Pseudoprogression in Glioblastoma: Role of Metabolic and Functional MRI-Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Definition of Pseudoprogression
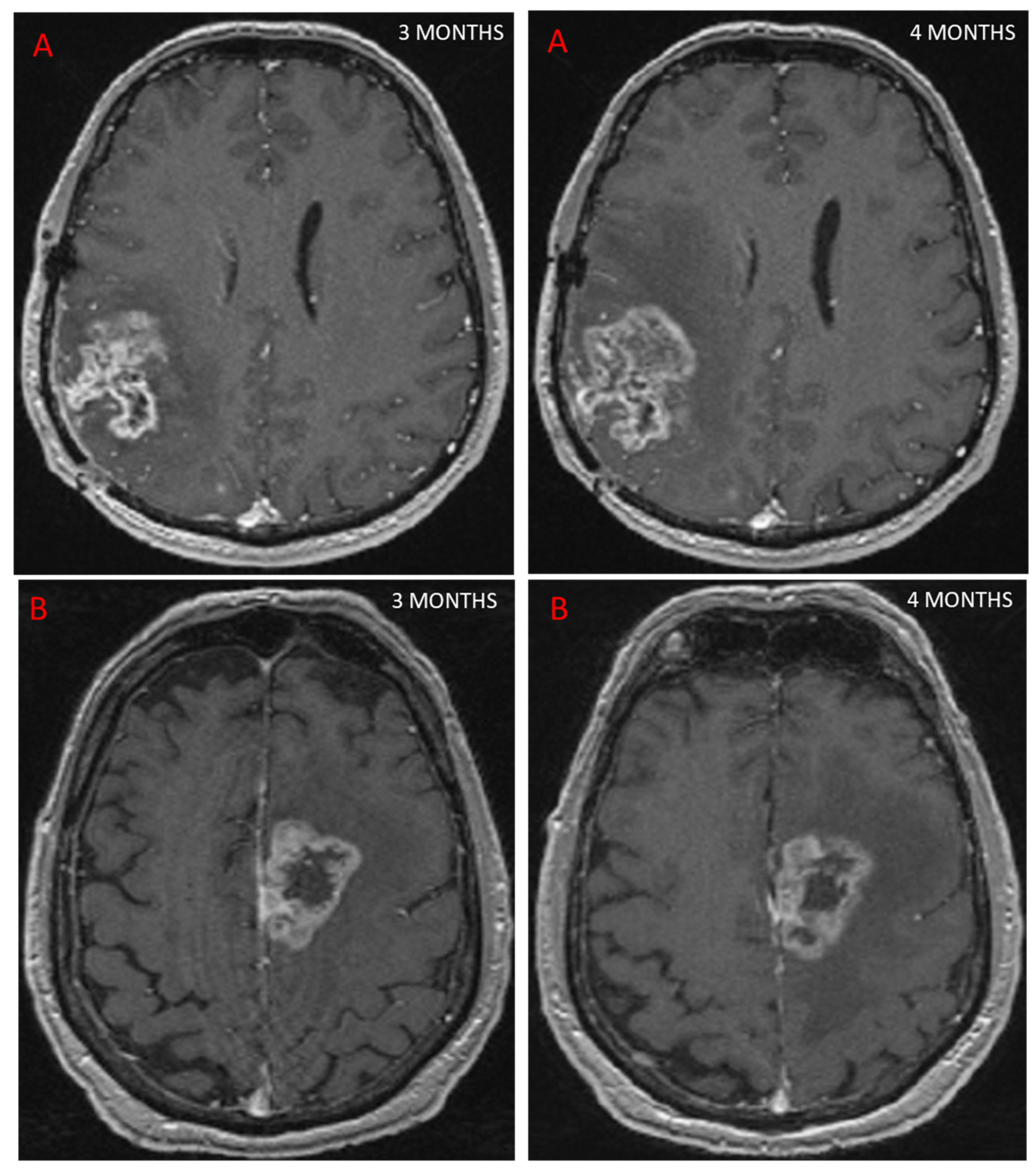
3.2. Advanced MRI and PsP
3.2.1. Diffusion Imaging Including Diffusion Tensor Imaging (DTI) and Diffusion Weighted Imaging (DWI)
3.2.2. Perfusion-Weighted Imaging (PWI)
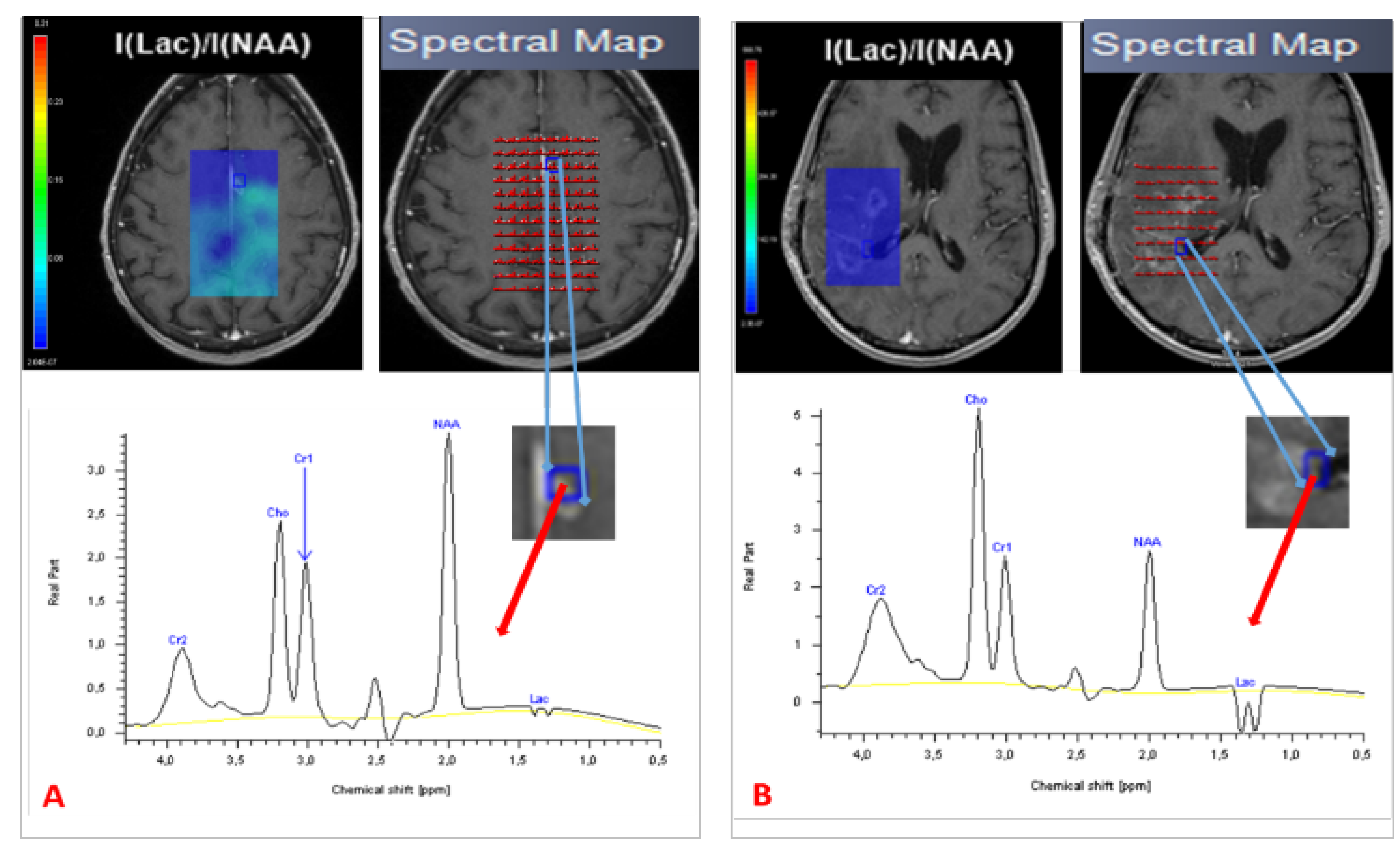
3.2.3. Spectroscopy
3.2.4. Radiomics and Pseudoprogression
3.2.5. Differentiation between PsP and TP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Stetson, L.; Virk, S.; Barnholtz-Sloan, J.S. Epidemiology of Intracranial Gliomas. Intracranial Gliomas Part I—Surg. 2018, 30, 1–11. [Google Scholar] [CrossRef]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; la Fougère, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology Working Group and European Association for Neuro-Oncology Recommendations for the Clinical Use of PET Imaging in Gliomas. Neuro Oncol. 2016, 18, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Baldi, I.; Huchet, A.; Bauchet, L.; Loiseau, H. Epidemiology of glioblastoma. Neurochirurgie 2010, 56, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The Epidemiology of Glioma in Adults: A “State of the Science” Review. Neuro Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, F.; Vicario, N.; Spitale, F.M.; Cammarata, F.P.; Minafra, L.; Salvatorelli, L.; Russo, G.; Cuttone, G.; Valable, S.; Gulino, R.; et al. The Role of Hypoxia and SRC Tyrosine Kinase in Glioblastoma Invasiveness and Radioresistance. Cancers 2020, 12, 2860. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Shang, E.; Westhoff, M.-A.; Karpel-Massler, G.; Siegelin, M.D. Methodological Approaches for Assessing Metabolomic Changes in Glioblastomas. Methods Mol. Biol. 2022, 2445, 305–328. [Google Scholar] [CrossRef]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical Features, Mechanisms, and Management of Pseudoprogression in Malignant Gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Brandes, A.A.; Franceschi, E.; Tosoni, A.; Blatt, V.; Pession, A.; Tallini, G.; Bertorelle, R.; Bartolini, S.; Calbucci, F.; Andreoli, A.; et al. MGMT Promoter Methylation Status Can Predict the Incidence and Outcome of Pseudoprogression after Concomitant Radiochemotherapy in Newly Diagnosed Glioblastoma Patients. J. Clin. Oncol. 2008, 26, 2192–2197. [Google Scholar] [CrossRef] [Green Version]
- Taal, W.; Brandsma, D.; de Bruin, H.G.; Bromberg, J.E.; Swaak-Kragten, A.T.; Smitt, P.A.E.S.; van Es, C.A.; van den Bent, M.J. Incidence of Early Pseudo-Progression in a Cohort of Malignant Glioma Patients Treated with Chemoirradiation with Temozolomide. Cancer 2008, 113, 405–410. [Google Scholar] [CrossRef]
