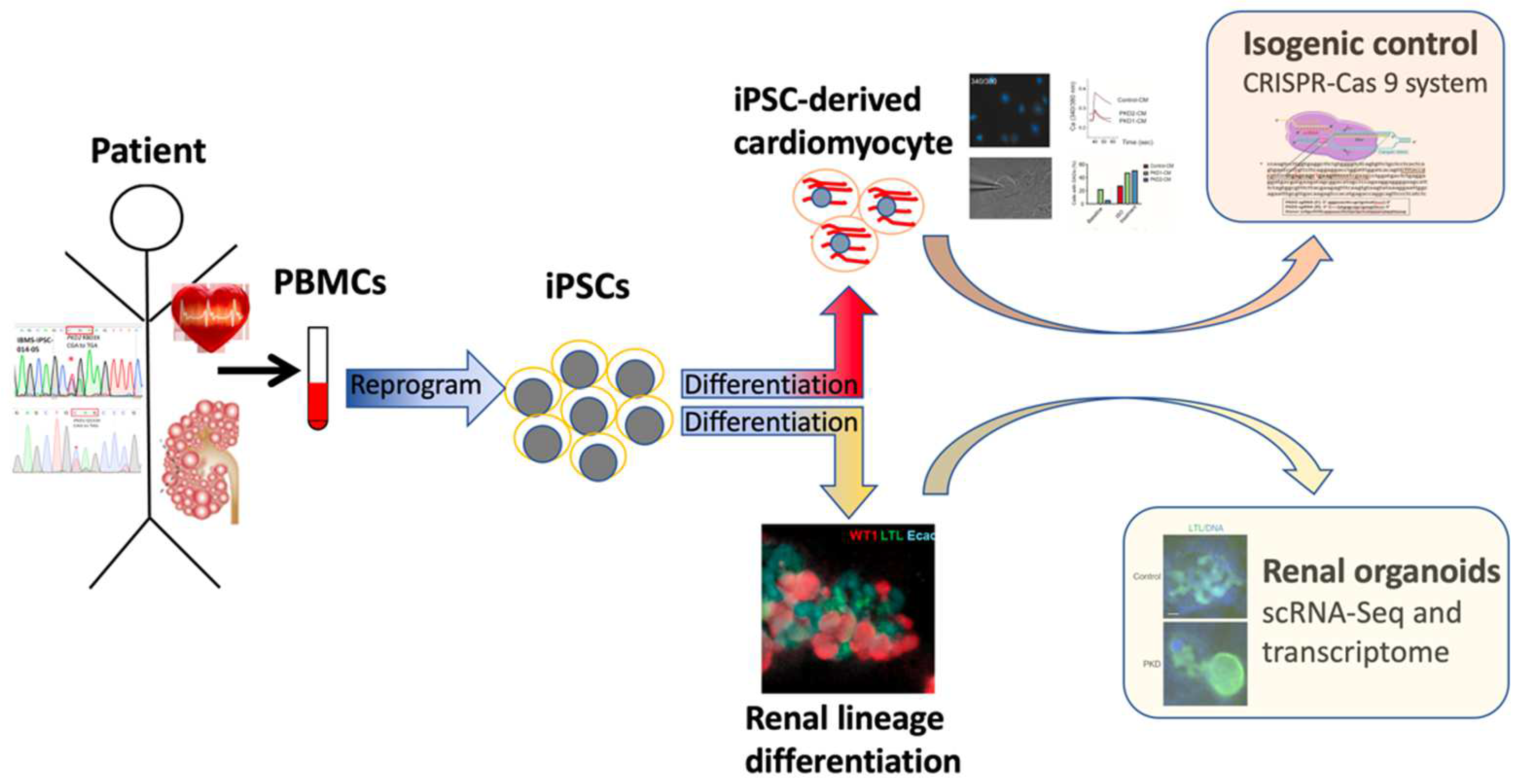
:1. Introduction
2. The Evolving iPSC Technology: Generation, Characterization and Banking
3. iPSC-Based Human Disease Modeling: Kidney Differentiation and ADPKD
4. Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slack, J.M.W. The Science of Stem Cells; Wiley-Blackwell: Hoboken, NJ, USA, 2018; Chapter 6; pp. 93–106. [Google Scholar]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, P.; Takahashi, K.; Saito, M.; Yoshida, Y.; Okita, K.; Watanabe, A.; Inoue, H.; Yamashita, J.K.; Todani, M.; Nakagawa, M.; et al. Induced Pluripotent Stem Cells and Their Use in Human Models of Disease and Development. Physiol. Rev. 2019, 99, 79–114. [Google Scholar] [CrossRef] [PubMed]
- Osafune, K. iPSC technology-based regenerative medicine for kidney diseases. Clin. Exp. Nephrol. 2021, 25, 574–584. [Google Scholar] [CrossRef]
- Haeckel, E. Natürliche Schöpfungsgeschichte; Georg Reimer: Berlin, Germany, 1868. [Google Scholar]
- Ramalho-Santos, M.; Willenbring, H. On the origin of the term “stem cell”. Cell Stem Cell 2007, 1, 35–38. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Beltcheva, M.; Gontarz, P.; Zhang, B.; Popli, P.; Fischer, L.A.; Khan, S.A.; Park, K.M.; Yoon, E.J.; Xing, X.; et al. Derivation of trophoblast stem cells from naïve human pluripotent stem cells. eLife 2020, 9, e52504. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, T.; Ma, L.; Zhang, Y.; Zhao, Y.; Lye, K.; Xiao, L.; Chen, C.; Wang, Z.; Ma, Y.; et al. Efficient derivation of human trophoblast stem cells from primed pluripotent stem cells. Sci. Adv. 2021, 7, eabf4416. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Tsubooka, N.; Ichisaka, T.; Okita, K.; Takahashi, K.; Nakagawa, M.; Yamanaka, S. Roles of Sall4 in the generation of pluripotent stem cells from blastocysts and fibroblasts. Genes Cells 2009, 14, 683–694. [Google Scholar] [CrossRef]
- Heng, J.C.; Feng, B.; Han, J.; Jiang, J.; Kraus, P.; Ng, J.H.; Orlov, Y.L.; Huss, M.; Yang, L.; Lufkin, T.; et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell 2010, 6, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, M.; Yamaguchi, K.; Nakamura, T.; Shibukawa, R.; Kodanaka, I.; Ichisaka, T.; Kawamura, Y.; Mochizuki, H.; Goshima, N.; Yamanaka, S. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature 2011, 474, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Fusaki, N.; Ban, H.; Nishiyama, A.; Saeki, K.; Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 348–362. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, K.; Sano, M.; Ohtaka, M.; Furuta, B.; Umemura, Y.; Nakajima, Y.; Ikehara, Y.; Kobayashi, T.; Segawa, H.; Takayasu, S.; et al. Development of defective and persistent Sendai virus vector: A unique gene delivery/expression system ideal for cell reprogramming. J. Biol. Chem. 2011, 286, 4760–4771. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.J.; Ho, M.C.; Huang, C.Y.; Wen, C.H.; Cheng, Y.C.; Hsu, Y.H.; Hwang, D.Y.; Lu, H.E.; Chen, H.C.; Hsieh, P.C.H. Induced pluripotent stem cells derived from an autosomal dominant polycystic kidney disease patient carrying a PKD1 Q533X mutation. Stem Cell Res. 2017, 25, 83–87. [Google Scholar] [CrossRef]
