L-Arginine Depletion Improves Spinal Cord Injury via Immunomodulation and Nitric Oxide Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Human Samples
2.2. Experimental Spinal Cord Injury and Recombinant Arginase-I Treatment
2.3. Assessment of Locomotor Recovery after Spinal Cord Injury
2.4. Plasma Preparation and L-Arginine ELISA
2.5. Immunohistochemistry
2.6. Quantitative Image Analysis
2.7. Splenocyte and Peripheral Blood Mononuclear Cell Isolation
2.8. T-Cell Characterization after Spinal Cord Injury
2.9. T-Cell Proliferation Assay
2.10. Quantitative PCR
2.11. Western Blotting
2.12. Microglial Cell Isolation
2.13. Isolation and Culture of Bone Marrow-Derived Macrophages
2.14. Griess Assay
2.15. MTT Viability Assay
2.16. Experimental Design and Statistical Analysis
3. Results
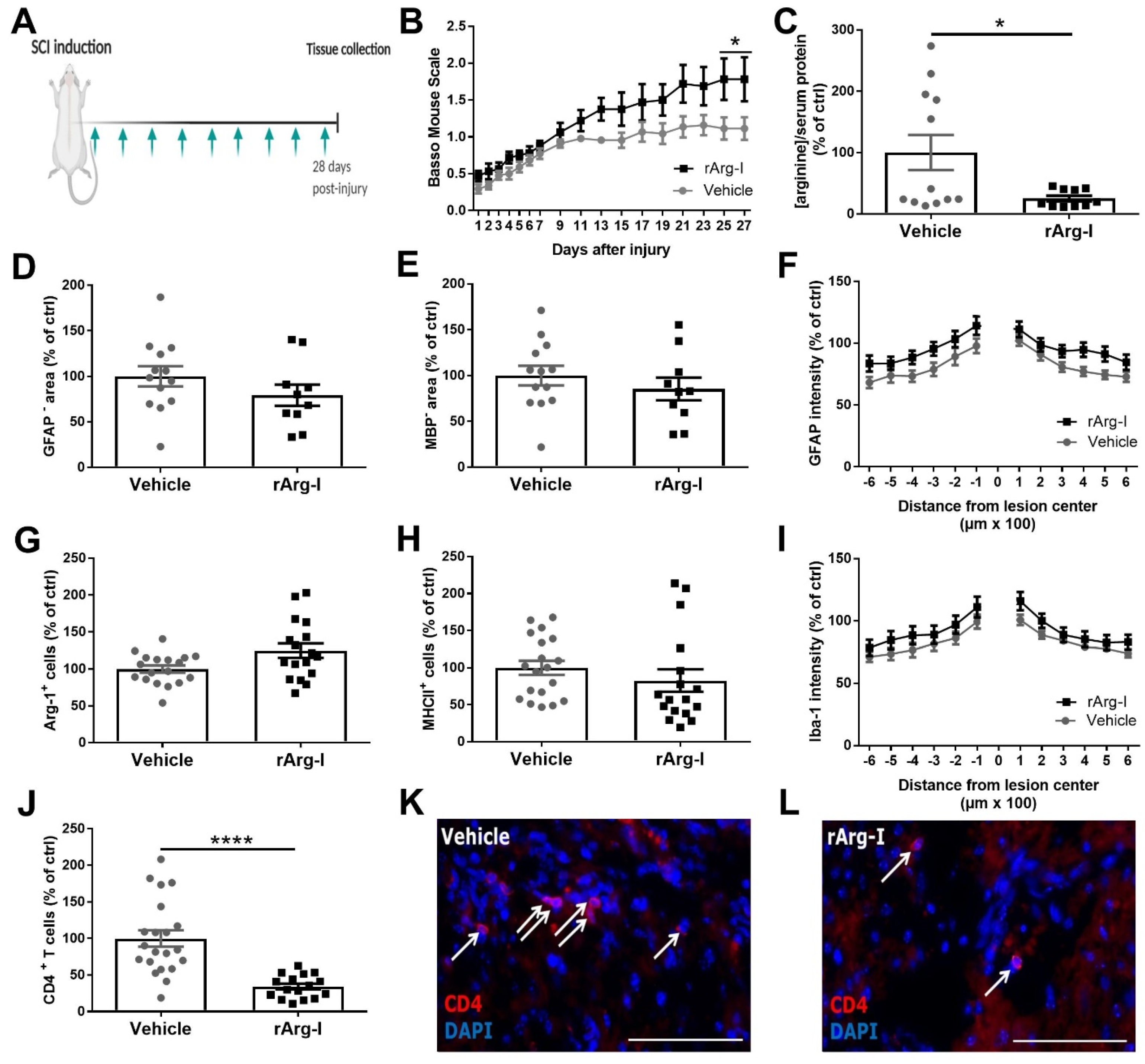
3.1. Systemic L-Arginine Depletion via rArg-I Administration Results in Improved Functional Recovery and Strongly Reduced CD4+ T-Cell Numbers after Spinal Cord Injury
3.2. rArg-I Treatment Alters the Systemic Immune Response after Spinal Cord Injury
3.3. L-Arginine Is Essential for T-Cell Activation
3.4. L-Arginine Depletion Reduces NO Production, Neurotoxicity, and the Number of Phagocyte/Axon Contacts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouanet, C.; Reges, D.; Rocha, E.; Gagliardi, V.; Silva, G.S. Traumatic spinal cord injury: Current concepts and treatment update. Arq. Neuropsiquiatr. 2017, 75, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Beck, K.D.; Nguyen, H.X.; Galvan, M.D.; Salazar, D.L.; Woodruff, T.M.; Anderson, A.J. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 2010, 133 (Pt. 2), 433–447. [Google Scholar] [CrossRef]
- Kigerl, K.A.; McGaughy, V.M.; Popovich, P.G. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J. Comp. Neurol. 2006, 494, 578–594. [Google Scholar] [CrossRef] [Green Version]
- Popovich, P.G.; Wei, P.; Stokes, B.T. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 1997, 377, 443–464. [Google Scholar] [CrossRef]
- Sroga, J.M.; Jones, T.B.; Kigerl, K.A.; McGaughy, V.M.; Popovich, P.G. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J. Comp. Neurol. 2003, 462, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.L.; Parrish, M.E.; Springer, J.E.; Doty, K.; Dossett, L. Acute inflammatory response in spinal cord following impact injury. Exp. Neurol. 1998, 151, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B. Lymphocytes and autoimmunity after spinal cord injury. Exp. Neurol. 2014, 258, 78–90. [Google Scholar] [CrossRef]
- Hendrix, S.; Nitsch, R. The role of T helper cells in neuroprotection and regeneration. J. Neuroimmunol. 2007, 184, 100–112. [Google Scholar] [CrossRef]
- Hauben, E.; Nevo, U.; Yoles, E.; Moalem-Taylor, G.; Agranov, E.; Mor, F.; Akselrod, S.; Neeman, M.; Cohen, I.; Schwartz, M. Autoimmune T cells as potential neuroprotective therapy for spinal cord injury. Lancet 2000, 355, 286–287. [Google Scholar] [CrossRef]
- Schwartz, M.; Hauben, E. Chapter 29 T cell-based therapeutic vaccination for spinal cord injury. Prog. Brain Res. 2002, 137, 401–406. [Google Scholar] [CrossRef]
