Folate-Targeted Liposomal Formulations Improve Effects of Methotrexate in Murine Collagen-Induced Arthritis
Abstract
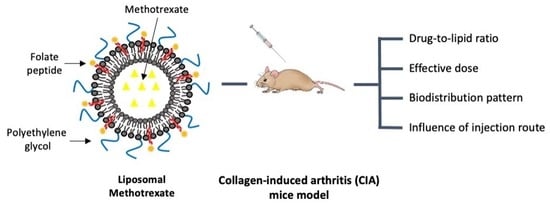
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liposomes Preparation
2.3. Determination of Size Distribution
2.4. In Vivo Studies
2.4.1. Collagen-Induced Arthritis Protocol
2.4.2. Clinical Score Evaluation
2.4.3. Experimental Design
2.4.4. Mouse Collagen-Induced Arthritis Treatment Scheme
2.4.5. Nuclear Medicine Imaging
2.4.6. In Vivo Biodistribution
2.5. Statistical Methods
3. Results
3.1. Study of the Drug-to-Lipid Ratio
3.2. Effective Dose in the Murine CIA Model
3.3. Targeting of Inflamed Paws
3.4. MTX Distribution in Non-Targeted Tissues
3.5. Subcutaneous Injection Route
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shrivastava, A.K.; Pandey, A. Inflammation and rheumatoid arthritis. J. Physiol. Biochem. 2013, 69, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Kitas, G.D.; Gabrie, S.E. Cardiovascular disease in rheumatoid arthritis: State of the art and future perspectives. Ann. Rheum. Dis. 2011, 70, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Koota, K.; Isomäki, H.; Mutru, O. Death rate and causes of death in RA patients during a period of five years. Scand. J. Rheumatol. 1977, 6, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Myllykangas-Luosujvi, R.A.; Aho, K.; Isomäki, H.A. Mortality in rheumatoid arthritis. Semin. Arthritis Rheum. 1995, 25, 193–202. [Google Scholar] [CrossRef]
- Colmegna, I.; Ohata, B.R.; Menard, H.A. Current understanding of rheumatoid arthritis therapy. Clin. Pharmacol. Ther. 2012, 91, 607–620. [Google Scholar] [CrossRef]
- Kremer, J.M.; Alarcón, G.S.; Lightfoot, R.W.; Willkens, R.F.; Furst, D.E.; Williams, H.J.; Dent, P.B.; Weinblatt, M.E. Methotrexate for Rheumatoid Arthritis. Arthritis Rheum. 1994, 37, 316–328. [Google Scholar] [CrossRef]
- Bulatović, M.; Heijstek, M.W.; Verkaaik, M.; Van Dijkhuizen, E.H.P.; Armbrust, W.; Hoppenreijs, E.P.A.; Kamphuis, S.; Kuis, W.; Egberts, T.C.G.; Sinnema, G.; et al. High prevalence of methotrexate intolerance in juvenile idiopathic arthritis: Development and validation of a methotrexate intolerance severity score. Arthritis Rheum. 2011, 63, 2007–2013. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.; Bijlsma, J.; Burmester, G.; Chatzidionysiou, K.; Dougados, M.; Nam, J.; Ramiro, S.; Voshaar, M.; Van Vollenhoven, R.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 2017, 76, 960–977. [Google Scholar] [CrossRef]
- Van Ede, A.E.; Laan, R.F.J.M.; Blom, H.J.; De Abreu, R.A.; Van de Putte, L.B.A. Methotrexate in rheumatoid arthritis: An update with focus on mechanisms involved in toxicity. Semin. Arthritis Rheum. 1998, 27, 277–292. [Google Scholar] [CrossRef]
- Świerkot, J.; Batko, B.; Wiland, P.; Jędrzejewski, M.; Stajszczyk, M. Methotrexate treatment for rheumatoid arthritis in Poland: Retrospective analysis of patients in routine clinical practice. Reumatologia 2018, 56, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Curtis, J.R.; Wallenstein, G.; Takiya, L.; Gruben, D.; Chen, C.; Shan, Y.; Blachley, T.; Dandreo, K.J.; Kremer, J. Patterns of methotrexate use and discontinuation in a U.S. Rheumatoid arthritis registry [abstract]. Arthritis Rheumatol. 2017, 69 (Suppl. 10), 111. [Google Scholar]
- Putrik, P.; Ramiro, S.; Kvien, T.K.; Sokka, T.; Pavlova, M.; Uhlig, T.; Boonen, A.; Tafaj, A.; Harutyunyan, R.; Radner, H.; et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann. Rheum. Dis. 2014, 73, 198–206. [Google Scholar] [CrossRef]
- Merkesdal, S.; Ruof, J.; Mittendorf, T.; Zeidler, H. Cost-effectiveness of TNF-A-blocking agents in the treatment of rheumatoid arthritis. Expert Opin. Pharmacother. 2004, 5, 1881–1886. [Google Scholar] [CrossRef]