- Huang, C.Y.; Liu, C.L.; Ting, C.Y.; Chiu, Y.T.; Cheng, Y.C.; Nicholson, M.W.; Hsieh, P.C.H. Human iPSC banking: Barriers and opportunities. J. Biomed. Sci. 2019, 26, 87. [Google Scholar] [CrossRef] [Green Version]
- Schlaeger, T.M.; Daheron, L.; Brickler, T.R.; Entwisle, S.; Chan, K.; Cianci, A.; DeVine, A.; Ettenger, A.; Fitzgerald, K.; Godfrey, M.; et al. A comparison of non-integrating reprogramming methods. Nat. Biotechnol. 2015, 33, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.Y.; Li, L.H.; Hsu, W.T.; Cheng, Y.C.; Nicholson, M.W.; Liu, C.L.; Ting, C.Y.; Ko, H.W.; Syu, S.H.; Wen, C.H.; et al. Copy number variant hotspots in Han Taiwanese population induced pluripotent stem cell lines—Lessons from establishing the Taiwan human disease iPSC Consortium Bank. J. Biomed. Sci. 2020, 27, 92. [Google Scholar] [CrossRef]
- Umekage, M.; Sato, Y.; Takasu, N. Overview: An iPS cell stock at CiRA. Inflamm. Regen. 2019, 39, 17. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kurtz, A.; Yuan, B.Z.; Zeng, F.; Lomax, G.; Loring, J.F.; Crook, J.; Ju, J.H.; Clarke, L.; Inamdar, M.S.; et al. Report of the International Stem Cell Banking Initiative Workshop Activity: Current Hurdles and Progress in Seed-Stock Banking of Human Pluripotent Stem Cells. Stem Cells Transl. Med. 2017, 6, 1956–1962. [Google Scholar] [CrossRef]
- De Sousa, P.A.; Steeg, R.; Wachter, E.; Bruce, K.; King, J.; Hoeve, M.; Khadun, S.; McConnachie, G.; Holder, J.; Kurtz, A.; et al. Rapid establishment of the European Bank for induced Pluripotent Stem Cells (EBiSC)—The Hot Start experience. Stem Cell Res. 2017, 20, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.M.; Hei, D.; Stacey, G. The International Stem Cell Banking Initiative (ISCBI): Raising standards to bank on. In Vitro Cell Dev. Biol. Anim. 2010, 46, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.Y.; Huang, C.Y.; Chen, H.C.; Chiu, Y.W.; Hsieh, P.C.H.; Lee, J.J. Generation of induced pluripotent stem cells from a Bardet-Biedl syndrome patient carrying a homologous BBS2 c.534 + 1G > T mutation. Stem Cell Res. 2021, 55, 102480. [Google Scholar] [CrossRef] [PubMed]
- Dimos, J.T.; Rodolfa, K.T.; Niakan, K.K.; Weisenthal, L.M.; Mitsumoto, H.; Chung, W.; Croft, G.F.; Saphier, G.; Leibel, R.; Goland, R.; et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 2008, 321, 1218–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.Y.; Yoshida, M.; Li, L.T.; Ikenaka, A.; Oshima, S.; Nakagawa, K.; Sakurai, H.; Matsui, E.; Nakahata, T.; Saito, M.K. iPSC-derived functional human neuromuscular junctions model the pathophysiology of neuromuscular diseases. JCI Insight 2019, 4, e124299. [Google Scholar] [CrossRef]
- Takahashi, J. iPS cell-based therapy for Parkinson’s disease: A Kyoto trial. Regen. Ther. 2020, 13, 18–22. [Google Scholar] [CrossRef]
