Pyrvinium Pamoate: Past, Present, and Future as an Anti-Cancer Drug
Abstract
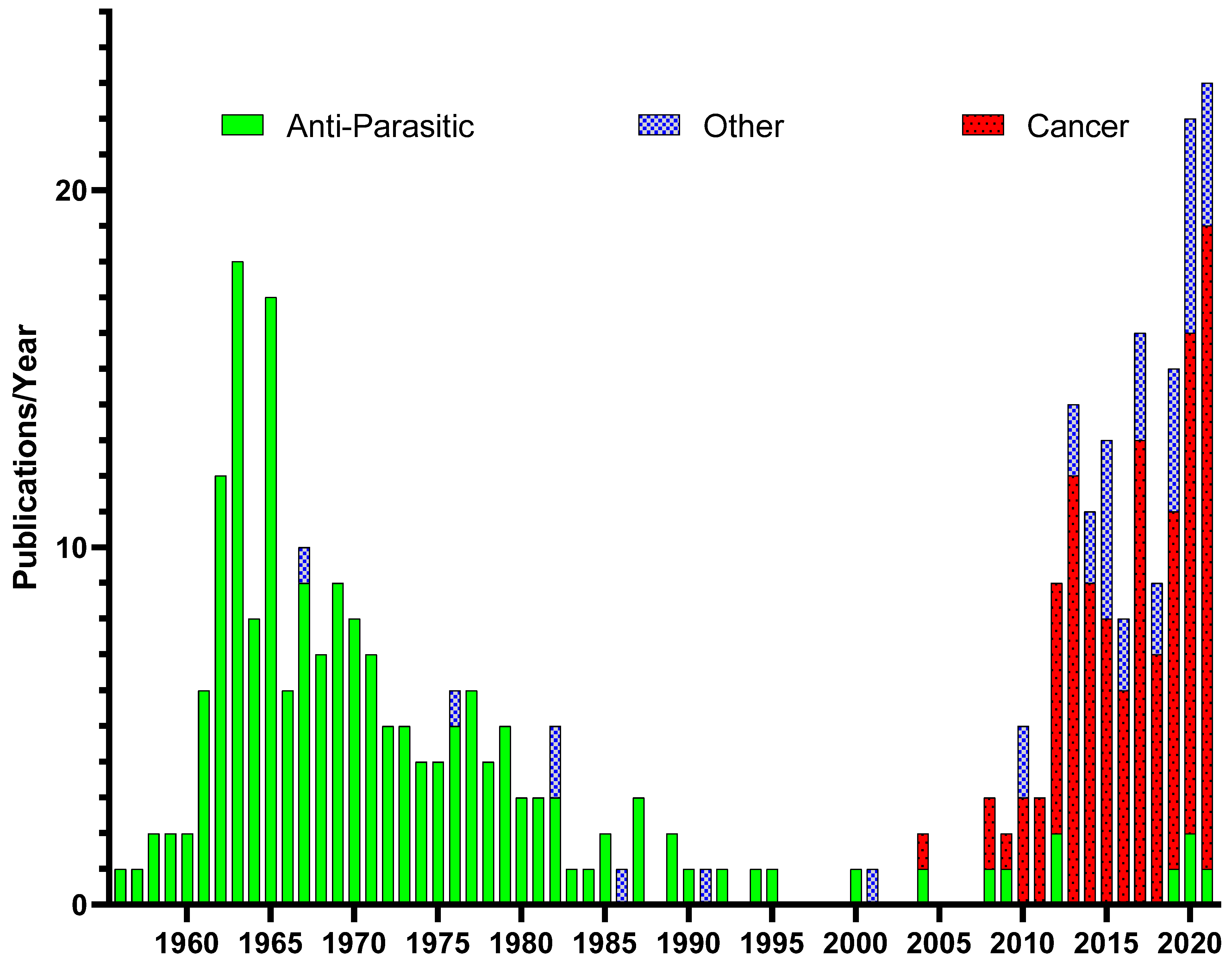
:1. Introduction
Historical Use of Pyrvinium Pamoate
2. PP Mechanisms of Action as an Anti-Cancer Agent
2.1. WNT Signaling
2.2. Mitochondrial Inhibition
2.3. Tumor Stemness
2.4. ELAVL1/HuR Inhibition
2.5. Androgen Receptor Inhibition
2.6. Unfolded Protein Response
2.7. Attenuation of Hedgehog Signaling
2.8. Inhibition of PD-1/PDL-1 Interaction
3. Delivery of Pyrvinium Pamoate
4. Perspectives and Future of PP as a Therapeutic
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sil, D.; Panja, S.; Jogdeo, C.M.; Kumar, R.; Yu, A.; Holbert, C.E.; Ding, L.; Foley, J.R.; Stewart, T.M.; Casero, R.A., Jr.; et al. Self-Assembled Alkylated Polyamine Analogs as Supramolecular Anticancer Agents. Molecules 2022, 27, 2441. [Google Scholar] [CrossRef]
- Thomas, T.; Balabhadrapathruni, S.; Gallo, M.A.; Thomas, T.J. Development of polyamine analogs as cancer therapeutic agents. Oncol. Res. 2002, 13, 123–135. [Google Scholar] [PubMed]
- Smith, M.A.; Maris, J.M.; Lock, R.; Kolb, E.A.; Gorlick, R.; Keir, S.T.; Carol, H.; Morton, C.L.; Reynolds, C.P.; Kang, M.H.; et al. Initial testing (stage 1) of the polyamine analog PG11047 by the pediatric preclinical testing program. Pediatr. Blood Cancer 2011, 57, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.P.; Zhang, H.; Fang, B.; Minna, J.D.; Gomika Udugamasooriya, D. Unbiased peptoid cell screen identifies a peptoid targeting newly appeared cell surface vimentin on tumor transformed early lung cancer cells. Bioorg. Med. Chem. 2022, 58, 116673. [Google Scholar] [CrossRef] [PubMed]
- Raudszus, R.; Nowotny, R.; Gertzen, C.G.W.; Scholer, A.; Krizsan, A.; Gockel, I.; Kalwa, H.; Gohlke, H.; Thieme, R.; Hansen, F.K. Fluorescent analogs of peptoid-based HDAC inhibitors: Synthesis, biological activity and cellular uptake kinetics. Bioorg. Med. Chem. 2019, 27, 115039. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.J.; Udugamasooriya, D.G. A comprehensive lipid binding and activity validation of a cancer-specific peptide-peptoid hybrid PPS1. Biochem. Biophys. Res. Commun. 2017, 486, 545–550. [Google Scholar] [CrossRef] [Green Version]
- Herlan, C.N.; Sonnefeld, A.; Gloge, T.; Bruckel, J.; Schlee, L.C.; Muhle-Goll, C.; Nieger, M.; Brase, S. Macrocyclic Tetramers-Structural Investigation of Peptide-Peptoid Hybrids. Molecules 2021, 26, 4548. [Google Scholar] [CrossRef]
- Nichugovskiy, A.; Maksimova, V.; Trapeznikova, E.; Eshtukova-Shcheglova, E.; Ivanov, I.; Yakubovskaya, M.; Kirsanov, K.; Cheshkov, D.; Tron, G.C.; Maslov, M. Synthesis of Novel Lipophilic Polyamines via Ugi Reaction and Evaluation of Their Anticancer Activity. Molecules 2022, 27, 6218. [Google Scholar] [CrossRef]
- Aparna, E.P.; Devaky, K.S. Advances in the Solid-Phase Synthesis of Pyrimidine Derivatives. ACS Comb. Sci. 2019, 21, 35–68. [Google Scholar] [CrossRef]
- Nam, H.Y.; Hong, J.A.; Choi, J.; Shin, S.; Cho, S.K.; Seo, J.; Lee, J. Mitochondria-Targeting Peptoids. Bioconjug. Chem. 2018, 29, 1669–1676. [Google Scholar] [CrossRef]
- Welch, A.D.; Peters, L.; Bueding, E.; Valk, A., Jr.; Higashi, A. A New Class of Antifilarial Compounds. Science 1947, 105, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.; Welch, A.D.; Higashi, A. The antifilarial action of cyanine dyes; selection of 1’-ethyl-3,6-dimethyl-2-phenyl-4-pyrimido-2’-cyanine chloride (863) for further study as a potential antifilarial agent. J. Pharmacol. Exp. Ther. 1949, 96, 460–471. [Google Scholar] [PubMed]
- Hales, D.R.; Welch, A.D. A preliminary study of the anthelmintic activity of cyanine dye 715 in dogs. J. Pharmacol. Exp. Ther. 1953, 107, 310–314. [Google Scholar]