- Abbasi, A.W.; Westerlaan, H.E.; Holtman, G.A.; Aden, K.M.; van Laar, P.J.; van der Hoorn, A. Incidence of Tumour Progression and Pseudoprogression in High-Grade Gliomas: A Systematic Review and Meta-Analysis. Clin. Neuroradiol. 2018, 28, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Chinot, O.L.; Bendszus, M.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Revil, C.; Kerloeguen, Y.; Cloughesy, T. Evaluation of Pseudoprogression Rates and Tumor Progression Patterns in a Phase III Trial of Bevacizumab plus Radiotherapy/Temozolomide for Newly Diagnosed Glioblastoma. Neuro Oncol. 2016, 18, 1434–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Mieghem, E.; Wozniak, A.; Geussens, Y.; Menten, J.; De Vleeschouwer, S.; Van Calenbergh, F.; Sciot, R.; Van Gool, S.; Bechter, O.E.; Demaerel, P.; et al. Defining Pseudoprogression in Glioblastoma Multiforme. Eur. J. Neurol. 2013, 20, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Leao, D.J.; Craig, P.G.; Godoy, L.F.; Leite, C.C.; Policeni, B. Response Assessment in Neuro-Oncology Criteria for Gliomas: Practical Approach Using Conventional and Advanced Techniques. AJNR Am. J. Neuroradiol. 2020, 41, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; Glenn, C.A.; Javan, R.; Olson, J.J. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines Update on the Role of Imaging in the Management of Progressive Glioblastoma in Adults. J. Neuro-Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Thust, S.C.; van den Bent, M.J.; Smits, M. Pseudoprogression of Brain Tumors. J. Magn. Reson. Imaging 2018, 48, 571–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen-Baas, K.M.; Moen, G.; Fluge, Ø.; Storstein, A. Pseudoprogression in High-Grade Glioma. Acta Neurol. Scand. Suppl. 2013, 127, 31–37. [Google Scholar] [CrossRef]
- Le Fèvre, C.; Lhermitte, B.; Ahle, G.; Chambrelant, I.; Cebula, H.; Antoni, D.; Keller, A.; Schott, R.; Thiery, A.; Constans, J.-M.; et al. Pseudoprogression versus True Progression in Glioblastoma Patients: A Multiapproach Literature Review: Part 1—Molecular, Morphological and Clinical Features. Crit. Rev. Oncol. Hematol. 2020, 157, 103188. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- da Cruz, L.C.H.; Rodriguez, I.; Domingues, R.C.; Gasparetto, E.L.; Sorensen, A.G. Pseudoprogression and Pseudoresponse: Imaging Challenges in the Assessment of Posttreatment Glioma. Am. J. Neuroradiol. 2011, 32, 1978–1985. [Google Scholar] [CrossRef] [Green Version]
- Kucharczyk, M.J.; Parpia, S.; Whitton, A.; Greenspoon, J.N. Evaluation of Pseudoprogression in Patients with Glioblastoma. Neuro-Oncol. Pract. 2017, 4, 120–134. [Google Scholar] [CrossRef] [PubMed]
- van Dijken, B.R.J.; van Laar, P.J.; Holtman, G.A.; van der Hoorn, A. Diagnostic Accuracy of Magnetic Resonance Imaging Techniques for Treatment Response Evaluation in Patients with High-Grade Glioma, a Systematic Review and Meta-Analysis. Eur. Radiol. 2017, 27, 4129–4144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tye, K.; Engelhard, H.H.; Slavin, K.V.; Nicholas, M.K.; Chmura, S.J.; Kwok, Y.; Ho, D.S.; Weichselbaum, R.R.; Koshy, M. An Analysis of Radiation Necrosis of the Central Nervous System Treated with Bevacizumab. J. Neuro-Oncol. 2014, 117, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Sanghera, P.; Perry, J.; Sahgal, A.; Symons, S.; Aviv, R.; Morrison, M.; Lam, K.; Davey, P.; Tsao, M.N. Pseudoprogression Following Chemoradiotherapy for Glioblastoma Multiforme. Can. J. Neurol. Sci. 2010, 37, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, R.J.; Gupta, A.; Shah, A.D.; Graber, J.J.; Zhang, Z.; Shi, W.; Holodny, A.I.; Omuro, A.M.P. Potential Utility of Conventional MRI Signs in Diagnosing Pseudoprogression in Glioblastoma. Neurology 2011, 76, 1918–1924. [Google Scholar] [CrossRef] [Green Version]