- Hu, J.-G.; Shi, L.-L.; Chen, Y.-J.; Xie, X.-M.; Zhang, N.; Zhu, A.-Y.; Jiang, Z.-S.; Feng, Y.-F.; Zhang, C.; Xi, J.; et al. Differential effects of myelin basic protein-activated Th1 and Th2 cells on the local immune microenvironment of injured spinal cord. Exp. Neurol. 2016, 277, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B.; Ankeny, D.P.; Guan, Z.; McGaughy, V.; Fisher, L.C.; Basso, D.M.; Popovich, P.G. Passive or Active Immunization with Myelin Basic Protein Impairs Neurological Function and Exacerbates Neuropathology after Spinal Cord Injury in Rats. J. Neurosci. 2004, 24, 3752–3761. [Google Scholar] [CrossRef]
- Ishii, H.; Jin, X.; Ueno, M.; Tanabe, S.; Kubo, T.; Serada, S.; Naka, T.; Yamashita, T. Adoptive transfer of Th1-conditioned lymphocytes promotes axonal remodeling and functional recovery after spinal cord injury. Cell Death Dis. 2012, 3, e363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, J.; Wang, D.; Niu, X.; Hu, J.; Zhou, Y.; Li, Z. MicroRNA-155 Deficiency Suppresses Th17 Cell Differentiation and Improves Locomotor Recovery after Spinal Cord Injury. Scand. J. Immunol. 2015, 81, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, R.; Glaser, J.; Liu, M.T.; Lane, T.E.; Keirstead, H.S. Reducing inflammation decreases secondary degeneration and func-tional deficit after spinal cord injury. Exp. Neurol. 2003, 184, 456–463. [Google Scholar] [CrossRef]
- Potas, J.R.; Zheng, Y.; Moussa, C.; Venn, M.; Gorrie, C.A.; Deng, C.; Waite, P.M.E. Augmented locomotor recovery after spinal cord injury in the athymic nude rat. J. Neurotrauma 2006, 23, 660–673. [Google Scholar] [CrossRef]
- Wu, B.; Matic, D.; Djogo, N.; Szpotowicz, E.; Schachner, M.; Jakovcevski, I. Improved regeneration after spinal cord injury in mice lacking functional T- and B-lymphocytes. Exp. Neurol. 2012, 237, 274–285. [Google Scholar] [CrossRef]
- Luchetti, S.; Beck, K.D.; Galvan, M.D.; Silva, R.; Cummings, B.J.; Anderson, A.J. Comparison of Immunopathology and Locomotor Recovery in C57BL/6, BUB/BnJ, and NOD-SCID Mice after Contusion Spinal Cord Injury. J. Neurotrauma 2010, 27, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Imagama, T.; Ogino, K.; Takemoto, K.; Kato, Y.; Kataoka, H.; Suzuki, H.; Ran, Z.; Setiawan, H.; Fujikura, Y.; Taguchi, T. Regulation of nitric oxide generation by up-regulated arginase I in rat spinal cord injury. J. Clin. Biochem. Nutr. 2012, 51, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Nemoto, T.; Sekikawa, T.; Suzuki, T.; Moriya, H.; Nakaya, H. Inhibition of nitric oxide synthesis accelerates the recovery of polysynaptic reflex potentials after transient spinal cord ischemia in cats. Naunyn Schmiedebergs Arch. Pharmacol. 1997, 355, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Pearse, D.D.; Chatzipanteli, K.; Marcillo, A.E.; Bunge, M.B.; Dietrich, W.D. Comparison of iNOS inhibition by antisense and phar-macological inhibitors after spinal cord injury. J. Neuropathol. Exp. Neurol. 2003, 62, 1096–1107. [Google Scholar] [CrossRef] [Green Version]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronte, V.; Serafini, P.; Mazzoni, A.; Segal, D.M.; Zanovello, P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003, 24, 301–305. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Quiceno, D.G.; Ochoa, A.C. l-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007, 109, 1568–1573. [Google Scholar] [CrossRef] [Green Version]