- Beck, J.W. Treatment of Pinworm Infections with Reduced Single Dose of Pyrvinium Pamoate. JAMA 1964, 189, 511. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.W.; Saavedra, D.; Antell, G.J.; Tejeiro, B. The treatment of pinworm infections in humans (enterobiasis) with pyrvinium chloride and pyrvinium pamoate. Am. J. Trop. Med. Hyg. 1959, 8, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.C.; Kinkel, A.W.; Gryczko, C.M.; Goulet, J.R. Absorption of pyrvinium pamoate. Clin. Pharmacol. Ther. 1976, 19, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A.; Johnson, P.E., Jr. Pyrvinium pamoate in the treatment of pinworm infection (enterobiasis) in the home. J. Pediatr. 1962, 60, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, B.D.; Garcia, E.G. Studies on enterobiasis in the Philippines. VII. Experience with pyrivinium pamoate (Vanquin) in the treatment of enterobiasis. Philipp J Surg 1961, 16, 145–148. [Google Scholar]
- Thompson, P.E.; Worley, D.E.; Meisenhelder, J.E. Anthelmintic studies on pyrvinium pamoate (Povan) and other drugs in rodents, dogs, and monkeys. Am. J. Trop. Med. Hyg. 1962, 11, 89–95. [Google Scholar] [CrossRef]
- Wagner, E.D. Pyrvinium pamoate in the treatment of strongyloidiasis. Am. J. Trop. Med. Hyg. 1963, 12, 60–61. [Google Scholar] [CrossRef]
- Wang, C.C.; Galli, G.A. Strongyloidiasis Treated with Pyrvinium Pamoate. JAMA 1965, 193, 847–848. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S. Single-dose treatment of oxyuriasis with pyrvinium embonate. BMJ 1962, 2, 1583–1585. [Google Scholar] [CrossRef] [PubMed]
- Garin, J.P. Treatment of oxyuriasis in children under 10 years of age by a single dose of pyrvinium embonate (Povanyl). (Apropos of 25 recent cases). Pediatrie 1962, 17, 551–559. [Google Scholar] [PubMed]
- Baranski, M.C.; Carneiro Filho, M.; Gusso, J.F.; Tarran, A.F. Treatment of enterobiasis with pyrantel pamoate. Comparative study with pyrvinium pamoate. Rev. Inst. Med. Trop. Sao Paulo 1971, 13, 422–427. [Google Scholar]
- Nassif, S.; Bell, W.J.; Prescott, J.E. Comparison of pyrantel pamoate syrup and pyrvinium pamoate syrup in the treatment of enterobiasis in Egypt. J. Trop. Med. Hyg. 1974, 77, 270–271. [Google Scholar]
- Alekseeva, M.I.; Lysenko, A. Comparative effectiveness and tolerance in the vanquin, combantrin and vermox treatments of enterobiasis. Med. Parazitol. 1980, 49, 34–38. [Google Scholar]
- Shen, L.; Niu, J.; Wang, C.; Huang, B.; Wang, W.; Zhu, N.; Deng, Y.; Wang, H.; Ye, F.; Cen, S.; et al. High-Throughput Screening and Identification of Potent Broad-Spectrum Inhibitors of Coronaviruses. J. Virol. 2019, 93, e00023-19. [Google Scholar] [CrossRef] [Green Version]
- Glanz, A.; Chawla, K.; Fabry, S.; Subramanian, G.; Garcia, J.; Jay, B.; Ciricillo, J.; Chakravarti, R.; Taylor, R.T.; Chattopadhyay, S. High Throughput Screening of FDA-Approved Drug Library Reveals the Compounds that Promote IRF3-Mediated Pro-Apoptotic Pathway Inhibit Virus Replication. Viruses 2020, 12, 442. [Google Scholar] [CrossRef] [Green Version]
- Niu, H.; Yee, R.; Cui, P.; Tian, L.; Zhang, S.; Shi, W.; Sullivan, D.; Zhu, B.; Zhang, W.; Zhang, Y. Identification of Agents Active against Methicillin-Resistant Staphylococcus aureus USA300 from a Clinical Compound Library. Pathogens 2017, 6, 44. [Google Scholar] [CrossRef] [Green Version]
- Gaikwad, V.R.; Karale, U.B.; Govindarajalu, G.; Adhikari, N.; Krishna, E.V.; Krishna, V.S.; Misra, S.; Sriram, D.; Sijwali, P.S.; Rode, H.B. Synthesis and efficacy of pyrvinium-inspired analogs against tuberculosis and malaria pathogens. Bioorg. Med. Chem. Lett. 2020, 30, 127037. [Google Scholar] [CrossRef]