- Bammer, R. Basic Principles of Diffusion-Weighted Imaging. Eur. J. Radiol. 2003, 45, 169–184. [Google Scholar] [CrossRef]
- Ogura, A.; Hatano, I.; Osakabe, K.; Yamaguchi, N.; Koyama, D.; Watanabe, H. Importance of Fractional b Value for Calculating Apparent Diffusion Coefficient in DWI. AJR Am. J. Roentgenol. 2016, 207, 1239–1243. [Google Scholar] [CrossRef]
- Park, M.Y.; Byun, J.Y. Understanding the Mathematics Involved in Calculating Apparent Diffusion Coefficient Maps. AJR Am. J. Roentgenol. 2012, 199, W784. [Google Scholar] [CrossRef]
- Zakhari, N.; Taccone, M.S.; Torres, C.; Chakraborty, S.; Sinclair, J.; Woulfe, J.; Jansen, G.H.; Nguyen, T.B. Diagnostic Accuracy of Centrally Restricted Diffusion in the Differentiation of Treatment-Related Necrosis from Tumor Recurrence in High-Grade Gliomas. AJNR Am. J. Neuroradiol. 2018, 39, 260–264. [Google Scholar] [CrossRef]
- Chu, H.H.; Choi, S.H.; Ryoo, I.; Kim, S.C.; Yeom, J.A.; Shin, H.; Jung, S.C.; Lee, A.L.; Yoon, T.J.; Kim, T.M.; et al. Differentiation of True Progression from Pseudoprogression in Glioblastoma Treated with Radiation Therapy and Concomitant Temozolomide: Comparison Study of Standard and High-b-Value Diffusion-Weighted Imaging. Radiology 2013, 269, 831–840. [Google Scholar] [CrossRef]
- Kazda, T.; Bulik, M.; Pospisil, P.; Lakomy, R.; Smrcka, M.; Slampa, P.; Jancalek, R. Advanced MRI Increases the Diagnostic Accuracy of Recurrent Glioblastoma: Single Institution Thresholds and Validation of MR Spectroscopy and Diffusion Weighted MR Imaging. Neuroimage Clin. 2016, 11, 316–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimer, C.; Deike, K.; Graf, M.; Reimer, P.; Wiestler, B.; Floca, R.O.; Kickingereder, P.; Schlemmer, H.-P.; Wick, W.; Bendszus, M.; et al. Differentiation of Pseudoprogression and Real Progression in Glioblastoma Using ADC Parametric Response Maps. PLoS ONE 2017, 12, e0174620. [Google Scholar] [CrossRef] [PubMed]
- Prager, A.J.; Martinez, N.; Beal, K.; Omuro, A.; Zhang, Z.; Young, R.J. Diffusion and Perfusion MRI to Differentiate Treatment-Related Changes Including Pseudoprogression from Recurrent Tumors in High-Grade Gliomas with Histopathologic Evidence. AJNR Am. J. Neuroradiol. 2015, 36, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Sundgren, P.C.; Fan, X.; Weybright, P.; Welsh, R.C.; Carlos, R.C.; Petrou, M.; McKeever, P.E.; Chenevert, T.L. Differentiation of Recurrent Brain Tumor versus Radiation Injury Using Diffusion Tensor Imaging in Patients with New Contrast-Enhancing Lesions. Magn. Reson. Imaging 2006, 24, 1131–1142. [Google Scholar] [CrossRef]