- Lee, J.J.; Cheng, S.J.; Huang, C.Y.; Chen, C.Y.; Feng, L.; Hwang, D.Y.; Kamp, T.J.; Chen, H.C.; Hsieh, P.C.H. Primary cardiac manifestation of autosomal dominant polycystic kidney disease revealed by patient induced pluripotent stem cell-derived cardiomyocytes. eBioMedicine 2019, 40, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Hnatiuk, A.P.; Briganti, F.; Staudt, D.W.; Mercola, M. Human iPSC modeling of heart disease for drug development. Cell Chem. Biol. 2021, 28, 271–282. [Google Scholar] [CrossRef]
- Huang, C.Y.; Nicholson, M.W.; Wang, J.Y.; Ting, C.Y.; Tsai, M.H.; Cheng, Y.C.; Liu, C.L.; Chan, D.Z.H.; Lee, Y.C.; Hsu, C.C.; et al. Population-based high-throughput toxicity screen of human iPSC-derived cardiomyocytes and neurons. Cell Rep. 2022, 39, 110643. [Google Scholar] [CrossRef]
- Chong, J.J.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef]
- Liu, Y.W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef]
- Miyagawa, S.; Kainuma, S.; Kawamura, T.; Suzuki, K.; Ito, Y.; Iseoka, H.; Ito, E.; Takeda, M.; Sasai, M.; Mochizuki-Oda, N.; et al. Case report: Transplantation of human induced pluripotent stem cell-derived cardiomyocyte patches for ischemic cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 950829. [Google Scholar] [CrossRef]
- Mae, S.; Shono, A.; Shiota, F.; Yasuno, T.; Kajiwara, M.; Gotoda-Nishimura, N.; Arai, S.; Sato-Otubo, A.; Toyoda, T.; Takahashi, K.; et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent-stemcells. Nat. Commun. 2013, 4, 1367. [Google Scholar] [CrossRef] [Green Version]
- Araoka, T.; Mae, S.; Kurose, Y.; Uesugi, M.; Ohta, A.; Yamanaka, S.; Osafune, K. Efficient and rapid induction of human iPSCs/ESCs into nephrogenic intermediate mesoderm using small molecule-based differentiation methods. PLoS ONE 2014, 9, e84881. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, A.; Kaku, Y.; Ohmori, T.; Sharmin, S.; Ogawa, M.; Sasaki, H.; Nishinakamura, R. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 2014, 14, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; de Sousa, C.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015, 526, 564–568. [Google Scholar] [CrossRef]
- Takasato, M.; Little, M.H. Making a Kidney Organoid Using the Directed Differentiation of Human Pluripotent Stem Cells. Methods Mol. Biol. 2017, 1597, 195–206. [Google Scholar] [CrossRef]
- Morizane, R.; Lam, A.Q.; Freedman, B.S.; Kishi, S.; Valeius, M.T.; Bonventre, J.V. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 2015, 33, 1193–1200. [Google Scholar] [CrossRef] [Green Version]
- Freedman, B.S.; Brooks, C.R.; Lam, A.Q.; Fu, H.; Morizane, R.; Agrawal, V.; Saad, A.F.; Li, M.K.; Hughes, M.R.; Werff, R.V.; et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat. Commun. 2015, 6, 8715. [Google Scholar] [CrossRef] [Green Version]
- Morizane, R.; Bonventre, J.V. Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nat. Protoc. 2017, 12, 195–207. [Google Scholar] [CrossRef]
- Taguchi, A.; Nishinakamura, R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 2017, 21, 730–746.e6. [Google Scholar] [CrossRef] [Green Version]