- Guan, Q.; Zhan, L.; Liu, Z.H.; Pan, Q.; Chen, X.L.; Xiao, Z.; Qin, C.; Zhang, X.L. Identification of pyrvinium pamoate as an anti-tuberculosis agent in vitro and in vivo by SOSA approach amongst known drugs. Emerg. Microbes Infect. 2020, 9, 302–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teguh, S.C.; Klonis, N.; Duffy, S.; Lucantoni, L.; Avery, V.M.; Hutton, C.A.; Baell, J.B.; Tilley, L. Novel conjugated quinoline-indoles compromise Plasmodium falciparum mitochondrial function and show promising antimalarial activity. J. Med. Chem. 2013, 56, 6200–6215. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Sun, Y.; He, C.; Zeng, T.; Li, M. Synergy between Pyrvinium Pamoate and Azoles against Exophiala dermatitidis. Antimicrob. Agents Chemother. 2018, 62, e02361-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Gao, L.; Yuan, M.; Yuan, L.; Yang, J.; Zeng, T. In vitro and in vivo Study of Antifungal Effect of Pyrvinium Pamoate Alone and in Combination With Azoles Against Exophiala dermatitidis. Front. Cell Infect. Microbiol. 2020, 10, 576975. [Google Scholar] [CrossRef] [PubMed]
- Simm, C.; Weerasinghe, H.; Thomas, D.R.; Harrison, P.F.; Newton, H.J.; Beilharz, T.H.; Traven, A. Disruption of Iron Homeostasis and Mitochondrial Metabolism Are Promising Targets to Inhibit Candida auris. Microbiol. Spectr. 2022, 10, e0010022. [Google Scholar] [CrossRef]
- Talaam, K.K.; Inaoka, D.K.; Hatta, T.; Tsubokawa, D.; Tsuji, N.; Wada, M.; Saimoto, H.; Kita, K.; Hamano, S. Mitochondria as a Potential Target for the Development of Prophylactic and Therapeutic Drugs against Schistosoma mansoni Infection. Antimicrob. Agents Chemother. 2021, 65, e0041821. [Google Scholar] [CrossRef]
- Srivastava, S.; Yadav, S.; Singh, G.; Bajwa, S.S. Wnt/beta-catenin antagonist pyrvinium rescues high dose isoproterenol induced cardiotoxicity in rats: Biochemical and immunohistological evidences. Chem. Biol. Interact. 2022, 358, 109902. [Google Scholar] [CrossRef]
- Faheem, S.A.; El-Sayed, N.M.; Moustafa, Y.M.; Saeed, N.M.; Hazem, R.M. Pyrvinium pamoate ameliorates cyclosporin A-induced hepatotoxicity via the modulation of Wnt/beta-catenin signaling and upregulation of PPAR-gamma. Int. Immunopharmacol. 2022, 104, 108538. [Google Scholar] [CrossRef]
- Liu, C.T.; Hsu, S.C.; Hsieh, H.L.; Chen, C.H.; Chen, C.Y.; Sue, Y.M.; Chen, T.H.; Hsu, Y.H.; Lin, F.Y.; Shih, C.M.; et al. Inhibition of beta-catenin signaling attenuates arteriovenous fistula thickening in mice by suppressing myofibroblasts. Mol. Med. 2022, 28, 7. [Google Scholar] [CrossRef]
- Zhou, S.; Obianom, O.N.; Huang, J.; Guo, D.; Yang, H.; Li, Q.; Shu, Y. Pyrvinium Treatment Confers Hepatic Metabolic Benefits via beta-Catenin Downregulation and AMPK Activation. Pharmaceutics 2021, 13, 330. [Google Scholar] [CrossRef]
- Sen, P.; Gupta, K.; Kumari, A.; Singh, G.; Pandey, S.; Singh, R. Wnt/beta-Catenin Antagonist Pyrvinium Exerts Cardioprotective Effects in Polymicrobial Sepsis Model by Attenuating Calcium Dyshomeostasis and Mitochondrial Dysfunction. Cardiovasc. Toxicol. 2021, 21, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Hwang, J.Y.; Gertner, M.; Pontarelli, F.; Zukin, R.S. Casein kinase 1 suppresses activation of REST in insulted hippocampal neurons and halts ischemia-induced neuronal death. J. Neurosci. 2014, 34, 6030–6039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aur, R.J. Treatment of parasitic infestation in children with malignant neoplasms. J. Pediatr. 1971, 78, 129–131. [Google Scholar] [CrossRef]
- Udaka, M.; Maehara, N.; Tamaki, K.; Fukuhara, H.; Kaneshima, H.; Nakamura, H.; Irabu, Y.; Shimoji, K.; Kitsukawa, K.; Shigeno, Y.; et al. A case of Pneumocystis carinii pneumonia with hyperinfection of Strongyloides stercoralis complicated with smoldering adult T-cell leukemia. Kansenshogaku Zasshi 1990, 64, 630–635. [Google Scholar] [CrossRef]
- Esumi, H.; Lu, J.; Kurashima, Y.; Hanaoka, T. Antitumor activity of pyrvinium pamoate, 6-(dimethylamino)-2-[2-(2,5-dimethyl-1-phenyl-1H-pyrrol-3-yl)ethenyl]-1-methyl-qu inolinium pamoate salt, showing preferential cytotoxicity during glucose starvation. Cancer Sci. 2004, 95, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Thomas Jefferson, U. A Study to Determine if the Drug, Pyrvinium Pamoate, is Safe and Tolerable in Patients With Pancreatic Cancer. 2023.