- Ferré, J.-C.; Bannier, E.; Raoult, H.; Mineur, G.; Carsin-Nicol, B.; Gauvrit, J.-Y. Arterial Spin Labeling (ASL) Perfusion: Techniques and Clinical Use. Diagn. Interv. Imaging 2013, 94, 1211–1223. [Google Scholar] [CrossRef]
- Barbier, E.L.; Lamalle, L.; Décorps, M. Methodology of Brain Perfusion Imaging. J. Magn. Reson. Imaging 2001, 13, 496–520. [Google Scholar] [CrossRef] [Green Version]
- Rempp, K.A.; Brix, G.; Wenz, F.; Becker, C.R.; Gückel, F.; Lorenz, W.J. Quantification of Regional Cerebral Blood Flow and Volume with Dynamic Susceptibility Contrast-Enhanced MR Imaging. Radiology 1994, 193, 637–641. [Google Scholar] [CrossRef]
- Seeger, A.; Braun, C.; Skardelly, M.; Paulsen, F.; Schittenhelm, J.; Ernemann, U.; Bisdas, S. Comparison of Three Different MR Perfusion Techniques and MR Spectroscopy for Multiparametric Assessment in Distinguishing Recurrent High-Grade Gliomas from Stable Disease. Acad. Radiol. 2013, 20, 1557–1565. [Google Scholar] [CrossRef]
- McGehee, B.E.; Pollock, J.M.; Maldjian, J.A. Brain Perfusion Imaging: How Does It Work and What Should I Use? J. Magn. Reson. Imaging 2012, 36, 1257–1272. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Chen, S.; Xiao, H.-F.; Li, Y.; Wang, Y.; Liu, G.; Lou, X.; Ma, L. Differentiation between Radiation-Induced Brain Injury and Glioma Recurrence Using 3D PCASL and Dynamic Susceptibility Contrast-Enhanced Perfusion-Weighted Imaging. Radiother. Oncol. 2018, 129, 68–74. [Google Scholar] [CrossRef]
- Young, R.J.; Gupta, A.; Shah, A.D.; Graber, J.J.; Chan, T.A.; Zhang, Z.; Shi, W.; Beal, K.; Omuro, A.M. MRI Perfusion in Determining Pseudoprogression in Patients with Glioblastoma. Clin. Imaging 2013, 37, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Boxerman, J.L.; Ellingson, B.M.; Jeyapalan, S.; Elinzano, H.; Harris, R.J.; Rogg, J.M.; Pope, W.B.; Safran, H. Longitudinal DSC-MRI for Distinguishing Tumor Recurrence from Pseudoprogression in Patients with a High-Grade Glioma. Am. J. Clin. Oncol. 2017, 40, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Rowe, L.S.; Butman, J.A.; Mackey, M.; Shih, J.H.; Cooley-Zgela, T.; Ning, H.; Gilbert, M.R.; Smart, D.K.; Camphausen, K.; Krauze, A.V. Differentiating Pseudoprogression from True Progression: Analysis of Radiographic, Biologic, and Clinical Clues in GBM. J. Neurooncol. 2018, 139, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.A.; Arevalo-Perez, J.; Kaley, T.; Lyo, J.; Peck, K.K.; Shi, W.; Zhang, Z.; Young, R.J. Dynamic Contrast Enhanced T1 MRI Perfusion Differentiates Pseudoprogression from Recurrent Glioblastoma. J. Neurooncol. 2015, 125, 183–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, T.J.; Park, C.-K.; Kim, T.M.; Lee, S.-H.; Kim, J.-H.; Sohn, C.-H.; Park, S.-H.; Kim, I.H.; Choi, S.H. Glioblastoma Treated with Concurrent Radiation Therapy and Temozolomide Chemotherapy: Differentiation of True Progression from Pseudoprogression with Quantitative Dynamic Contrast-Enhanced MR Imaging. Radiology 2015, 274, 830–840. [Google Scholar] [CrossRef]