- Sahin, E.; Haubenwallner, S.; Kuttke, M.; Kollmann, I.; Halfmann, A.; Dohnal, A.M.; Chen, L.; Cheng, P.; Hoesel, B.; Einwallner, E.; et al. Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses. J. Immunol. 2014, 193, 1717–1727. [Google Scholar] [CrossRef] [Green Version]
- Farooque, M.; Hillered, L.; Holtz, A.; Olsson, Y. Changes of extracellular levels of amino acids after graded compression trau-ma to the spinal cord: An experimental study in the rat using microdialysis. J. Neurotrauma 1996, 13, 537–548. [Google Scholar] [CrossRef]
- Liu, D.; McAdoo, D.J. Methylprednisolone reduces excitatory amino acid release following experimental spinal cord injury. Brain Res. 1993, 609, 293–297. [Google Scholar] [CrossRef]
- Esquivel-Aguilar, A.; Castañeda-Hernández, G.; Martínez-Cruz, A.; Franco-Bourland, R.E.; Madrazo, I.; Guizar-Sahagun, G. Early Administration of l-Arginine in Experimental Acute Spinal Cord Injury Impairs Long-Term Motor Function Recovery. J. Trauma Acute Care Surg. 2011, 70, 1198–1202. [Google Scholar] [CrossRef]
- Tuncer, M.C.; Hatipoglu, E.S.; Ozturk, H.; Kervancioglu, P.; Buyukbayram, H. The Effects of L-Arginine on Neurological Function, Histopathology, and Expression of Hypoxia-Inducible Factor-1 Alpha following Spinal Cord Ischemia in Rats. Eur. Surg. Res. 2005, 37, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Savas, S.; Savas, C.; Altuntas, I.; Adiloglu, A. The correlation between nitric oxide and vascular endothelial growth factor in spinal cord injury. Spinal Cord. 2008, 46, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Yüceer, N.; Attar, A.; Sargon, M.F.; Egemen, N.; Türker, R.K.; Demirel-Yilmaz, E. The early protective effects of L-arginine and Ng-nitro-L-arginine methyl ester after experimental acute spinal cord injury. A light and electron microscopic study. J. Clin. Neurosci. 2000, 7, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-M.; Lam, T.-L.; Lam, W.-M.; Tsui, S.-M.; Cheng, A.W.-M.; Lo, W.-H.; Leung, Y.-C. Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res. 2007, 67, 309–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, D.; Corstjens, I.; Sanchez, S.; Dooley, D.; Lemmens, S.; Van Broeckhoven, J.; Bogie, J.; Vanmierlo, T.; Vidal, P.M.; Rose-John, S.; et al. ADAM17-deficiency on microglia but not on macrophages promotes phagocytosis and functional recovery after spinal cord injury. Brain Behav. Immun. 2019, 80, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Vangansewinkel, T.; Geurts, N.; Quanten, K.; Nelissen, S.; Lemmens, S.; Geboes, L.; Dooley, D.; Vidal, P.M.; Pejler, G.; Hendrix, S. Mast cells promote scar remodeling and functional recovery after spinal cord injury via mouse mast cell protease. FASEB J. 2016, 30, 2040–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, N.; Man Leung, Y.; Chung, L.O.; Lo, W. Bio-Cancer Treatment International Limited [CN]/[CN], assignee. Pharmaceutical preparation and method of treatment of human malignancies with arginine deprivation. U.S. Patent 20050244398A1, 20 June 2002. [Google Scholar]