- Li, B.; Flaveny, C.A.; Giambelli, C.; Fei, D.L.; Han, L.; Hang, B.I.; Bai, F.; Pei, X.H.; Nose, V.; Burlingame, O.; et al. Repurposing the FDA-approved pinworm drug pyrvinium as a novel chemotherapeutic agent for intestinal polyposis. PLoS ONE 2014, 9, e101969. [Google Scholar] [CrossRef] [Green Version]
- Thorne, C.A.; Hanson, A.J.; Schneider, J.; Tahinci, E.; Orton, D.; Cselenyi, C.S.; Jernigan, K.K.; Meyers, K.C.; Hang, B.I.; Waterson, A.G.; et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat. Chem. Biol. 2010, 6, 829–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mologni, L.; Brussolo, S.; Ceccon, M.; Gambacorti-Passerini, C. Synergistic effects of combined Wnt/KRAS inhibition in colorectal cancer cells. PLoS ONE 2012, 7, e51449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegering, A.; Uthe, F.W.; Huttenrauch, M.; Muhling, B.; Linnebacher, M.; Krummenast, F.; Germer, C.T.; Thalheimer, A.; Otto, C. The impact of pyrvinium pamoate on colon cancer cell viability. Int. J. Colorectal. Dis. 2014, 29, 1189–1198. [Google Scholar] [CrossRef]
- Song, P.; Feng, L.; Li, J.; Dai, D.; Zhu, L.; Wang, C.; Li, J.; Li, L.; Zhou, Q.; Shi, R.; et al. β-catenin represses miR455-3p to stimulate m6A modification of HSF1 mRNA and promote its translation in colorectal cancer. Mol. Cancer 2020, 19, 129. [Google Scholar] [CrossRef]
- Faux, M.C.; King, L.E.; Kane, S.R.; Love, C.; Sieber, O.M.; Burgess, A.W. APC regulation of ESRP1 and p120-catenin isoforms in colorectal cancer cells. Mol. Biol. Cell 2021, 32, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Barham, W.; Frump, A.L.; Sherrill, T.P.; Garcia, C.B.; Saito-Diaz, K.; VanSaun, M.N.; Fingleton, B.; Gleaves, L.; Orton, D.; Capecchi, M.R.; et al. Targeting the Wnt pathway in synovial sarcoma models. Cancer Discov. 2013, 3, 1286–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Lacerda, L.; Debeb, B.G.; Atkinson, R.L.; Solley, T.N.; Li, L.; Orton, D.; McMurray, J.S.; Hang, B.I.; Lee, E.; et al. The antihelmintic drug pyrvinium pamoate targets aggressive breast cancer. PLoS ONE 2013, 8, e71508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lou, Y.; Zheng, X.; Wang, H.; Sun, J.; Dong, Q.; Han, B. Wnt blockers inhibit the proliferation of lung cancer stem cells. Drug Des. Dev. Ther. 2015, 9, 2399–2407. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zheng, X.; Lou, Y.; Wang, H.; Xu, J.; Zhang, Y.; Han, B. β-catenin inhibitors suppress cells proliferation and promote cells apoptosis in PC9 lung cancer stem cells. Int. J. Clin. Exp. Pathol. 2017, 10, 11968–11978. [Google Scholar]
- Venugopal, C.; Hallett, R.; Vora, P.; Manoranjan, B.; Mahendram, S.; Qazi, M.A.; McFarlane, N.; Subapanditha, M.; Nolte, S.M.; Singh, M.; et al. Pyrvinium Targets CD133 in Human Glioblastoma Brain Tumor-Initiating Cells. Clin. Cancer Res. 2015, 21, 5324–5337. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Jin, R.; Xu, H.; Li, Y.; Chen, Y.; Zhao, G. Pyrvinium pamoate regulates MGMT expression through suppressing the Wnt/beta-catenin signaling pathway to enhance the glioblastoma sensitivity to temozolomide. Cell Death Discov. 2021, 7, 288. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Zhang, S.; Wang, W.; Hu, P. Targeting of Wnt/beta-Catenin by Anthelmintic Drug Pyrvinium Enhances Sensitivity of Ovarian Cancer Cells to Chemotherapy. Med. Sci. Monit. 2017, 23, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.M.; McMellen, A.; Watson, Z.L.; Aguilera, J.; Ferguson, R.; Nurmemmedov, E.; Thakar, T.; Moldovan, G.L.; Kim, H.; Cittelly, D.M.; et al. Activation of Wnt signaling promotes olaparib resistant ovarian cancer. Mol. Carcinog. 2019, 58, 1770–1782. [Google Scholar] [CrossRef]
- Stoddart, A.; Wang, J.; Hu, C.; Fernald, A.A.; Davis, E.M.; Cheng, J.X.; Le Beau, M.M. Inhibition of WNT signaling in the bone marrow niche prevents the development of MDS in the Apc(del/+) MDS mouse model. Blood 2017, 129, 2959–2970. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z.; Xu, L.; Mo, G.; Duan, G.; Wang, Y.; Sun, Z.; Chen, H. Targeting the eIF4E/beta-catenin axis sensitizes cervical carcinoma squamous cells to chemotherapy. Am. J. Transl. Res. 2017, 9, 1203–1212. [Google Scholar] [PubMed]
- Polosukhina, D.; Love, H.D.; Moses, H.L.; Lee, E.; Zent, R.; Clark, P.E. Pharmacologic Inhibition of beta-Catenin With Pyrvinium Inhibits Murine and Human Models of Wilms Tumor. Oncol. Res. 2017, 25, 1653–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Liu, Y.; Pan, J. Inhibitory effect of pyrvinium pamoate on uveal melanoma cells involves blocking of Wnt/beta-catenin pathway. Acta Biochim. Biophys. Sin. 2017, 49, 890–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Zhao, J.; Liu, J. Pyrvinium Sensitizes Clear Cell Renal Cell Carcinoma Response to Chemotherapy Via Casein Kinase 1alpha-Dependent Inhibition of Wnt/beta-Catenin. Am. J. Med. Sci. 2018, 355, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhu, Y.; Lu, Y.; Yu, Z.; Zhong, J.; Li, Y.; Pan, J. Anthelmintic pyrvinium pamoate blocks Wnt/beta-catenin and induces apoptosis in multiple myeloma cells. Oncol. Lett. 2018, 15, 5871–5878. [Google Scholar] [CrossRef] [PubMed]
- Wu, C. beta-catenin inhibitors ICG-001 and pyrvinium sensitize bortezomib-resistant multiple myeloma cells to bortezomib. Oncol. Lett. 2022, 24, 205. [Google Scholar] [CrossRef] [PubMed]