- Yoo, R.-E.; Choi, S.H.; Kim, T.M.; Park, C.-K.; Park, S.-H.; Won, J.-K.; Kim, I.H.; Lee, S.T.; Choi, H.J.; You, S.-H.; et al. Dynamic Contrast-Enhanced MR Imaging in Predicting Progression of Enhancing Lesions Persisting after Standard Treatment in Glioblastoma Patients: A Prospective Study. Eur. Radiol. 2017, 27, 3156–3166. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, H.S.; Jahng, G.-H.; Kim, S.J.; Suh, D.C. Pseudoprogression in Patients with Glioblastoma: Added Value of Arterial Spin Labeling to Dynamic Susceptibility Contrast Perfusion MR Imaging. Acta Radiol. 2013, 54, 448–454. [Google Scholar] [CrossRef]
- Manning, P.; Daghighi, S.; Rajaratnam, M.K.; Parthiban, S.; Bahrami, N.; Dale, A.M.; Bolar, D.; Piccioni, D.E.; McDonald, C.R.; Farid, N. Differentiation of Progressive Disease from Pseudoprogression Using 3D PCASL and DSC Perfusion MRI in Patients with Glioblastoma. J. Neurooncol. 2020, 147, 681–690. [Google Scholar] [CrossRef]
- Tsakiris, C.; Siempis, T.; Alexiou, G.A.; Zikou, A.; Sioka, C.; Voulgaris, S.; Argyropoulou, M.I. Differentiation Between True Tumor Progression of Glioblastoma and Pseudoprogression Using Diffusion-Weighted Imaging and Perfusion-Weighted Imaging: Systematic Review and Meta-Analysis. World Neurosurg. 2020, 144, e100–e109. [Google Scholar] [CrossRef]
- Posse, S.; Otazo, R.; Dager, S.R.; Alger, J. MR Spectroscopic Imaging: Principles and Recent Advances. J. Magn. Reson. Imaging 2013, 37, 1301–1325. [Google Scholar] [CrossRef]
- Öz, G.; Alger, J.R.; Barker, P.B.; Bartha, R.; Bizzi, A.; Boesch, C.; Bolan, P.J.; Brindle, K.M.; Cudalbu, C.; Dinçer, A.; et al. Clinical Proton MR Spectroscopy in Central Nervous System Disorders. Radiology 2014, 270, 658–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tensaouti, F.; Desmoulin, F.; Gilhodes, J.; Martin, E.; Ken, S.; Lotterie, J.-A.; Noël, G.; Truc, G.; Sunyach, M.-P.; Charissoux, M.; et al. Quality Control of 3D MRSI Data in Glioblastoma: Can We Do without the Experts? Magn. Reson. Med 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Horská, A.; Barker, P.B. Imaging of Brain Tumors: MR Spectroscopy and Metabolic Imaging. Neuroimaging Clin. N. Am. 2010, 20, 293–310. [Google Scholar] [CrossRef] [Green Version]
- Mascalchi, M.; Montomoli, M.; Guerrini, R. Neuroimaging in Mitochondrial Disorders. Essays Biochem. 2018, 62, 409–421. [Google Scholar] [CrossRef]
- McKnight, T.R.; von dem Bussche, M.H.; Vigneron, D.B.; Lu, Y.; Berger, M.S.; McDermott, M.W.; Dillon, W.P.; Graves, E.E.; Pirzkall, A.; Nelson, S.J. Histopathological Validation of a Three-Dimensional Magnetic Resonance Spectroscopy Index as a Predictor of Tumor Presence. J. Neurosurg. 2002, 97, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Laprie, A.; Catalaa, I.; Cassol, E.; McKnight, T.R.; Berchery, D.; Marre, D.; Bachaud, J.-M.; Berry, I.; Moyal, E.C.-J. Proton Magnetic Resonance Spectroscopic Imaging in Newly Diagnosed Glioblastoma: Predictive Value for the Site of Postradiotherapy Relapse in a Prospective Longitudinal Study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 773–781. [Google Scholar] [CrossRef]