- Kuraoka, S.; Tanigawa, S.; Taguchi, A.; Hotta, A.; Nakazato, H.; Osafune, K.; Kobayashi, A.; Nishinakamura, R. PKD1-Dependent Renal Cystogenesis in Human Induced Pluripotent Stem Cell-Derived Ureteric Bud/Collecting Duct Organoids. J. Am. Soc. Nephrol. 2020, 31, 2355–2371. [Google Scholar] [CrossRef]
- Mae, S.I.; Ryosaka, M.; Toyoda, T.; Matsuse, K.; Oshima, Y.; Tsujimoto, H.; Okumura, S.; Shibasaki, A.; Osafune, K. Generation of branching ureteric bud tissues from human pluripotent stem cells. Biochem. Biophys. Res. Commun. 2018, 495, 954–961. [Google Scholar] [CrossRef]
- Mae, S.I.; Ryosaka, M.; Sakamoto, S.; Matsuse, K.; Nozaki, A.; Igami, M.; Kabai, R.; Watanabe, A.; Osafune, K. Expansion of human iPSC-derived ureteric bud organoids with repeated branching potential. Cell Rep. 2020, 32, 107963. [Google Scholar] [CrossRef]
- Ong, A.C.; Devuyst, O.; Knebelmann, B.; Walz, G.; ERA-EDTA Working Group for Inherited Kidney Diseases. Autosomal dominant polycystic kidney disease: The changing face of clinical management. Lancet 2015, 385, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Harris, P.C.; Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Chapin, H.C.; Caplan, M.J. The cell biology of polycystic kidney disease. J. Cell Biol. 2010, 191, 701–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, B.S.; Lam, A.Q.; Sundsbak, J.L.; Iatrino, R.; Su, X.; Koon, S.J.; Wu, M.; Daheron, L.; Harris, P.C.; Zhou, J.; et al. Reduced ciliary polycystin-2 in induced pluripotent stem cells from polycystic kidney disease patients with PKD1 mutations. J. Am. Soc. Nephrol. 2013, 24, 1571–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, T.A.; Howden, S.E.; Lawlor, K.; Phipson, B.; Maksimovic, J.; Hale, L.; Wilson, S.; Quinlan, C.; Ho, G.; Holman, K.; et al. Patient-iPSC-Derived Kidney Organoids Show Functional Validation of a Ciliopathic Renal Phenotype and Reveal Underlying Pathogenetic Mechanisms. Am. J. Hum. Genet. 2018, 102, 816–831. [Google Scholar] [CrossRef] [Green Version]
- Tsujimoto, H.; Kasahara, T.; Sueta, S.; Araoka, T.; Sakamoto, S.; Okada, C.; Mae, S.I.; Nakajima, T.; Okamoto, N.; Taura, D.; et al. A modular differentiation system maps multiple human kidney lineage from pluripotent stem cells. Cell Rep. 2020, 31, 107476. [Google Scholar] [CrossRef]
- Shimizu, T.; Mae, S.I.; Araoka, T.; Okita, K.; Hotta, A.; Yamagata, K.; Osafune, K. A novel ADPKD model using kidney organoids derived from disease-specific human iPSCs. Biochem. Biophys. Res. Commun. 2020, 529, 1186–1194. [Google Scholar] [CrossRef]
- Ajay, A.K. Functional Drug Screening using Kidney Cells On-A-Chip: Advances in Disease Modeling and Development of Biomarkers. Kidney360 2022, 3, 194–198. [Google Scholar] [CrossRef]
- Koslowski, S.; Latapy, C.; Auvray, P.; Blondel, M.; Meijer, L. An Overview of In Vivo and In Vitro Models for Autosomal Dominant Polycystic Kidney Disease: A Journey from 3D-Cysts to Mini-Pigs. Int. J. Mol. Sci. 2020, 21, 4537. [Google Scholar] [CrossRef]