- Matteson, E.L.; Lowe, V.J.; Prendergast, F.G.; Crowson, C.S.; Moder, K.G.; Morgenstern, D.E.; Messmann, R.A.; Low, P.S. Assessment of disease activity in rheumatoid arthritis using a novel folate targeted radiopharmaceutical Folatescan TM. Clin. Exp. Rheumatol. 2009, 27, 253–259. [Google Scholar]
- Bilthariya, U.; Jain, N.; Rajoriya, V.; Jain, A.K. Folate-conjugated albumin nanoparticles for rheumatoid arthritis-targeted delivery of etoricoxib. Drug Dev. Ind. Pharm. 2015, 41, 95–104. [Google Scholar] [CrossRef]
- Xia, W.; Hilgenbrink, A.R.; Matteson, E.L.; Lockwood, M.B.; Cheng, J.X.; Low, P.S. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood 2009, 113, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Paulos, C.M.; Turk, M.J.; Breur, G.J.; Low, P.S. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv. Drug Deliv. Rev. 2004, 56, 1205–1217. [Google Scholar] [CrossRef]
- Jesorka, A.; Orwar, O. Liposomes: Technologies and analytical applications. Annu. Rev. Anal. Chem. 2008, 1, 801–832. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar]
- Lian, T.; Ho, R.J.Y. Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef]
- Nogueira, E.; Mangialavori, I.C.; Loureiro, A.; Azoia, N.G.; Sárria, M.P.; Nogueira, P.; Freitas, J.; Härmark, J.; Shimanovich, U.; Rollett, A.; et al. Peptide Anchor for Folate-Targeted Liposomal Delivery. Biomacromolecules 2015, 16, 2904–2910. [Google Scholar] [CrossRef] [Green Version]
- Hegen, M.; Keith, J.C.; Collins, M.; Nickerson-Nutter, C.L. Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 1505–1515. [Google Scholar] [CrossRef]
- Nogueira, E.; Lager, F.; Le Roux, D.; Nogueira, P.; Freitas, J.; Charvet, C.; Renault, G.; Loureiro, A.; Almeida, C.R.; Ohradanova-Repic, A.; et al. Enhancing methotrexate tolerance with folate tagged liposomes in arthritic mice. J. Biomed. Nanotechnol. 2015, 11, 2243–2252. [Google Scholar] [CrossRef] [Green Version]
- Guimaraes, D.; Noro, J.; Loureiro, A.; Lager, F.; Renault, G.; Cavaco-Paulo, A. Increased Encapsulation Efficiency of Methotrexate in Liposomes for Rheumatoid Arthritis Therapy. Biomedicines 2020, 8, 630. [Google Scholar] [CrossRef]
- Rosloniec, E.F.; Cremer, M.; Kang, A.H.; Myers, L.K.; Brand, D.D. Collagen-induced arthritis. Curr. Protoc. Immunol. 2010, 20, 15. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Naziris, N.; Pippa, N.; Demetzos, C. The significance of drug-to-lipid ratio to the development of optimized liposomal formulation. J. Liposome Res. 2018, 28, 249–258. [Google Scholar] [CrossRef]
- Chen, M.; Kambere Amerigos Daddy, J.C.; Su, Z.; El Islem Guissi, N.; Xiao, Y.; Zong, L.; Ping, Q. Folate receptor-targeting and reactive oxygen species-responsive liposomal formulation of methotrexate for treatment of rheumatoid arthritis. Pharmaceutics 2019, 11, 582. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.; Goodfellow, R.; Topley, N.; Amos, N.; Williams, B. The suppression of rat collagen-induced arthritis and inhibition of macrophage derived mediator release by liposomal methotrexate formulations. Inflamm. Res. 2000, 49, 155–161. [Google Scholar] [CrossRef]
- Rauscher, A.; Frindel, M.; Maurel, C.; Maillasson, M.; Le Saëc, P.; Rajerison, H.; Gestin, J.F.; Barbet, J.; Faivre-Chauvet, A.; Mougin-Degraef, M. Influence of pegylation and hapten location at the surface of radiolabelled liposomes on tumour immunotargeting using bispecific antibody. Nucl. Med. Biol. 2014, 41, e66–e74. [Google Scholar] [CrossRef]
- Clavel, G.; Marchiol-Fournigault, C.; Renault, G.; Boissier, M.C.; Fradelizi, D.; Bessis, N. Ultrasound and Doppler micro-imaging in a model of rheumatoid arthritis in mice. Ann. Rheum. Dis. 2008, 67, 1765–1772. [Google Scholar] [CrossRef]