- Deviers, A.; Ken, S.; Filleron, T.; Rowland, B.; Laruelo, A.; Catalaa, I.; Lubrano, V.; Celsis, P.; Berry, I.; Mogicato, G.; et al. Evaluation of the Lactate-to-N-Acetyl-Aspartate Ratio Defined with Magnetic Resonance Spectroscopic Imaging before Radiation Therapy as a New Predictive Marker of the Site of Relapse in Patients with Glioblastoma Multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 385–393. [Google Scholar] [CrossRef]
- Laprie, A.; Ken, S.; Filleron, T.; Lubrano, V.; Vieillevigne, L.; Tensaouti, F.; Catalaa, I.; Boetto, S.; Khalifa, J.; Attal, J.; et al. Dose-Painting Multicenter Phase III Trial in Newly Diagnosed Glioblastoma: The SPECTRO-GLIO Trial Comparing Arm A Standard Radiochemotherapy to Arm B Radiochemotherapy with Simultaneous Integrated Boost Guided by MR Spectroscopic Imaging. BMC Cancer 2019, 19, 167. [Google Scholar] [CrossRef] [Green Version]
- Dowling, C.; Bollen, A.W.; Noworolski, S.M.; McDermott, M.W.; Barbaro, N.M.; Day, M.R.; Henry, R.G.; Chang, S.M.; Dillon, W.P.; Nelson, S.J.; et al. Preoperative Proton MR Spectroscopic Imaging of Brain Tumors: Correlation with Histopathologic Analysis of Resection Specimens. AJNR Am. J. Neuroradiol. 2001, 22, 604–612. [Google Scholar]
- Delorme, S.; Weber, M.-A. Applications of MRS in the Evaluation of Focal Malignant Brain Lesions. Cancer Imaging 2006, 6, 95–99. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, I.; Verma, N.; Aggarwal, P.; Ojha, R. Magnetic Resonance Spectroscopy—Revisiting the Biochemical and Molecular Milieu of Brain Tumors. BBA Clin. 2016, 5, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.A.; Carlos, R.C.; Junck, L.R.; Tsien, C.I.; Elias, A.; Sundgren, P.C. Developing a Clinical Decision Model: MR Spectroscopy to Differentiate between Recurrent Tumor and Radiation Change in Patients with New Contrast-Enhancing Lesions. AJR Am. J. Roentgenol. 2009, 192, W45–W52. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.E.; Carlos, R.C.; Smith, E.A.; Frechtling, D.; George, B.; Maly, P.; Sundgren, P.C. MR Spectroscopy Using Normalized and Non-Normalized Metabolite Ratios for Differentiating Recurrent Brain Tumor from Radiation Injury. Acad. Radiol. 2011, 18, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Bulik, M.; Kazda, T.; Slampa, P.; Jancalek, R. The Diagnostic Ability of Follow-Up Imaging Biomarkers after Treatment of Glioblastoma in the Temozolomide Era: Implications from Proton MR Spectroscopy and Apparent Diffusion Coefficient Mapping. Biomed. Res. Int. 2015, 2015, 641023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawlani, V.; Taylor, R.; Rowley, K.; Redfern, R.; Martin, J.; Poptani, H. Magnetic Resonance Spectroscopy for Differentiating Pseudo-Progression from True Progression in GBM on Concurrent Chemoradiotherapy. Neuroradiol. J. 2012, 25, 575–586. [Google Scholar] [CrossRef]
- Anbarloui, M.R.; Ghodsi, S.M.; Khoshnevisan, A.; Khadivi, M.; Abdollahzadeh, S.; Aoude, A.; Naderi, S.; Najafi, Z.; Faghih-Jouibari, M. Accuracy of Magnetic Resonance Spectroscopy in Distinction between Radiation Necrosis and Recurrence of Brain Tumors. Iran J. Neurol. 2015, 14, 29–34. [Google Scholar]