- Hwang, S.H.; Lee, S.; Park, J.Y.; Jeon, J.S.; Cho, Y.J.; Kim, S. Potential of Drug Efficacy Evaluation in Lung and Kidney Cancer Models Using Organ-on-a-Chip Technology. Micromachines 2021, 12, 215. [Google Scholar] [CrossRef]
- Linn, A.K.; Maneepitasut, W.; Tubsuwan, A.; Kitiyanant, N.; Phakdeekitcharoen, B.; Borwornpinyo, S.; Hongeng, S.; Phanthong, P. Establishment and Characterization of MUi027-A: A Novel Patient-Derived Cell Line of Polycystic Kidney Disease with PKD1 Mutation. J. Pers. Med. 2022, 12, 766. [Google Scholar] [CrossRef]
- Uchimura, K.; Wu, H.; Yoshimura, Y.; Humphreys, B.D. Human Pluripotent Stem Cell-Derived Kidney Organoids with Improved Collecting Duct Maturation and Injury Modeling. Cell Rep. 2020, 33, 108514. [Google Scholar] [CrossRef]
- Huang, C.Y.; Ho, M.C.; Lee, J.J.; Hwang, D.Y.; Ko, H.W.; Cheng, Y.C.; Hsu, Y.H.; Lu, H.E.; Chen, H.C.; Hsieh, P.C.H. Generation of induced pluripotent stem cells derived from an autosomal dominant polycystic kidney disease patient with a p.Ser1457fs mutation in PKD1. Stem Cell Res. 2017, 24, 139–143. [Google Scholar] [CrossRef]
- Ho, M.C.; Huang, C.Y.; Lee, J.J.; Hsu, S.H.; Cheng, Y.C.; Hsu, Y.H.; Hwang, D.Y.; Lu, H.E.; Chen, H.C.; Hsieh, P.C.H. Generation of an induced pluripotent stem cell line, IBMS-iPSC-014-05, from a female autosomal dominant polycystic kidney disease patient carrying a common mutation of R803X in PKD2. Stem Cell Res. 2017, 25, 38–41. [Google Scholar] [CrossRef]
- Liu, C.L.; Huang, C.Y.; Chen, H.C.; Lu, H.E.; Hsieh, P.C.H.; Lee, J.J. Generation of a gene corrected human isogenic IBMS-iPSC-014-C from polycystic-kidney-disease induced pluripotent stem cell line using CRISPR/Cas9. Stem Cell Res. 2020, 45, 101784. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Braun, F.; Lütgehetmann, M.; Pfefferle, S.; Wong, M.N.; Carsten, A.; Lindenmeyer, M.T.; Nörz, D.; Heinrich, F.; Meißner, K.; Wichmann, D.; et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 2020, 396, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the Nervous System. Cell 2020, 183, 16–27.e1. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Liu, X.; Chiu, M.C.; Zhao, X.; Wang, D.; Wei, Y.; Lee, A.; Zhang, A.J.; Chu, H.; et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat. Med. 2020, 26, 1077–1083. [Google Scholar] [CrossRef]
- Ramani, A.; Müller, L.; Ostermann, P.N.; Gabriel, E.; Abida-Islam, P.; Müller-Schiffmann, A.; Mariappan, A.; Goureau, O.; Gruell, H.; Walker, A.; et al. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020, 39, e106230. [Google Scholar] [CrossRef]
- Sharma, A.; Garcia GJr Wang, Y.; Plummer, J.T.; Morizono, K.; Arumugaswami, V.; Svendsen, C.N. Human iPSC-Derived Cardiomyocytes Are Susceptible to SARS-CoV-2 Infection. Cell Rep. Med. 2020, 1, 100052. [Google Scholar] [CrossRef]
- Yang, L.; Han, Y.; Nilsson-Payant, B.E.; Gupta, V.; Wang, P.; Duan, X.; Tang, X.; Zhu, J.; Zhao, Z.; Jaffré, F.; et al. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell 2020, 27, 125–136.e7. [Google Scholar] [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913.e7. [Google Scholar] [CrossRef]
