Prognostic and Predictive Biomarkers in Head and Neck Squamous Cell Carcinoma Treated with Radiotherapy—A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
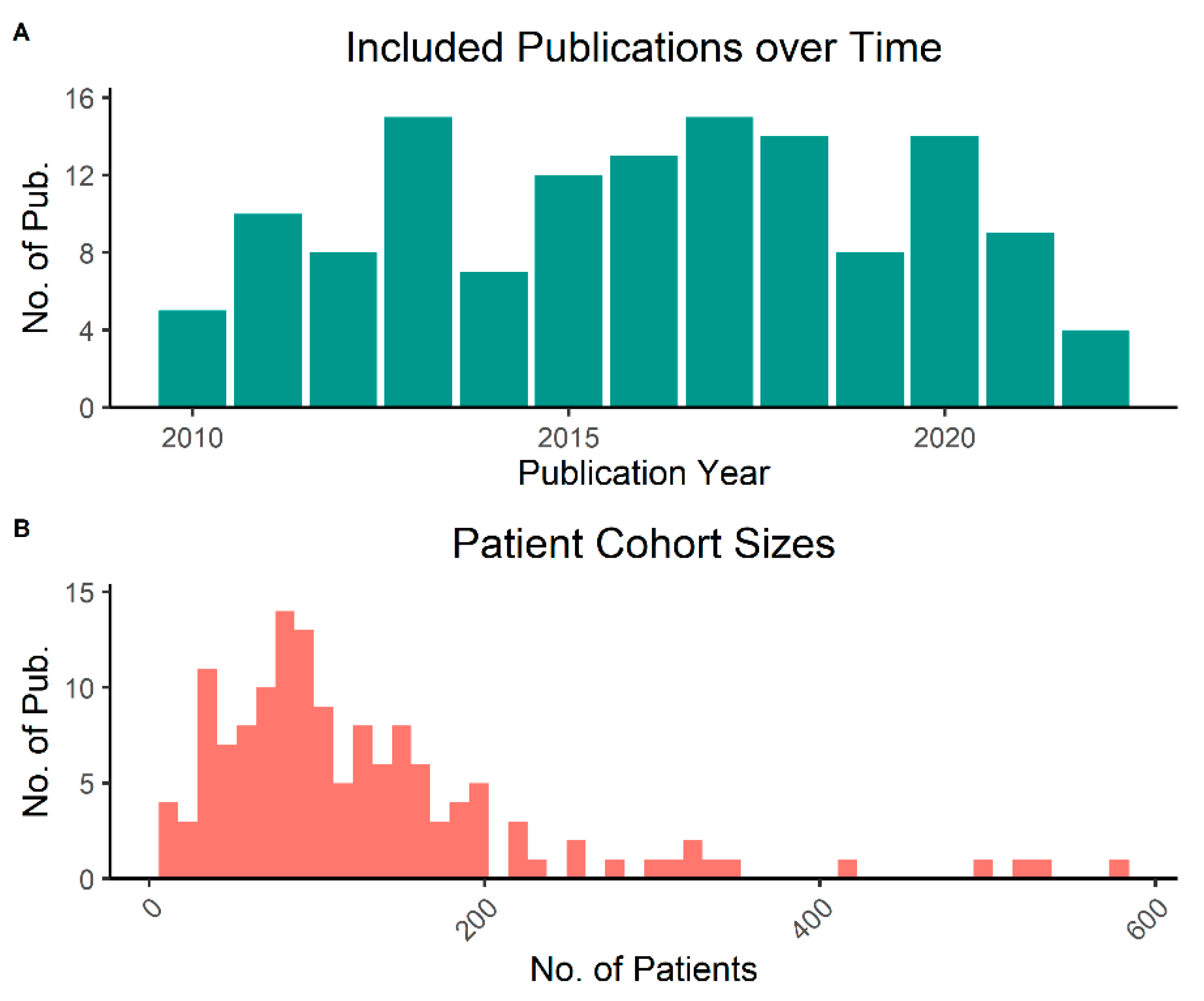
3.1. Literature Search and General Characteristics
3.2. Data Quality and Missingness
3.3. General Description of Included Studies
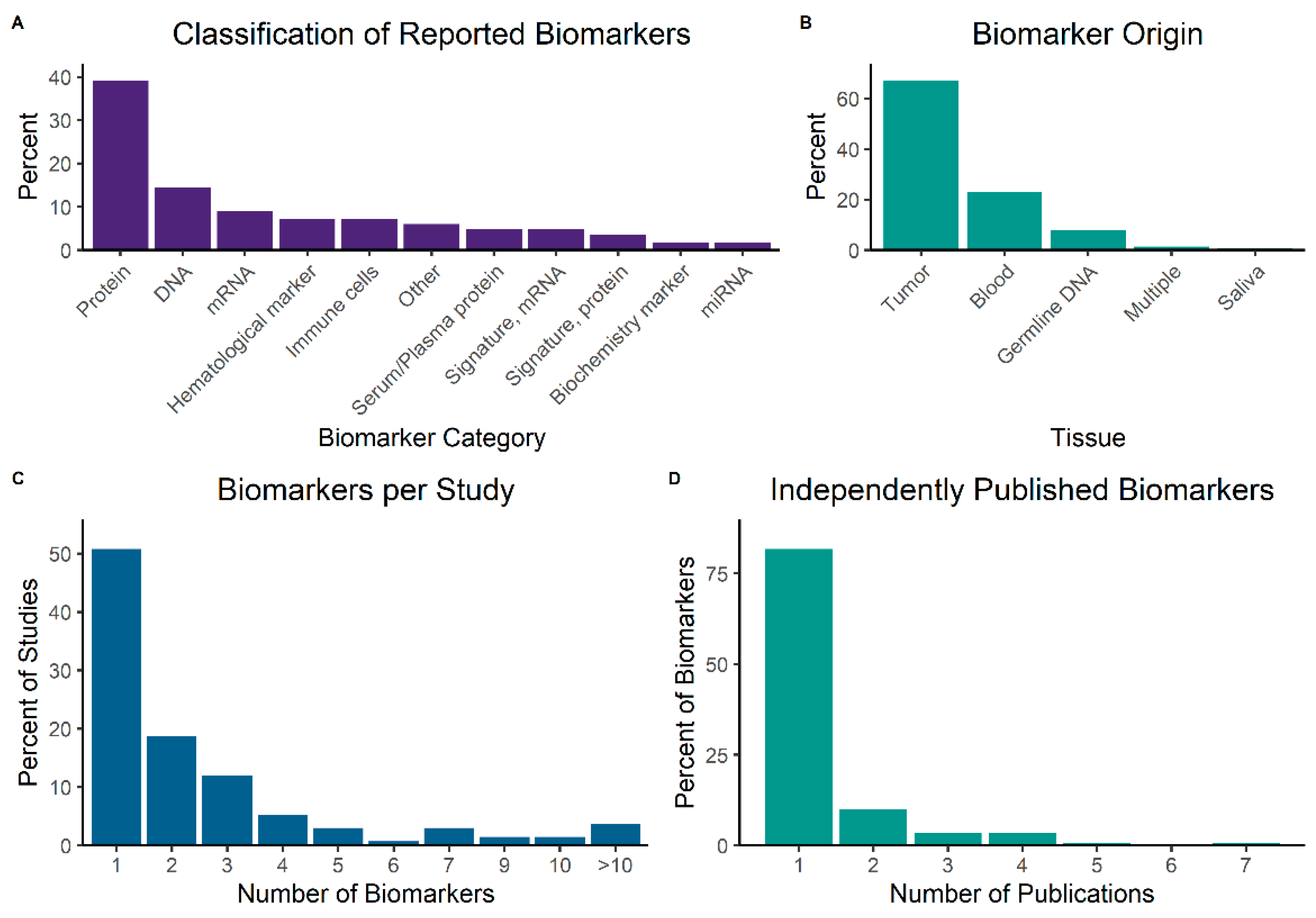
3.4. Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFR | Any failure rate |
| CSS | Cancer-specific survival |
| DFFS | Distant failure-free survival |
| DFS | Disease-free survival |
| DMFS | Distant metastasis-free survival |
| DRR | Distant recurrence rate |
| DSM | Disease-specific mortality |
| DSS | Disease-specific survival |
| EFS | Event-free survival |
| FFDM | Freedom from distant metastasis |
| FFR | Freedom from relapse |
| FFS | Failure-free survival |
| GOF | Goodness of fit |
| GOF | Goodness of fit |
| LC | Local control |
| LF | Local failure |
| LFFS | Local failure-free survival |
| LPFS | Local progression-free survival |
| LR | Local recurrence |
| LRC | Locoregional recurrence |
| LRF | Locoregional failure |
| LRFS | Local relapse-free survival |
| LRR | Locoregional recurrence |
| OS | Overall survival |
| PFS | Progression-free survival |
| RFS | Recurrence-free survival |
| RRFS | Regional recurrence-free survival |
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.; Srivastava, R.M.; Concha-Benavente, F.; Ferrone, S.; Garcia-Bates, T.M.; Li, J.; Ferris, R.L. Anti-EGFR Targeted Monoclonal Antibody Isotype Influences Antitumor Cellular Immunity in Head and Neck Cancer Patients. Clin. Cancer Res. 2016, 22, 5229–5237. [Google Scholar] [CrossRef] [Green Version]
- Kerr, K.M.; Bibeau, F.; Thunnissen, E.; Botling, J.; Ryška, A.; Wolf, J.; Öhrling, K.; Burdon, P.; Malapelle, U.; Büttner, R. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer 2021, 154, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Deacon, D.C.; Smith, E.A.; Judson-Torres, R.L. Molecular Biomarkers for Melanoma Screening, Diagnosis and Prognosis: Current State and Future Prospects. Front. Med. 2021, 8, 642380. [Google Scholar] [CrossRef]
- Budach, V.; Tinhofer, I. Novel prognostic clinical factors and biomarkers for outcome prediction in head and neck cancer: A systematic review. Lancet Oncol. 2019, 20, e313–e326. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.R.; Partridge, M. A mechanistic investigation of the oxygen fixation hypothesis and oxygen enhancement ratio. Biomed. Phys. Eng. Express 2015, 1, 045209. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.S.; Horsman, M.R. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10, 562. [Google Scholar] [CrossRef] [Green Version]