- Ikemoto, M.; Tabata, M.; Murachi, T.; Totani, M. Purification and Properties of Human Erythrocyte Arginase. Ann. Clin. Biochem. Int. J. Lab. Med. 1989, 26, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Ensor, C.M.; Holtsberg, F.W.; Bomalaski, J.S.; A Clark, M. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002, 62, 5443–5450. [Google Scholar] [PubMed]
- Morris, S.M., Jr. Arginine Metabolism Revisited. J. Nutr. 2016, 146, 2579s–2586s. [Google Scholar] [CrossRef]
- Basso, D.M.; Fisher, L.C.; Anderson, A.J.; Jakeman, L.B.; McTigue, D.M.; Popovich, P.G. Basso Mouse Scale for locomotion detects dif-ferences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 2006, 23, 635–659. [Google Scholar] [CrossRef]
- Tamashiro, T.T.; Dalgard, C.L.; Byrnes, K.R. Primary Microglia Isolation from Mixed Glial Cell Cultures of Neonatal Rat Brain Tissue. J. Vis. Exp. 2012, 66. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, S.; Lemmens, S.; Baeten, P.; Sommer, D.; Dooley, D.; Hendrix, S.; Gou-Fabregas, M. HDAC3 Inhibition Promotes Alternative Activation of Macrophages but Does Not Affect Functional Recovery after Spinal Cord Injury. Exp. Neurobiol. 2018, 27, 437–452. [Google Scholar] [CrossRef]
- Badurdeen, S.; Mulongo, M.; Berkley, J.A. Arginine depletion increases susceptibility to serious infections in preterm newborns. Pediatr. Res. 2014, 77, 290–297. [Google Scholar] [CrossRef]
- Xu, J.; Kim, G.-M.; Chen, S.; Yan, P.; Ahmed, S.H.; Ku, G.; Beckman, J.S.; Xu, X.M.; Hsu, C.Y. iNOS and Nitrotyrosine Expression After Spinal Cord Injury. J. Neurotrauma 2001, 18, 523–532. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Zea, A.H.; Culotta, K.S.; Zabaleta, J.; Ochoa, J.B.; Ochoa, A.C. Regulation of T cell receptor CD3zeta chain expres-sion by L-arginine. J. Biol. Chem. 2002, 277, 21123–21129. [Google Scholar] [CrossRef] [Green Version]
- Zea, A.H.; Rodriguez, P.C.; Culotta, K.S.; Hernandez, C.P.; DeSalvo, J.; Ochoa, J.B.; Park, H.; Zabaleta, J.; Ochoa, A.C. L-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell Immunol. 2004, 232, 21–31. [Google Scholar] [CrossRef]
- Liu, D.; Ling, X.; Wen, J.; Liu, J. The role of reactive nitrogen species in secondary spinal cord injury: Formation of nitric oxide, peroxynitrite, and nitrated protein. J. Neurochem. 2000, 75, 2144–2154. [Google Scholar] [CrossRef]
- Ishkitiev, N.; Yaegaki, K.; Imai, T.; Tanaka, T.; Nakahara, T.; Ishikawa, H.; Mitev, V.; Haapasalo, M. High-purity Hepatic Lineage Differentiated from Dental Pulp Stem Cells in Serum-free Medium. J. Endod. 2012, 38, 475–480. [Google Scholar] [CrossRef]