- Jansen, J.; Reimer, K.C.; Nagai, J.S.; Varghese, F.S.; Overheul, G.J.; de Beer, M.; Roverts, R.; Daviran, D.; Fermin, L.A.S.; Willemsen, B.; et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell 2022, 29, 217–231.e8. [Google Scholar] [CrossRef]
- Czerniecki, S.M.; Cruz, N.M.; Harder, J.L.; Menon, R.; Annis, J.; Otto, E.A.; Gulieva, R.E.; Islas, L.V.; Kim, Y.K.; Tran, L.M.; et al. High-Throughput Screening Enhances Kidney Organoid Differentiation from Human Pluripotent Stem Cells and Enables Automated Multidimensional Phenotyping. Cell Stem Cell 2018, 22, 929–940.e4. [Google Scholar] [CrossRef] [Green Version]
- Ebert, A.D.; Liang, P.; Wu, J.C. Induced pluripotent stem cells as a disease modeling and drug screening platform. J. Cardiovasc. Pharmacol. 2012, 60, 408–416. [Google Scholar] [CrossRef]
- Liu, C.; Oikonomopoulos, A.; Sayed, N.; Wu, J.C. Modeling human diseases with induced pluripotent stem cells: From 2D to 3D and beyond. Development 2018, 145, dev156166. [Google Scholar] [CrossRef] [Green Version]
- Little, M.H.; Combes, A.N. Kidney organoids: Accurate models or fortunate accidents. Genes Dev. 2019, 33, 1319–1345. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Coburn, J.M.; Davis-Knowlton, J.; Kimmerling, E.; Kaplan, D.L.; Oxburgh, L. Scaffolding kidney organoids on silk. J. Tissue Eng. Regen. Med. 2019, 13, 812–822. [Google Scholar] [CrossRef]
- Garreta, E.; Prado, P.; Tarantino, C.; Oria, R.; Fanlo, L.; Martí, E.; Zalvidea, D.; Trepat, X.; Roca-Cusachs, P.; Gavaldà-Navarro, A.; et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 2019, 18, 397–405. [Google Scholar] [CrossRef]
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Sances, S.; Workman, M.J.; Svendsen, C.N. Multi-lineage Human iPSC-Derived Platforms for Disease Modeling and Drug Discovery. Cell Stem Cell 2020, 26, 309–329. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov 2021, 20, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.K.; Koenig, P.; Countryman, S.; Thummel, K.E.; Himmelfarb, J.; Kelly, E.J. Tissue Chips in Space-Challenges and Opportunities. Clin. Transl. Sci. 2020, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Tran, F.; Klein, C.; Arlt, A.; Imm, S.; Knappe, E.; Simmons, A.; Rosenstiel, P.; Seibler, P. Stem Cells and Organoid Technology in Precision Medicine in Inflammation: Are We There Yet? Front. Immunol. 2020, 11, 573562. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ebert, A.; Liang, P. Human-induced pluripotent stem cells as models for rare cardiovascular diseases: From evidence-based medicine to precision medicine. Pflug. Arch.-Eur. J. Physiol. 2021, 473, 1151–1165. [Google Scholar] [CrossRef]
- Chun, Y.S.; Byun, K.; Lee, B. Induced pluripotent stem cells and personalized medicine: Current progress and future perspectives. Anat. Cell Biol. 2011, 44, 245–255. [Google Scholar] [CrossRef]