- Overgaard, J.; Thoustrup, K.G. DAHANCA 30: A Randomized Non-inferiority Trial of Hypoxia-Profile Guided Hypoxic Modification of Radiotherapy of HNSCC. Available online: https://clinicaltrials.gov/ct2/show/NCT02661152 (accessed on 27 November 2022).
- de Jong, M.C.; Pramana, J.; van der Wal, J.E.; Lacko, M.; Peutz-Kootstra, C.J.; de Jong, J.M.; Takes, R.P.; Kaanders, J.H.; van der Laan, B.F.; Wachters, J.; et al. CD44 Expression Predicts Local Recurrence after Radiotherapy in Larynx Cancer. Clin. Cancer Res. 2010, 16, 5329–5338. [Google Scholar] [CrossRef] [Green Version]
- Linge, A.; Löck, S.; Gudziol, V.; Nowak, A.; Lohaus, F.; von Neubeck, C.; Jütz, M.; Abdollahi, A.; Debus, J.; Tinhofer, I.; et al. Low Cancer Stem Cell Marker Expression and Low Hypoxia Identify Good Prognosis Subgroups in HPV(−) HNSCC after Postoperative Radiochemotherapy: A Multicenter Study of the DKTK-ROG. Clin. Cancer Res. 2016, 22, 2639–2649. [Google Scholar] [CrossRef]
- Linge, A.; Lohaus, F.; Löck, S.; Nowak, A.; Gudziol, V.; Valentini, C.; von Neubeck, C.; Jütz, M.; Tinhofer, I.; Budach, V.; et al. HPV status, cancer stem cell marker expression, hypoxia gene signatures and tumour volume identify good prognosis subgroups in patients with HNSCC after primary radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother. Oncol. 2016, 121, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, S.H.; Kang, S.-G.; Moon, J.H.; Cho, J.; Suh, C.-O.; Yoon, H.I.; Chang, J.H. ATM mutations improve radio-sensitivity in wild-type isocitrate dehydrogenase-associated high-grade glioma: Retrospective analysis using next-generation sequencing data. Radiat. Oncol 2020, 15, 184. [Google Scholar] [CrossRef] [PubMed]
- Lussier, D.M.; Alspach, E.; Ward, J.P.; Miceli, A.P.; Runci, D.; White, J.M.; Mpoy, C.; Arthur, C.D.; Kohlmiller, H.N.; Jacks, T.; et al. Radiation-induced neoantigens broaden the immunotherapeutic window of cancers with low mutational loads. Proc. Natl. Acad. Sci. USA 2021, 118, e2102611118. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.G.L.; Sterne, J.A.C.; Bailey, M.; Heyderman, R.S.; Birchall, M.A.; Thomas, S.J. Human papillomavirus and head and neck cancer: A systematic review and meta-analysis. Clin. Otolaryngol. 2006, 31, 259–266. [Google Scholar] [CrossRef] [PubMed]