- Verma, G.; Chawla, S.; Mohan, S.; Wang, S.; Nasrallah, M.; Sheriff, S.; Desai, A.; Brem, S.; O’Rourke, D.M.; Wolf, R.L.; et al. Three-Dimensional Echo Planar Spectroscopic Imaging for Differentiation of True Progression from Pseudoprogression in Patients with Glioblastoma. NMR Biomed. 2019, 32, e4042. [Google Scholar] [CrossRef]
- Le Fèvre, C.; Constans, J.-M.; Chambrelant, I.; Antoni, D.; Bund, C.; Leroy-Freschini, B.; Schott, R.; Cebula, H.; Noël, G. Pseudoprogression versus True Progression in Glioblastoma Patients: A Multiapproach Literature Review. Part 2—Radiological Features and Metric Markers. Crit. Rev. Oncol. Hematol. 2021, 159, 103230. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting More Information from Medical Images Using Advanced Feature Analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Kickingereder, P.; Neuberger, U.; Bonekamp, D.; Piechotta, P.L.; Götz, M.; Wick, A.; Sill, M.; Kratz, A.; Shinohara, R.T.; Jones, D.T.W.; et al. Radiomic Subtyping Improves Disease Stratification beyond Key Molecular, Clinical, and Standard Imaging Characteristics in Patients with Glioblastoma. Neuro Oncol. 2018, 20, 848–857. [Google Scholar] [CrossRef]
- Chaddad, A.; Kucharczyk, M.J.; Daniel, P.; Sabri, S.; Jean-Claude, B.J.; Niazi, T.; Abdulkarim, B. Radiomics in Glioblastoma: Current Status and Challenges Facing Clinical Implementation. Front. Oncol. 2019, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The Bridge between Medical Imaging and Personalized Medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Bani-Sadr, A.; Eker, O.F.; Berner, L.-P.; Ameli, R.; Hermier, M.; Barritault, M.; Meyronet, D.; Guyotat, J.; Jouanneau, E.; Honnorat, J.; et al. Conventional MRI Radiomics in Patients with Suspected Early- or Pseudo-Progression. Neurooncol. Adv. 2019, 1, vdz019. [Google Scholar] [CrossRef]
- Sun, Y.-Z.; Yan, L.-F.; Han, Y.; Nan, H.-Y.; Xiao, G.; Tian, Q.; Pu, W.-H.; Li, Z.-Y.; Wei, X.-C.; Wang, W.; et al. Differentiation of Pseudoprogression from True Progressionin Glioblastoma Patients after Standard Treatment: A Machine Learning Strategy Combinedwith Radiomics Features from T1-Weighted Contrast-Enhanced Imaging. BMC Med. Imaging 2021, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Elshafeey, N.; Kotrotsou, A.; Hassan, A.; Elshafei, N.; Hassan, I.; Ahmed, S.; Abrol, S.; Agarwal, A.; El Salek, K.; Bergamaschi, S.; et al. Multicenter Study Demonstrates Radiomic Features Derived from Magnetic Resonance Perfusion Images Identify Pseudoprogression in Glioblastoma. Nat. Commun. 2019, 10, 3170. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.; Hill, V.; Statsevych, V.; Huang, R.; Prasanna, P.; Correa, R.; Singh, G.; Bera, K.; Beig, N.; Thawani, R.; et al. Shape Features of the Lesion Habitat to Differentiate Brain Tumor Progression from Pseudoprogression on Routine Multiparametric MRI: A Multisite Study. AJNR Am. J. Neuroradiol. 2018, 39, 2187–2193. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Park, J.E.; Jo, Y.; Shim, W.H.; Nam, S.J.; Kim, J.H.; Yoo, R.-E.; Choi, S.H.; Kim, H.S. Incorporating Diffusion- and Perfusion-Weighted MRI into a Radiomics Model Improves Diagnostic Performance for Pseudoprogression in Glioblastoma Patients. Neuro Oncol. 2019, 21, 404–414. [Google Scholar] [CrossRef]