- Feng, Z.T.; Yang, T.; Hou, X.Q.; Wu, H.Y.; Feng, J.T.; Ou, B.J.; Cai, S.J.; Li, J.; Mei, Z.G. Sinomenine mitigates collagen-induced arthritis mice by inhibiting angiogenesis. Biomed. Pharmacother. 2019, 113, 108759. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; He, Y.; Liang, J.; Cheng, Z.; Zhang, M.; Zhu, Y.; Hong, C.; Qin, J.; Xu, X.; Wang, J. Role of Liposome Size, Surface Charge, and PEGylation on Rheumatoid Arthritis Targeting Therapy. ACS Appl. Mater. Interfaces 2019, 11, 20304–20315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, X. Recent advances in nanomedicines for the treatment of rheumatoid arthritis. Biomater. Sci. 2017, 5, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Pirmardvand Chegini, S.; Varshosaz, J.; Taymouri, S. Recent approaches for targeted drug delivery in rheumatoid arthritis diagnosis and treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 502–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, E.; Gomes, A.C.; Preto, A.; Cavaco-Paulo, A. Folate-targeted nanoparticles for rheumatoid arthritis therapy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1113–1126. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, I.M.; Gonçalves, C.; Reis, R.L.; Oliveira, J.M. Engineering nanoparticles for targeting rheumatoid arthritis: Past, present, and future trends. Nano Res. 2018, 11, 4489–4506. [Google Scholar] [CrossRef] [Green Version]
- Zuckier, L.S.; Martineau, P. Altered biodistribution of radiopharmaceuticals used in bone scintigraphy. Semin. Nucl. Med. 2015, 45, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Gregoriadis, G. Liposome Technology: Interactions of Liposomes with the Biological Milieu; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780849397288. [Google Scholar]
- Yuan, D.; He, H.; Wu, Y.; Fan, J.; Cao, Y. Physiologically Based Pharmacokinetic Modeling of Nanoparticles. J. Pharm. Sci. 2019, 108, 58–72. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle–Liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; Han, S.; Inocencio, I.; Cao, E.; Hong, J.; Phillips, A.R.J.; Windsor, J.A.; Porter, C.J.H.; Trevaskis, N.L. Lymphatic Uptake of Liposomes after Intraperitoneal Administration Primarily Occurs via the Diaphragmatic Lymphatics and is Dependent on Liposome Surface Properties. Mol. Pharm. 2019, 16, 4987–4999. [Google Scholar] [CrossRef]
- Allen, T.M.; Hansen, C.B.; Guo, L.S.S. Subcutaneous administration of liposomes: A comparison with the intravenous and intraperitoneal routes of injection. BBA Biomembr. 1993, 1150, 9–16. [Google Scholar] [CrossRef]
- Harrington, K.J.; Rowlinson-Busza, G.; Syrigos, K.N.; Uster, P.S.; Vile, R.G.; Stewart, J.S.W. Pegylated liposomes have potential as vehicles for intratumoral and subcutaneous drug delivery. Clin. Cancer Res. 2000, 6, 2528–2537. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, D.; Lager, F.; Renault, G.; Guezguez, J.; Burnet, M.; Cunha, J.; Cavaco-Paulo, A.; Nogueira, E. Folate-Targeted Liposomal Formulations Improve Effects of Methotrexate in Murine Collagen-Induced Arthritis. Biomedicines 2022, 10, 229. https://doi.org/10.3390/biomedicines10020229
Guimarães D, Lager F, Renault G, Guezguez J, Burnet M, Cunha J, Cavaco-Paulo A, Nogueira E. Folate-Targeted Liposomal Formulations Improve Effects of Methotrexate in Murine Collagen-Induced Arthritis. Biomedicines. 2022; 10(2):229. https://doi.org/10.3390/biomedicines10020229
Chicago/Turabian StyleGuimarães, Diana, Franck Lager, Gilles Renault, Jamil Guezguez, Michael Burnet, Joana Cunha, Artur Cavaco-Paulo, and Eugénia Nogueira. 2022. "Folate-Targeted Liposomal Formulations Improve Effects of Methotrexate in Murine Collagen-Induced Arthritis" Biomedicines 10, no. 2: 229. https://doi.org/10.3390/biomedicines10020229
APA StyleGuimarães, D., Lager, F., Renault, G., Guezguez, J., Burnet, M., Cunha, J., Cavaco-Paulo, A., & Nogueira, E. (2022). Folate-Targeted Liposomal Formulations Improve Effects of Methotrexate in Murine Collagen-Induced Arthritis. Biomedicines, 10(2), 229. https://doi.org/10.3390/biomedicines10020229