- O’Rorke, M.A.; Ellison, M.V.; Murray, L.J.; Moran, M.; James, J.; Anderson, L.A. Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral. Oncol. 2012, 48, 1191–1201. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Chen, L.; Guo, R.; Zhou, G.; Tang, L.; Mao, Y.; Li, W.; Liu, X.; Du, X.; et al. The Clinical Utility of Plasma Epstein–Barr Virus DNA Assays in Nasopharyngeal Carcinoma. Medicine 2015, 94, e845. [Google Scholar] [CrossRef] [PubMed]
- Campo, F.; Zocchi, J.; Moretto, S.; Mazzola, F.; Petruzzi, G.; Donà, M.G.; Benevolo, M.; Iocca, O.; Virgilio, A.D.; Pichi, B.; et al. Cell-Free Human Papillomavirus-DNA for Monitoring Treatment Response of Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Laryngoscope 2022, 132, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.W.M.; Lee, V.H.F.; Ng, W.-T.; Strojan, P.; Saba, N.F.; Rinaldo, A.; Willems, S.M.; Rodrigo, J.P.; Forastiere, A.A.; Ferlito, A. A systematic review and recommendations on the use of plasma EBV DNA for nasopharyngeal carcinoma. Eur. J. Cancer 2021, 153, 109–122. [Google Scholar] [CrossRef]
- Bartoš, F.; Schimmack, U. Z-Curve.2.0: Estimating Replication Rates and Discovery Rates. Meta-Psychology 2022, 6. [Google Scholar] [CrossRef]
- Brunner, J.; Schimmack, U. Estimating Population Mean Power Under Conditions of Heterogeneity and Selection for Significance. Meta-Psychology 2020, 4. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, O.M.; Vandenbroucke, J.P.; Cevallos, M.; Renehan, A.G.; Altman, D.G.; Egger, M. COSMOS-E: Guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019, 16, e1002742. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Gao, J.; Phillips, S.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; Luca, A.D.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; Feo, G.D.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Marinoni, J.; Roy, R.; Vermeulen, W.; Miniou, P.; Lutz, Y.; Weeda, G.; Seroz, T.; Gomez, D.M.; Hoeijmakers, J.H.J.; Egly, J. Cloning and characterization of p52, the fifth subunit of the core of the transcription/DNA repair factor TFIIH. EMBO J. 1997, 16, 1093–1102. [Google Scholar] [CrossRef]
- Zhang, H.; Ge, J.; Hong, H.; Bi, L.; Sun, Z. Genetic polymorphisms in ERCC1 and ERCC2 genes are associated with response to chemotherapy in osteosarcoma patients among Chinese population: A meta-analysis. World J. Surg. Oncol. 2017, 15, 75. [Google Scholar] [CrossRef] [Green Version]
- Moisan, F.; Laroche-Clary, A.; Auzanneau, C.; Ricard, N.; Pourquier, P.; Robert, J.; Morvan, V.L. Deciphering the role of the ERCC2 gene polymorphism on anticancer drug sensitivity. Carcinogenesis 2012, 33, 962–968. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Damish, A.W.; Frazier, Z.; Liu, D.; Reznichenko, E.; Kamburov, A.; Bell, A.; Zhao, H.; Jordan, E.J.; Gao, S.P.; et al. ERCC2 Helicase Domain Mutations Confer Nucleotide Excision Repair Deficiency and Drive Cisplatin Sensitivity in Muscle-Invasive Bladder Cancer. Clin. Cancer Res. 2019, 25, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Zafeer, M.; Mahjabeen, I.; Kayani, M.A. Increased Expression of ERCC2 Gene in Head and Neck Cancer is Associated with Aggressive Tumors: A Systematic Review and Case-Control Study. Int. J. Biol. Mark. 2015, 31, 17–25. [Google Scholar] [CrossRef]
- Villavicencio, E.H.; Walterhouse, D.O.; Iannaccone, P.M. The Sonic Hedgehog–Patched–Gli Pathway in Human Development and Disease. Am. J. Hum. Genet. 2000, 67, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Ghosh, S.; Maiti, G.P.; Mukherjee, S.; Mukherjee, N.; Chakraborty, J.; Roy, A.; Roychoudhury, S.; Panda, C.K. Association of FANCC and PTCH1 with the Development of Early Dysplastic Lesions of the Head and Neck. Ann. Surg. Oncol. 2012, 19 (Suppl. 3), 528–538. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.F.; Romano, M.V.; Martinez, A.P.; González, A.; Muchnik, C.; Stengel, F.M.; Mazzuoccolo, L.D.; Azurmendi, P.J. Nevoid Basal Cell Carcinoma Syndrome: PTCH1 Mutation Profile and Expression of Genes Involved in the Hedgehog Pathway in Argentinian Patients. Cells 2019, 8, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassompierre, A.; Dalac, S.; Dreno, B.; Neidhardt, E.M.; Maubec, E.; Capelle, C.; Andre, F.; Behal, H.; Dziwniel, V.; Bens, G.; et al. Efficacy of sonic hedgehog inhibitors rechallenge, after initial complete response in recurrent advanced basal cell carcinoma: A retrospective study from the CARADERM database. ESMO Open 2021, 6, 100284. [Google Scholar] [CrossRef]
- Nakajima, E.C.; Drezner, N.; Li, X.; Mishra-Kalyani, P.S.; Liu, Y.; Zhao, H.; Bi, Y.; Liu, J.; Rahman, A.; Wearne, E.; et al. FDA Approval Summary: Sotorasib for KRAS G12C-Mutated Metastatic NSCLCFDA Approval: Sotorasib for KRAS G12C-Mutated NSCLC. Clin. Cancer Res. 2021, 28, 1482–1486. [Google Scholar] [CrossRef]
- Consortium, T.A.P.G.; André, F.; Arnedos, M.; Baras, A.S.; Baselga, J.; Bedard, P.L.; Berger, M.F.; Bierkens, M.; Calvo, F.; Cerami, E.; et al. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef] [Green Version]
- FDA-NIH. Understanding Prognostic versus Predictive Biomarkers. BEST (Biomarkers, EndpointS, and Other Tools) Resource [Internet]. 22 November 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK402284/ (accessed on 27 November 2022).
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef]