- Vincent, V.A.M.; Tilders, F.J.H.; Van Dam, A.-M. Production, regulation and role of nitric oxide in glial cells. Mediat. Inflamm. 1998, 7, 239–255. [Google Scholar] [CrossRef]
- Khandoker, A.R.; Mahbub, H.; Ji-Eun, S. Severity of the autoimmune encephalomyelitis symptoms in mouse model by inhi-bition of LAT-1 transporters. J. Pharm. Investig. 2020, 50, 481–491. [Google Scholar]
- Boato, F.; Hendrix, S.; Huelsenbeck, S.C.; Hofmann, F.; Große, G.; Djalali, S.; Klimaschewski, L.; Auer, M.; Just, I.; Ahnert-Hilger, G.; et al. C3 peptide enhances recovery from spinal cord injury by improved regenerative growth of descending fiber tracts. J. Cell Sci. 2010, 123, 1652–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuszynski, M.H.; Steward, O. Concepts and Methods for the Study of Axonal Regeneration in the CNS. Neuron 2012, 74, 777–791. [Google Scholar] [CrossRef] [Green Version]
- Seifter, E.; Rettura, G.; Barbul, A.; Levenson, S.M. Arginine: An essential amino acid for injured rats. Surgery 1978, 84, 224–230. [Google Scholar] [PubMed]
- Ahmadi, S.A.; Jafari, M.; Darabi, M.R.; Chehrei, A.; Rezaei, M.; Mirsalehi, M. The Effect of l-Arginine on Dural Healing After Experimentally Induced Dural Defect in a Rat Model. World Neurosurg. 2017, 97, 98–103. [Google Scholar] [CrossRef]
- Dusart, I.; Schwab, M.E. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur. J. Neurosci. 1994, 6, 712–724. [Google Scholar] [CrossRef]
- Ghirnikar, R.S.; Lee, Y.L.; Eng, L.F. Chemokine antagonist infusion promotes axonal sparing after spinal cord contusion injury in rat. J. Neurosci. Res. 2001, 64, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B.; Hart, R.P.; Popovich, P.G. Molecular Control of Physiological and Pathological T-Cell Recruitment after Mouse Spinal Cord Injury. J. Neurosci. 2005, 25, 6576–6583. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yu, W.-B.; Tao, L.-Y.; Xu, Q. Myeloid-derived suppressor cells mediate immune suppression in spinal cord injury. J. Neuroimmunol. 2016, 290, 96–102. [Google Scholar] [CrossRef]
- Werner, A.; Amann, E.; Schnitzius, V.; Habermeier, A.; Luckner-Minden, C.; Leuchtner, N.; Rupp, J.; Closs, E.I.; Munder, M. Induced arginine transport via cationic amino acid transporter-1 is necessary for human T-cell proliferation. Eur. J. Immunol. 2015, 46, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Hoeks, C.; Vanheusden, M.; Peeters, L.M.; Stinissen, P.; Broux, B.; Hellings, N. Treg-Resistant Cytotoxic CD4+ T Cells Dictate T Helper Cells in Their Vicinity: TH17 Skewing and Modulation of Proliferation. Int. J. Mol. Sci. 2021, 22, 5660. [Google Scholar] [CrossRef]
- Werner, A.; Koschke, M.; Leuchtner, N.; Luckner-Minden, C.; Habermeier, A.; Rupp, J.; Heinrich, C.; Conradi, R.; Closs, E.I.; Munder, M. Reconstitution of T Cell Prolifera-tion under Arginine Limitation: Activated Human T Cells Take Up Citrulline via L-Type Amino Acid Transporter 1 and Use It to Regenerate Arginine after Induction of Argininosuccinate Synthase Expression. Front Immunol. 2017, 8, 864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geginat, J.; Lanzavecchia, A.; Sallusto, F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 2003, 101, 4260–4266. [Google Scholar] [CrossRef]