- Barbarino, M.; Cesari, D.; Intruglio, R.; Indovina, P.; Namagerdi, A.; Bertolino, F.M.; Bottaro, M.; Rahmani, D.; Bellan, C.; Giordano, A. Possible repurposing of pyrvinium pamoate for the treatment of mesothelioma: A pre-clinical assessment. J. Cell Physiol. 2018, 233, 7391–7401. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Y.; Wang, P.; Ke, S.; Yang, L.; Shen, Y. Casein kinase 1alpha-dependent inhibition of Wnt/beta-catenin selectively targets nasopharyngeal carcinoma and increases chemosensitivity. Anticancer Drugs 2019, 30, e0747. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Woo, Y.M.; Hwang, K.H.; Kim, H.S.; Lee, S.H. Niclosamide and Pyrvinium Are Both Potential Therapeutics for Osteosarcoma, Inhibiting Wnt-Axin2-Snail Cascade. Cancers 2021, 13, 4630. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Zhang, C.; Bliton, R.J.; Caldwell, B.; Caplan, L.; Presentation, K.S.; Park, D.J.; Kong, S.H.; Lee, H.S.; Washington, M.K.; et al. Dysplastic Stem Cell Plasticity Functions as a Driving Force for Neoplastic Transformation of Precancerous Gastric Mucosa. Gastroenterology 2022, 163, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Hu, J.; Lv, Y.; Bai, B.; Shan, L.; Chen, K.; Dai, S.; Zhu, H. Pyrvinium pamoate inhibits cell proliferation through ROS-mediated AKT-dependent signaling pathway in colorectal cancer. Med. Oncol. 2021, 38, 21. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Cheong, J.K.; Ang, S.H.; Teo, B.; Xu, P.; Asari, K.; Sun, W.T.; Than, H.; Bunte, R.M.; Virshup, D.M.; et al. Pyrvinium selectively targets blast phase-chronic myeloid leukemia through inhibition of mitochondrial respiration. Oncotarget 2015, 6, 33769–33780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Jin, Y.; Pan, J. Inhibitory effect of the anthelmintic drug pyrvinium pamoate on T315I BCRABLpositive CML cells. Mol. Med. Rep. 2017, 16, 9217–9223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, Y.; Ishii, I.; Hatake, K.; Kasahara, T. Pyrvinium pamoate inhibits proliferation of myeloma/erythroleukemia cells by suppressing mitochondrial respiratory complex I and STAT3. Cancer Lett. 2012, 319, 83–88. [Google Scholar] [CrossRef]
- Wander, P.; Arentsen-Peters, S.; Pinhanos, S.S.; Koopmans, B.; Dolman, M.E.M.; Ariese, R.; Bos, F.L.; Castro, P.G.; Jones, L.; Schneider, P.; et al. High-throughput drug screening reveals Pyrvinium pamoate as effective candidate against pediatric MLL-rearranged acute myeloid leukemia. Transl. Oncol. 2021, 14, 101048. [Google Scholar] [CrossRef]
- Tomitsuka, E.; Kita, K.; Esumi, H. An anticancer agent, pyrvinium pamoate inhibits the NADH-fumarate reductase system--a unique mitochondrial energy metabolism in tumour microenvironments. J. Biochem. 2012, 152, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.W.; McCarthy, G.A.; Nerwal, T.; Nevler, A.; DuHadaway, J.B.; McCoy, M.D.; Jiang, W.; Brown, S.Z.; Goetz, A.; Jain, A.; et al. The FDA-Approved Anthelmintic Pyrvinium Pamoate Inhibits Pancreatic Cancer Cells in Nutrient-Depleted Conditions by Targeting the Mitochondria. Mol. Cancer Ther. 2021, 20, 2166–2176. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, L.; Zhou, Y.; Rajoria, P.; Wang, C. Pyrvinium selectively induces apoptosis of lymphoma cells through impairing mitochondrial functions and JAK2/STAT5. Biochem. Biophys. Res. Commun. 2016, 469, 716–722. [Google Scholar] [CrossRef]
- Nair, R.R.; Piktel, D.; Hathaway, Q.A.; Rellick, S.L.; Thomas, P.; Saralkar, P.; Martin, K.H.; Geldenhuys, W.J.; Hollander, J.M.; Gibson, L.F. Pyrvinium Pamoate Use in a B cell Acute Lymphoblastic Leukemia Model of the Bone Tumor Microenvironment. Pharm. Res. 2020, 37, 43. [Google Scholar] [CrossRef]
- Aminzadeh-Gohari, S.; Weber, D.D.; Catalano, L.; Feichtinger, R.G.; Kofler, B.; Lang, R. Targeting Mitochondria in Melanoma. Biomolecules 2020, 10, 1395. [Google Scholar] [CrossRef]
- Datta, S.; Sears, T.; Cortopassi, G.; Woolard, K.; Angelastro, J.M. Repurposing FDA approved drugs inhibiting mitochondrial function for targeting glioma-stem like cells. Biomed. Pharmacother. 2021, 133, 111058. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Tseng, C.Y.; Lu, J.W.; Lu, W.H.; Lan, P.Q.; Chen, C.Y.; Ou, D.L.; Lin, L.I. Deciphering the Role of Pyrvinium Pamoate in the Generation of Integrated Stress Response and Modulation of Mitochondrial Function in Myeloid Leukemia Cells through Transcriptome Analysis. Biomedicines 2021, 9, 1869. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lv, J.; Chang, S.; Chen, Z.; Lu, W.; Xu, C.; Liu, M.; Pang, X. Inhibiting cytoplasmic accumulation of HuR synergizes genotoxic agents in urothelial carcinoma of the bladder. Oncotarget 2016, 7, 45249–45262. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ban, F.; Dalal, K.; Leblanc, E.; Frewin, K.; Ma, D.; Adomat, H.; Rennie, P.S.; Cherkasov, A. Discovery of small-molecule inhibitors selectively targeting the DNA-binding domain of the human androgen receptor. J. Med. Chem. 2014, 57, 6458–6467. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.O.; Bolton, E.C.; Huang, Y.; Feau, C.; Guy, R.K.; Yamamoto, K.R.; Hann, B.; Diamond, M.I. Non-competitive androgen receptor inhibition in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 7233–7238. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.K.; Tew, B.Y.; Lim, M.; Stankavich, B.; He, M.; Pufall, M.; Hu, W.; Chen, Y.; Jones, J.O. Mechanistic Investigation of the Androgen Receptor DNA-Binding Domain Inhibitor Pyrvinium. ACS Omega 2019, 4, 2472–2481. [Google Scholar] [CrossRef]
- Lim, M.; Otto-Duessel, M.; He, M.; Su, L.; Nguyen, D.; Chin, E.; Alliston, T.; Jones, J.O. Ligand-independent and tissue-selective androgen receptor inhibition by pyrvinium. ACS Chem. Biol. 2014, 9, 692–702. [Google Scholar] [CrossRef] [Green Version]
- Luu, H.H.; Zhang, R.; Haydon, R.C.; Rayburn, E.; Kang, Q.; Si, W.; Park, J.K.; Wang, H.; Peng, Y.; Jiang, W.; et al. Wnt/beta-catenin signaling pathway as a novel cancer drug target. Curr. Cancer Drug Targets 2004, 4, 653–671. [Google Scholar] [CrossRef]
- Jansson, E.A.; Are, A.; Greicius, G.; Kuo, I.C.; Kelly, D.; Arulampalam, V.; Pettersson, S. The Wnt/beta-catenin signaling pathway targets PPARgamma activity in colon cancer cells. Proc. Natl. Acad. Sci. USA 2005, 102, 1460–1465. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.G.; Luo, Y.; He, D.L.; Li, X.; Zhang, L.L.; Peng, T.; Li, M.C.; Lin, Y.H. Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int. J. Urol. 2007, 14, 1034–1039. [Google Scholar] [CrossRef]