- Tsai, C.J.; McBride, S.M.; Riaz, N.; Kang, J.J.; Spielsinger, D.J.; Waldenberg, T.; Gelblum, D.; Yu, Y.; Chen, L.C.; Zakeri, K.; et al. Evaluation of Substantial Reduction in Elective Radiotherapy Dose and Field in Patients With Human Papillomavirus–Associated Oropharyngeal Carcinoma Treated With Definitive Chemoradiotherapy. JAMA Oncol. 2022, 8, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Toustrup, K.; Sørensen, B.S.; Alsner, J.; Overgaard, J. Hypoxia Gene Expression Signatures as Prognostic and Predictive Markers in Head and Neck Radiotherapy. Semin. Radiat. Oncol. 2012, 22, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Berglund, A.; Schell, M.J.; Mihaylov, I.; Fulp, W.J.; Yue, B.; Welsh, E.; Caudell, J.J.; Ahmed, K.; Strom, T.S.; et al. A genome-based model for adjusting radiotherapy dose (GARD): A retrospective, cohort-based study. Lancet Oncol. 2017, 18, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Tawk, B.; Grosser, M.; Lohaus, F.; Gudziol, V.; Kemper, M.; Nowak, A.; Haim, D.; Tinhofer, I.; Budach, V.; et al. Analyses of molecular subtypes and their association to mechanisms of radioresistance in patients with HPV-negative HNSCC treated by postoperative radiochemotherapy. Radiother. Oncol. 2022, 167, 300–307. [Google Scholar] [CrossRef]
- Scott, J.G.; Sedor, G.; Ellsworth, P.; Scarborough, J.A.; Ahmed, K.A.; Oliver, D.E.; Eschrich, S.A.; Kattan, M.W.; Torres-Roca, J.F. Pan-cancer prediction of radiotherapy benefit using genomic-adjusted radiation dose (GARD): A cohort-based pooled analysis. Lancet Oncol. 2021, 22, 1221–1229. [Google Scholar] [CrossRef]
- Baker, M. 1500 scientists lift the lid on reproducibility. Nature 2016, 533, 452–454. [Google Scholar] [CrossRef] [Green Version]
- Open Science Collaboration. Estimating the reproducibility of psychological science. Science 2015, 349, aac4716. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.P.A. Contradicted and Initially Stronger Effects in Highly Cited Clinical Research. JAMA 2005, 294, 218–228. [Google Scholar] [CrossRef] [Green Version]
- Eytan, D.F.; Blackford, A.L.; Eisele, D.W.; Fakhry, C. Prevalence of Comorbidities among Older Head and Neck Cancer Survivors in the United States. Otolaryngol. Head Neck Surg. 2018, 160, 85–92. [Google Scholar] [CrossRef]
- Overgaard, J.; Hansen, H.S.; Specht, L.; Overgaard, M.; Grau, C.; Andersen, E.; Bentzen, J.; Bastholt, L.; Hansen, O.; Johansen, J.; et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6&7 randomised controlled trial. Lancet 2003, 362, 933–940. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK). J. Natl. Cancer Inst. 2005, 97, 1180–1184. [Google Scholar] [CrossRef] [PubMed]