| Name | Allies | Geographic Region | Products | Link |
|---|---|---|---|---|
| California Institute for Regenerative Medicine (CIRM) | Fujifilm Cellular Dynamics International (FCDI) | United States | 40 diseases including 239 neurodevelopmental disorders, 131 liver disease, 442 heart disease, 65 neurodegenerative disease, 175 eyes disease, 191 lung disease, and 302 controls | https://www.cirm.ca.gov/researchers/ipsc-repository/about (accessed on 30 October 2022) https://www.fujifilmcdi.com/cirm-ipsc-products/ (accessed on 30 October 2022) |
| Center for iPS Cell Research and Application (CiRA) | ATCC, RIKEN, RUCDR | Japan | 39 lines including 3 diseases: two neurodevelopmental diseases and a bone disorder | https://www.cira.kyoto-u.ac.jp/e/research/material_1.html (accessed on 30 October 2022) |
| European Bank for induced pluripotent Stem Cells (EBiSC) | HipSci | Europe | 36 diseases, 895 iPSC lines including 359 normal control lines | https://ebisc.org/search (accessed on 6 December 2022) |
| Human Induced pluripotent Stem Cell Initiative (HipSci) | ECACC, EBiSC | United Kingdom | 15 disease statuses, 339 disease lines, and 496 normal lines | https://www.hipsci.org/lines/#/lines (accessed on 30 October 2022) |
| Institute of Physical and Chemical Research (RIKEN) | Japan | 14 disease categories including 231 diseases, 753 patients, and 3110 iPSC lines; 718 health control lines | https://cell.brc.riken.jp/en/hps/patient_specific_ips (accessed on 30 October 2022) | |
| Human Disease iPSC Consortium Resource Center (Taiwan Human Disease iPSC Consortium) | BCRC | Taiwan | 10 normal lines, 74 disease lines of 23 diseases | http://ipsc.ibms.sinica.edu.tw/schedule.html (accessed on 30 October 2022) |
| WiCell Research Institute (WiCell) | N/A | United States | 1377 iPSC lines including 308 disease lines of 40 disease types | https://www.wicell.org/home/stem-cells/catalog-of-stem-cell-lines/advanced-search.cmsx (accessed on 30 October 2022) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-J.; Lin, C.-Y.; Chen, H.-C.; Hsieh, P.C.H.; Chiu, Y.-W.; Chang, J.-M. Opportunities and Challenges of Human IPSC Technology in Kidney Disease Research. Biomedicines 2022, 10, 3232. https://doi.org/10.3390/biomedicines10123232
Lee J-J, Lin C-Y, Chen H-C, Hsieh PCH, Chiu Y-W, Chang J-M. Opportunities and Challenges of Human IPSC Technology in Kidney Disease Research. Biomedicines. 2022; 10(12):3232. https://doi.org/10.3390/biomedicines10123232
Chicago/Turabian StyleLee, Jia-Jung, Chuang-Yu Lin, Hung-Chun Chen, Patrick C. H. Hsieh, Yi-Wen Chiu, and Jer-Ming Chang. 2022. "Opportunities and Challenges of Human IPSC Technology in Kidney Disease Research" Biomedicines 10, no. 12: 3232. https://doi.org/10.3390/biomedicines10123232
APA StyleLee, J. -J., Lin, C. -Y., Chen, H. -C., Hsieh, P. C. H., Chiu, Y. -W., & Chang, J. -M. (2022). Opportunities and Challenges of Human IPSC Technology in Kidney Disease Research. Biomedicines, 10(12), 3232. https://doi.org/10.3390/biomedicines10123232