- Yeh, C.T.; Yao, C.J.; Yan, J.L.; Chuang, S.E.; Lee, L.M.; Chen, C.M.; Yeh, C.F.; Li, C.H.; Lai, G.M. Apoptotic Cell Death and Inhibition of Wnt/beta-Catenin Signaling Pathway in Human Colon Cancer Cells by an Active Fraction (HS7) from Taiwanofungus camphoratus. Evid. Based Complement. Altern. Med. 2011, 2011, 750230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, T.D.; Suto, M.J.; Li, Y. The Wnt/beta-catenin signaling pathway: A potential therapeutic target in the treatment of triple negative breast cancer. J. Cell Biochem. 2012, 113, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magid, A.F. Wnt/beta-Catenin Signaling Pathway Inhibitors: A Promising Cancer Therapy. ACS Med. Chem. Lett. 2014, 5, 956–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gedaly, R.; Galuppo, R.; Daily, M.F.; Shah, M.; Maynard, E.; Chen, C.; Zhang, X.; Esser, K.A.; Cohen, D.A.; Evers, B.M.; et al. Targeting the Wnt/beta-catenin signaling pathway in liver cancer stem cells and hepatocellular carcinoma cell lines with FH535. PLoS ONE 2014, 9, e99272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatansever, H.S.; Gumus, B.; Aydogdu, O.; Sivrikoz, O.N.; Turkoz-Uluer, E.; Kivanc, M.; Atesci, Y.Z.; Bugdayci, H. The role of stem/progenitor cells and Wnt/beta-catenin signaling pathway in the patients with prostate cancer. Minerva Urol. Nefrol. 2014, 66, 249–255. [Google Scholar]
- Wang, R.; Sun, Q.; Wang, P.; Liu, M.; Xiong, S.; Luo, J.; Huang, H.; Du, Q.; Geller, D.A.; Cheng, B. Notch and Wnt/beta-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget 2016, 7, 5754–5768. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Meng, X.K.; Wang, W.X.; Zhang, R.M.; Zhang, T.; Ren, J.J. The Wnt/beta-catenin signaling pathway mechanism for pancreatic cancer chemoresistance in a three-dimensional cancer microenvironment. Am. J. Transl. Res. 2016, 8, 4490–4498. [Google Scholar]
- Shen, C.; Li, B.; Astudillo, L.; Deutscher, M.P.; Cobb, M.H.; Capobianco, A.J.; Lee, E.; Robbins, D.J. The CK1alpha Activator Pyrvinium Enhances the Catalytic Efficiency (kcat/Km) of CK1alpha. Biochemistry 2019, 58, 5102–5106. [Google Scholar] [CrossRef]
- Venerando, A.; Girardi, C.; Ruzzene, M.; Pinna, L.A. Pyrvinium pamoate does not activate protein kinase CK1, but promotes Akt/PKB down-regulation and GSK3 activation. Biochem. J. 2013, 452, 131–137. [Google Scholar] [CrossRef]
- Downey, A.S.; Chong, C.R.; Graczyk, T.K.; Sullivan, D.J. Efficacy of pyrvinium pamoate against Cryptosporidium parvum infection in vitro and in a neonatal mouse model. Antimicrob. Agents Chemother. 2008, 52, 3106–3112. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Gao, L.; Zhang, Y.; Yang, J.; Zeng, T. Synergistic Effect of Pyrvinium Pamoate and Azoles Against Aspergillus fumigatus in vitro and in vivo. Front. Microbiol. 2020, 11, 579362. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska, K.; Szewczuk, M.; Kazmierczak-Baranska, J.; Karwowski, B.T. The Similarities between Human Mitochondria and Bacteria in the Context of Structure, Genome, and Base Excision Repair System. Molecules 2020, 25, 2857. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh-Gohari, S.; Weber, D.D.; Vidali, S.; Catalano, L.; Kofler, B.; Feichtinger, R.G. From old to new—Repurposing drugs to target mitochondrial energy metabolism in cancer. Semin. Cell Dev. Biol. 2020, 98, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, F.; Wang, X.; Li, C.; Meng, Q.; Wang, C.; Huang, J.; Chen, S.; Zhu, Z. Antibiotic bedaquiline effectively targets growth, survival and tumor angiogenesis of lung cancer through suppressing energy metabolism. Biochem. Biophys. Res. Commun. 2018, 495, 267–272. [Google Scholar] [CrossRef]
- Tian, F.; Wang, C.; Tang, M.; Li, J.; Cheng, X.; Zhang, S.; Ji, D.; Huang, Y.; Li, H. The antibiotic chloramphenicol may be an effective new agent for inhibiting the growth of multiple myeloma. Oncotarget 2016, 7, 51934–51942. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.; Yan, X.; Song, L.; Yi, H.; Li, P.; Sun, G.; Yu, D.; Li, L.; Zeng, Z.; Guo, Z. Induction of Mitochondrial Dysfunction and Oxidative Damage by Antibiotic Drug Doxycycline Enhances the Responsiveness of Glioblastoma to Chemotherapy. Med. Sci. Monit. 2017, 23, 4117–4125. [Google Scholar] [CrossRef] [Green Version]
- Falabella, M.; Fernandez, R.J.; Johnson, F.B.; Kaufman, B.A. Potential Roles for G-Quadruplexes in Mitochondria. Curr. Med. Chem. 2019, 26, 2918–2932. [Google Scholar] [CrossRef]
- Falabella, M.; Kolesar, J.E.; Wallace, C.; de Jesus, D.; Sun, L.; Taguchi, Y.V.; Wang, C.; Wang, T.; Xiang, I.M.; Alder, J.K.; et al. G-quadruplex dynamics contribute to regulation of mitochondrial gene expression. Sci. Rep. 2019, 9, 5605. [Google Scholar] [CrossRef]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef]
- Haraguchi, N.; Inoue, H.; Tanaka, F.; Mimori, K.; Utsunomiya, T.; Sasaki, A.; Mori, M. Cancer stem cells in human gastrointestinal cancers. Hum. Cell 2006, 19, 24–29. [Google Scholar] [CrossRef]
- Collins, A.T.; Berry, P.A.; Hyde, C.; Stower, M.J.; Maitland, N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005, 65, 10946–10951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, R.; Ozsvari, B.; Lisanti, C.L.; Tanowitz, H.B.; Howell, A.; Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: Treating cancer like an infectious disease. Oncotarget 2015, 6, 4569–4584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dattilo, R.; Mottini, C.; Camera, E.; Lamolinara, A.; Auslander, N.; Doglioni, G.; Muscolini, M.; Tang, W.; Planque, M.; Ercolani, C.; et al. Pyrvinium Pamoate Induces Death of Triple-Negative Breast Cancer Stem-Like Cells and Reduces Metastases through Effects on Lipid Anabolism. Cancer Res. 2020, 80, 4087–4102. [Google Scholar] [CrossRef]