| Biomarker Category | Biomarker Name | PMID | Total Number of Patients | Outcome Category | Outcome Measure | Outcome Value | 95% CI Lower | 95% CI Upper | Outcomep-Value | OncoKB Drug (>=Level 3) | Ongoing Trials as of November 2022 (clinicaltrials.gov) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biochemistry marker | |||||||||||
| Beta-carotene | 20358469 | 29 | PFS | Hazard Ratio (HR) | 0.30 | 0.09 | 0.96 | 0.04 | |||
| Lutein | 20358469 | 29 | PFS | HR | 0.21 | 0.05 | 0.92 | 0.04 | |||
| CTCs | |||||||||||
| Circulating tumor cells (CTCs) | 25057171 | 144 | DFS | HR | 4.30 | 1.70 | 10.90 | 0.002 | NCT05008796, NCT03926468 | ||
| CTCs | 25057171 | 144 | OS | HR | 2.70 | 1.20 | 6.30 | 0.02 | |||
| DNA | |||||||||||
| CTLA4 | 22076708 | 531 | OS | aHR (additive model) | 1.32 | 1.08 | 1.62 | 0.01 | |||
| CTLA4 | 22076708 | 531 | OS | Global Wald test (co-dominant model) | 0.02 | ||||||
| Epigenetic Age Acceleration (EAA; end of RT) | 33882281 | 146 | OS | HR | 1.33 | 1.15 | 1.62 | <0.001 | |||
| EAA (end of RT) | 33882281 | 146 | PFS | HR | 1.32 | 1.16 | 1.54 | <0.001 | |||
| EAA (pre-RT) | 33882281 | 146 | PFS | HR | 1.13 | 1.03 | 1.24 | 0.01 | |||
| EAA (6 mo. post-RT) | 33882281 | 146 | PFS | HR | 1.08 | 1.02 | 1.14 | 0.03 | |||
| EAA (12 mo. post-RT) | 33882281 | 146 | OS | HR | 1.15 | 1.01 | 1.33 | 0.04 | |||
| ERCC1 | 22076708 | 531 | DFS | Global Wald test (co-dominant model) | 0.03 | NCT02128906 | |||||
| ERCC2 | 21890746 | 275 | OS | HR | 1.66 | 1.15 | 2.40 | <0.01 | Cisplatin | ||
| TNF | 29802455 | 62 | OS | HR | 2.14 | 1.12 | 4.08 | 0.021 | |||
| TP53 | 22076708 | 531 | DFS | aHR (additive model) | 1.28 | 1.02 | 1.60 | 0.03 | NCT02734537 | ||
| XRCC1 | 22076708 | 531 | OS | aHR (additive model) | 1.28 | 1.05 | 1.57 | 0.02 | |||
| XRCC1 | 22076708 | 531 | OS | Global Wald test (co-dominant model) | 0.03 | ||||||
| DNA/Epigenetic | |||||||||||
| FANCC | 23482805 | 84 | LRR | HR | 6.60 | 2.48 | 17.57 | 0.0002 | |||
| PTCH1 | 23482805 | 84 | LRR | HR | 5.98 | 2.37 | 15.07 | 0.0001 | Sonidegib, Vismodegib | ||
| Immune cells | |||||||||||
| TIL | 33753155 | 39 | LRC | HR | 0.31 | 0.11 | 0.83 | 0.02 | NCT05541016 | ||
| miRNA | |||||||||||
| MIR15A | 32266559 | 34 | LPFS | HR | 0.10 | 0.004 | 0.91 | 0.04 | |||
| mRNA | |||||||||||
| ATG12 | 34904929 | 103 | LRC | Log-rank | - | 0.03 | |||||
| ATG12 | 34904929 | 103 | LC | Log-rank | - | 0.04 | |||||
| SLC3A2 | 30993218 | 92 | OS | Concordance index | 0.65 | 0.57 | 0.73 | 0.01 | |||
| Protein | |||||||||||
| CXCR4 | 26374452 | 233 | DMFS | HR | 1.01 | 1.00 | 1.01 | 0.04 | NCT03784066 | ||
| DNMT1 | 21284050 | 95 | DFS | HR | 2.55 | 1.32 | 4.90 | 0.01 | |||