- Munder, M.; Choi, B.-S.; Rogers, M.; Kropf, P. L -Arginine deprivation impairs Leishmania major -specific T-cell responses. Eur. J. Immunol. 2009, 39, 2161–2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomster, L.V.; Brennan, F.H.; Lao, H.W.; Harle, D.W.; Harvey, A.R.; Ruitenberg, M.J. Mobilisation of the splenic monocyte reservoir and peripheral CX3CR1 deficiency adversely affects recovery from spinal cord injury. Exp. Neurol. 2013, 247, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Thawer, S.G.; Mawhinney, L.; Chadwick, K.; de Chickera, S.N.; Weaver, L.C.; Brown, A.; Dekaban, G.A. Temporal changes in monocyte and macrophage subsets and microglial macrophages following spinal cord injury in the lys-egfp-ki mouse model. J. Neuroimmunol. 2013, 261, 7–20. [Google Scholar] [CrossRef]
- Estévez, A.G.; Sahawneh, M.A.; Lange, P.S.; Bae, N.; Egea, M.; Ratan, R.R. Arginase 1 Regulation of Nitric Oxide Production Is Key to Survival of Trophic Factor-Deprived Motor Neurons. J. Neurosci. 2006, 26, 8512–8516. [Google Scholar] [CrossRef] [Green Version]
- Horn, K.P.; Busch, S.A.; Hawthorne, A.L.; van Rooijen, N.; Silver, J. Another barrier to regeneration in the CNS: Activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J. Neurosci. 2008, 28, 9330–9341. [Google Scholar] [CrossRef]
- Ravichandran, K.S.; Lorenz, U. Engulfment of apoptotic cells: Signals for a good meal. Nat. Rev. Immunol. 2007, 7, 964–974. [Google Scholar] [CrossRef]
- Love, S. Demyelinating diseases. J. Clin. Pathol. 2006, 59, 1151–1159. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erens, C.; Van Broeckhoven, J.; Hoeks, C.; Schabbauer, G.; Cheng, P.N.; Chen, L.; Hellings, N.; Broux, B.; Lemmens, S.; Hendrix, S. L-Arginine Depletion Improves Spinal Cord Injury via Immunomodulation and Nitric Oxide Reduction. Biomedicines 2022, 10, 205. https://doi.org/10.3390/biomedicines10020205
Erens C, Van Broeckhoven J, Hoeks C, Schabbauer G, Cheng PN, Chen L, Hellings N, Broux B, Lemmens S, Hendrix S. L-Arginine Depletion Improves Spinal Cord Injury via Immunomodulation and Nitric Oxide Reduction. Biomedicines. 2022; 10(2):205. https://doi.org/10.3390/biomedicines10020205
Chicago/Turabian StyleErens, Céline, Jana Van Broeckhoven, Cindy Hoeks, Gernot Schabbauer, Paul N. Cheng, Li Chen, Niels Hellings, Bieke Broux, Stefanie Lemmens, and Sven Hendrix. 2022. "L-Arginine Depletion Improves Spinal Cord Injury via Immunomodulation and Nitric Oxide Reduction" Biomedicines 10, no. 2: 205. https://doi.org/10.3390/biomedicines10020205
APA StyleErens, C., Van Broeckhoven, J., Hoeks, C., Schabbauer, G., Cheng, P. N., Chen, L., Hellings, N., Broux, B., Lemmens, S., & Hendrix, S. (2022). L-Arginine Depletion Improves Spinal Cord Injury via Immunomodulation and Nitric Oxide Reduction. Biomedicines, 10(2), 205. https://doi.org/10.3390/biomedicines10020205