- Cuevas, C.A.; Tapia-Rojas, C.; Cespedes, C.; Inestrosa, N.C.; Vio, C.P. beta-Catenin-Dependent Signaling Pathway Contributes to Renal Fibrosis in Hypertensive Rats. Biomed. Res. Int. 2015, 2015, 726012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuevas, C.A.; Gonzalez, A.A.; Inestrosa, N.C.; Vio, C.P.; Prieto, M.C. Angiotensin II increases fibronectin and collagen I through the beta-catenin-dependent signaling in mouse collecting duct cells. Am. J. Physiol. Renal. Physiol. 2015, 308, F358–F365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, K.I.; Phipps, R.P.; Sime, P.J.; Huxlin, K.R. Antifibrotic Actions of Peroxisome Proliferator-Activated Receptor gamma Ligands in Corneal Fibroblasts Are Mediated by beta-Catenin-Regulated Pathways. Am. J. Pathol. 2017, 187, 1660–1669. [Google Scholar] [CrossRef] [Green Version]
- Bastakoty, D.; Saraswati, S.; Cates, J.; Lee, E.; Nanney, L.B.; Young, P.P. Inhibition of Wnt/beta-catenin pathway promotes regenerative repair of cutaneous and cartilage injury. FASEB J. 2015, 29, 4881–4892. [Google Scholar] [CrossRef] [Green Version]
- Saraswati, S.; Alfaro, M.P.; Thorne, C.A.; Atkinson, J.; Lee, E.; Young, P.P. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS ONE 2010, 5, e15521. [Google Scholar] [CrossRef]
- Saraswati, S.; Deskins, D.L.; Holt, G.E.; Young, P.P. Pyrvinium, a potent small molecule Wnt inhibitor, increases engraftment and inhibits lineage commitment of mesenchymal stem cells (MSCs). Wound Repair Regen. 2012, 20, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Valle, S.; Alcala, S.; Martin-Hijano, L.; Cabezas-Sainz, P.; Navarro, D.; Munoz, E.R.; Yuste, L.; Tiwary, K.; Walter, K.; Ruiz-Canas, L.; et al. Exploiting oxidative phosphorylation to promote the stem and immunoevasive properties of pancreatic cancer stem cells. Nat. Commun. 2020, 11, 5265. [Google Scholar] [CrossRef]
- Li, H.; Feng, Z.; He, M.L. Lipid metabolism alteration contributes to and maintains the properties of cancer stem cells. Theranostics 2020, 10, 7053–7069. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.F.; Jimbo, M.; Wulfkuhle, J.; Gallagher, I.; Deng, J.; Enyenihi, L.; Meisner-Kober, N.; Londin, E.; Rigoutsos, I.; Sawicki, J.A.; et al. The mRNA-binding protein HuR promotes hypoxia-induced chemoresistance through posttranscriptional regulation of the proto-oncogene PIM1 in pancreatic cancer cells. Oncogene 2016, 35, 2529–2541. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, T.; Ohga, N.; Hida, Y.; Maishi, N.; Akiyama, K.; Kakuguchi, W.; Kuroshima, T.; Kondo, M.; Akino, T.; Totsuka, Y.; et al. HuR keeps an angiogenic switch on by stabilising mRNA of VEGF and COX-2 in tumour endothelium. Br. J. Cancer 2011, 104, 819–829. [Google Scholar] [CrossRef]
- Burkhart, R.A.; Pineda, D.M.; Chand, S.N.; Romeo, C.; Londin, E.R.; Karoly, E.D.; Cozzitorto, J.A.; Rigoutsos, I.; Yeo, C.J.; Brody, J.R.; et al. HuR is a post-transcriptional regulator of core metabolic enzymes in pancreatic cancer. RNA Biol. 2013, 10, 1312–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, S.; Burkhart, R.A.; Beeharry, N.; Bhattacharjee, V.; Londin, E.R.; Cozzitorto, J.A.; Romeo, C.; Jimbo, M.; Norris, Z.A.; Yeo, C.J.; et al. HuR posttranscriptionally regulates WEE1: Implications for the DNA damage response in pancreatic cancer cells. Cancer Res. 2014, 74, 1128–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarei, M.; Lal, S.; Parker, S.J.; Nevler, A.; Vaziri-Gohar, A.; Dukleska, K.; Mambelli-Lisboa, N.C.; Moffat, C.; Blanco, F.F.; Chand, S.N.; et al. Posttranscriptional Upregulation of IDH1 by HuR Establishes a Powerful Survival Phenotype in Pancreatic Cancer Cells. Cancer Res. 2017, 77, 4460–4471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.C.; Steitz, J.A. HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc. Natl. Acad. Sci. USA 1998, 95, 15293–15298. [Google Scholar] [CrossRef] [Green Version]
- Gorospe, M. HuR in the mammalian genotoxic response: Post-transcriptional multitasking. Cell Cycle 2003, 2, 412–414. [Google Scholar] [CrossRef]
- Jones, J.O.; Diamond, M.I. A cellular conformation-based screen for androgen receptor inhibitors. ACS Chem. Biol. 2008, 3, 412–418. [Google Scholar] [CrossRef]
- Rutkowski, D.T.; Wu, J.; Back, S.H.; Callaghan, M.U.; Ferris, S.P.; Iqbal, J.; Clark, R.; Miao, H.; Hassler, J.R.; Fornek, J.; et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev. Cell 2008, 15, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Socha, L.; Silva, D.; Lesage, S.; Goodnow, C.; Petrovsky, N. The role of endoplasmic reticulum stress in nonimmune diabetes: NOD.k iHEL, a novel model of beta cell death. Ann. N. Y. Acad. Sci. 2003, 1005, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Ron, D. Endoplasmic reticulum stress and the development of diabetes: A review. Diabetes 2002, 51 (Suppl. S3), S455–S461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral-Miranda, F.; Tamburini, G.; Martinez, G.; Ardiles, A.O.; Medinas, D.B.; Gerakis, Y.; Hung, M.D.; Vidal, R.; Fuentealba, M.; Miedema, T.; et al. Unfolded protein response IRE1/XBP1 signaling is required for healthy mammalian brain aging. EMBO J. 2022, 41, e111952. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Lindholm, D.; Ren, J.; Pratico, D. ER stress and UPR in Alzheimer’s disease: Mechanisms, pathogenesis, treatments. Cell Death Dis. 2022, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, F.; Lanzillotta, C. The disturbance of protein synthesis/degradation homeostasis is a common trait of age-related neurodegenerative disorders. Adv. Protein Chem. Struct. Biol. 2022, 132, 49–87. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.H.; Macdonald, J.; Liu, G.; Lee, A.S.; Ly, M.; Davis, T.; Ke, N.; Zhou, D.; Wong-Staal, F.; Li, Q.X. Pyrvinium targets the unfolded protein response to hypoglycemia and its anti-tumor activity is enhanced by combination therapy. PLoS ONE 2008, 3, e3951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Wang, J.; Levichkin, I.V.; Stasinopoulos, S.; Ryan, M.T.; Hoogenraad, N.J. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002, 21, 4411–4419. [Google Scholar] [CrossRef]