| EGFR | 19733016 | 148 | LRC | HR | 1.35 | 1.00 | 1.82 | 0.004 | Afatinib, Dacomitinib, Erlotinib, Erlotinib + Ramucirumab, Gefitinib, Osimertinib, Amivantamab, Mobocertinib, Erlotinib, Patritumab Deruxtecan, CLN-081, Poziotinib | NCT04456322 | |
| ERCC1 | 24064970 | 90 | PFS | HR | 3.00 | 1.20 | 7.80 | 0.02 | |||
| GLI2 | 27918595 | 36 | OS | HR | 0.40 | 0.16 | 0.95 | 0.03 | |||
| IL6 | 21284050 | 95 | DFS | HR | 2.00 | 1.06 | 3.73 | 0.03 | NCT03343236 | ||
| NME1 | 19733016 | 148 | LRC | HR | 1.65 | 1.05 | 2.59 | 0.01 | |||
| p-STAT3 | 21284050 | 95 | DFS | HR | 2.16 | 1.26 | 3.72 | 0.01 | |||
| PTEN | 22413021 | 147 | LRC | HR | 2.84 | 1.38 | 5.80 | 0.004 | NCT05172245 | ||
| SERPINE1 | 26359694 | 190 | PFS | HR | 1.92 | 1.03 | 3.59 | 0.04 | |||
| Serum/Plasma protein | |||||||||||
| CYFRA 21-1 | 28604997 | 185 | OS | HR | 2.33 | 1.14 | 4.73 | 0.02 | |||
| CYFRA 21-1 | 28604997 | 185 | DFS | HR | 2.25 | 1.13 | 4.46 | 0.02 | |||
| CXCL8 | 22383739 | 498 | OS | HR | 1.55 | 0.01 | |||||
| VEGFA | 29658000 | 86 | AFR | HR | 0.71 | 0.55 | 0.91 | 0.01 | |||
| Signature, mRNA | |||||||||||
| 15-gene hypoxia signature | 21846821 | 323 | LRC | HR | 1.41 | 1.03 | 1.94 | <0.05 | NCT02661152, NCT02976051, NCT03865277, NCT01212354, NCT02352792, NCT00568490, NCT03513042, NCT04724096, NCT03323463 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schanne, D.H.; Koch, A.; Elicin, O.; Giger, R.; Medová, M.; Zimmer, Y.; Aebersold, D.M. Prognostic and Predictive Biomarkers in Head and Neck Squamous Cell Carcinoma Treated with Radiotherapy—A Systematic Review. Biomedicines 2022, 10, 3288. https://doi.org/10.3390/biomedicines10123288
Schanne DH, Koch A, Elicin O, Giger R, Medová M, Zimmer Y, Aebersold DM. Prognostic and Predictive Biomarkers in Head and Neck Squamous Cell Carcinoma Treated with Radiotherapy—A Systematic Review. Biomedicines. 2022; 10(12):3288. https://doi.org/10.3390/biomedicines10123288
Chicago/Turabian StyleSchanne, Daniel H., Alexander Koch, Olgun Elicin, Roland Giger, Michaela Medová, Yitzhak Zimmer, and Daniel M. Aebersold. 2022. "Prognostic and Predictive Biomarkers in Head and Neck Squamous Cell Carcinoma Treated with Radiotherapy—A Systematic Review" Biomedicines 10, no. 12: 3288. https://doi.org/10.3390/biomedicines10123288
APA StyleSchanne, D. H., Koch, A., Elicin, O., Giger, R., Medová, M., Zimmer, Y., & Aebersold, D. M. (2022). Prognostic and Predictive Biomarkers in Head and Neck Squamous Cell Carcinoma Treated with Radiotherapy—A Systematic Review. Biomedicines, 10(12), 3288. https://doi.org/10.3390/biomedicines10123288