- Jiang, J.; Hui, C.C. Hedgehog signaling in development and cancer. Dev. Cell 2008, 15, 801–812. [Google Scholar] [CrossRef]
- Taipale, J.; Beachy, P.A. The Hedgehog and Wnt signalling pathways in cancer. Nature 2001, 411, 349–354. [Google Scholar] [CrossRef]
- Li, B.; Fei, D.L.; Flaveny, C.A.; Dahmane, N.; Baubet, V.; Wang, Z.; Bai, F.; Pei, X.H.; Rodriguez-Blanco, J.; Hang, B.; et al. Pyrvinium attenuates Hedgehog signaling downstream of smoothened. Cancer Res. 2014, 74, 4811–4821. [Google Scholar] [CrossRef] [Green Version]
- El-Derany, M.O.; El-Demerdash, E. Pyrvinium pamoate attenuates non-alcoholic steatohepatitis: Insight on hedgehog/Gli and Wnt/beta-catenin signaling crosstalk. Biochem. Pharmacol. 2020, 177, 113942. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Wang, X. Antagonism between Hedgehog and Wnt signaling pathways regulates tumorigenicity. Oncol. Lett. 2017, 14, 6327–6333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmaninejad, A.; Valilou, S.F.; Shabgah, A.G.; Aslani, S.; Alimardani, M.; Pasdar, A.; Sahebkar, A. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J. Cell Physiol. 2019, 234, 16824–16837. [Google Scholar] [CrossRef] [PubMed]
- Fattakhova, E.; Hofer, J.; DiFlumeri, J.; Cobb, M.; Dando, T.; Romisher, Z.; Wellington, J.; Oravic, M.; Radnoff, M.; Patil, S.P. Identification of the FDA-Approved Drug Pyrvinium as a Small-Molecule Inhibitor of the PD-1/PD-L1 Interaction. ChemMedChem 2021, 16, 2769–2774. [Google Scholar] [CrossRef]
- Buchanan, R.A.; Barrow, W.B.; Heffelfinger, J.C.; Kinkel, A.W.; Smith, T.C.; Turner, J.L. Pyrvinium pamoate. Clin. Pharmacol. Ther. 1974, 16, 716–719. [Google Scholar] [CrossRef]
- Deng, L.; Lei, Y.; Liu, R.; Li, J.; Yuan, K.; Li, Y.; Chen, Y.; Liu, Y.; Lu, Y.; Edwards, C.K., 3rd; et al. Pyrvinium targets autophagy addiction to promote cancer cell death. Cell Death Dis. 2013, 4, e614. [Google Scholar] [CrossRef] [Green Version]
- Rohner, N.A.; Nguyen, D.; von Recum, H.A. Affinity Effects on the Release of Non-Conventional Antifibrotics from Polymer Depots. Pharmaceutics 2020, 12, 275. [Google Scholar] [CrossRef]
- Hatamipour, M.; Jaafari, M.R.; Zangui, M.; Shakour, N.; Sahebkar, A. Anti-Tumor Efficacy of Pyrvinium Pamoate Nanoliposomes in an Experimental Model of Melanoma. Anticancer Agents Med. Chem. 2021, 21, 2379–2384. [Google Scholar] [CrossRef]
- Dhir, T.; Schultz, C.W.; Jain, A.; Brown, S.Z.; Haber, A.; Goetz, A.; Xi, C.; Su, G.H.; Xu, L.; Posey, J., 3rd; et al. Abemaciclib Is Effective Against Pancreatic Cancer Cells and Synergizes with HuR and YAP1 Inhibition. Mol. Cancer Res. 2019, 17, 2029–2041. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.; Bhatia, D.; Dave, V.; Sutariya, V.; Varghese Gupta, S. Salts of Therapeutic Agents: Chemical, Physicochemical, and Biological Considerations. Molecules 2018, 23, 1719. [Google Scholar] [CrossRef] [PubMed]
- Ferrandon, S.; Adams, A.; Aoun, R.J.; Devecchio, J.; Shao, X.; Liska, D.; Kalady, M. Pyrvinium Inhibits Familial Adenomatous Polyposis Patient-Derived 3D Adenoma Organoids . 2022; 2701 E. Insight Way Chandler, AZ 85286, Volume InSiGHT Biennial Conference Abstract Book. [Google Scholar]
- Sertkaya, A.; Birkenbach, A.; Berlind, A.; Eyraud, J. Examination of Clinical Trial Costs and Barriers for Drug Development; U.S. Department of Health and Human Services: Washington, DC, USA, 2014.

| Mechanism of Action | Cancer Type | In Vitro | In Vivo |
|---|---|---|---|
| WNT | Intestinal adenomas | [47] | |
| WNT | Colorectal | [48,49,50,51,52] | [50] |
| WNT | Synovial sarcoma | [53] | |
| WNT | Breast | [54] | [54] |
| WNT | Lung | [55,56] | |
| WNT | Glioblastoma | [57,58] | [57,58] |
| WNT | Ovarian | [59,60] | [59,60] |
| WNT | MDS | [61] | [61] |
| WNT | Cervical | [62] | [62] |
| WNT | Wilms tumor | [63] | [63] |
| WNT | Uveal melanoma | [64] | |
| WNT | Clear cell RCC | [65] | [65] |
| WNT | Multiple myeloma | [66,67] | |
| WNT | Malignant mesothelioma | [68] | |
| WNT | Nasopharyngeal carcinoma | [69] * | |
| WNT | Myelodysplastic syndrome | [61] * | |
| WNT | Osteosarcoma | [70] | |
| WNT | Gastric adeno | [71] | |
| WNT | Glioblastoma | [58] | |
| AKT/mTOR | Pancreatic | [45] | [45] |
| AKT/mTOR | Colorectal | [45] | [72] |
| Mitochondria | CML | [73,74] | [73] |
| Mitochondria | Multiple myeloma | [66,75] | |
| Mitochondria | AML | [76] | |
| Mitochondria | Pancreatic | [77,78] | [78] |
| Mitochondria | Hepatic | [77] | |
| Mitochondria | Colorectal | [77] | |
| Mitochondria | Lymphoma | [79] | |
| Mitochondria | B cell ALL | [80] | |
| Mitochondria | Melanoma | [81] | |
| Mitochondria | Glioma | [82] | |
| Mitochondria | AML | [83] | |
| HuR (through AMPK) | Pancreatic | [78] | [78] |
| HuR (through AMPK) | Bladder | [84] | [84] |
| Unfolded protein response | AML | [83] | |
| DNA-binding domain of the human androgen receptor | Prostate | [85] | |
| DNA-binding domain of the human androgen receptor | Prostate | [86,87,88] | [86,88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schultz, C.W.; Nevler, A. Pyrvinium Pamoate: Past, Present, and Future as an Anti-Cancer Drug. Biomedicines 2022, 10, 3249. https://doi.org/10.3390/biomedicines10123249
Schultz CW, Nevler A. Pyrvinium Pamoate: Past, Present, and Future as an Anti-Cancer Drug. Biomedicines. 2022; 10(12):3249. https://doi.org/10.3390/biomedicines10123249
Chicago/Turabian StyleSchultz, Christopher W., and Avinoam Nevler. 2022. "Pyrvinium Pamoate: Past, Present, and Future as an Anti-Cancer Drug" Biomedicines 10, no. 12: 3249. https://doi.org/10.3390/biomedicines10123249
APA StyleSchultz, C. W., & Nevler, A. (2022). Pyrvinium Pamoate: Past, Present, and Future as an Anti-Cancer Drug. Biomedicines, 10(12), 3249. https://doi.org/10.3390/biomedicines10123249