- Baine, M.; Burr, J.; Du, Q.; Zhang, C.; Liang, X.; Krajewski, L.; Zima, L.; Rux, G.; Zhang, C.; Zheng, D. The Potential Use of Radiomics with Pre-Radiation Therapy MR Imaging in Predicting Risk of Pseudoprogression in Glioblastoma Patients. J. Imaging 2021, 7, 17. [Google Scholar] [CrossRef]
- Hu, X.; Wong, K.K.; Young, G.S.; Guo, L.; Wong, S.T. Support Vector Machine (SVM) Multi-Parametric MRI Identification of Pseudoprogression from Tumor Recurrence in Patients with Resected Glioblastoma. J. Magn. Reson. Imaging 2011, 33, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Akbari, H.; Rathore, S.; Bakas, S.; Nasrallah, M.P.; Shukla, G.; Mamourian, E.; Rozycki, M.; Bagley, S.J.; Rudie, J.D.; Flanders, A.E.; et al. Histopathology-Validated Machine Learning Radiographic Biomarker for Noninvasive Discrimination between True Progression and Pseudo-Progression in Glioblastoma. Cancer 2020, 126, 2625–2636. [Google Scholar] [CrossRef]
- Jang, B.-S.; Park, A.J.; Jeon, S.H.; Kim, I.H.; Lim, D.H.; Park, S.-H.; Lee, J.H.; Chang, J.H.; Cho, K.H.; Kim, J.H.; et al. Machine Learning Model to Predict Pseudoprogression Versus Progression in Glioblastoma Using MRI: A Multi-Institutional Study (KROG 18-07). Cancers 2020, 12, 2706. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.-S.; Jeon, S.H.; Kim, I.H.; Kim, I.A. Prediction of Pseudoprogression versus Progression Using Machine Learning Algorithm in Glioblastoma. Sci. Rep. 2018, 8, 12516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, B.R.; Friedrich, F.; Fischer, C.; Paech, D.; Ladd, M.E. Beyond T2 and 3T: New MRI Techniques for Clinicians. Clin. Transl. Radiat. Oncol. 2019, 18, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Bashir, M.R.; Bhatti, L.; Marin, D.; Nelson, R.C. Emerging Applications for Ferumoxytol as a Contrast Agent in MRI. J. Magn. Reson. Imaging 2015, 41, 884–898. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Chung, C.; Pope, W.B.; Boxerman, J.L.; Kaufmann, T.J. Pseudoprogression, Radionecrosis, Inflammation or True Tumor Progression? Challenges Associated with Glioblastoma Response Assessment in an Evolving Therapeutic Landscape. J. Neuro-Oncol. 2017, 134, 495–504. [Google Scholar] [CrossRef] [Green Version]


| Study | N | Parameter | TP | PsP | p |
|---|---|---|---|---|---|
| Chu, 2013 | 30 | 5th percentile ADC 1000 5th percentile ADC 3000 | 906 × 10−6 mm2/s 587 × 10−6 mm2/s | 1030 × 10−6 mm2/s 719 × 10−6 mm2/s | 0.049 <0.001 |
| Prager, 2015 | 68 | ADC mean | 1380 × 10−6 mm2/s | 1590 × 10−6 mm2/s | 0.003 |
| Kazda, 2016 | 39 | ADC mean | 1155 × 10−6 mm2/s | 1372 × 10−6 mm2/s | <0.001 |
| Reimer, 2017 | 35 | rADC decrease | 59% | 18% | 0.005 |
| Zhakari, 2018 | 17 | ADC min in necrosis | 1756 × 10−6 mm2/s | 992 × 10−6 mm2/s | 0.027 |
| Study | N | Parameter | TP | PsP | p |
|---|---|---|---|---|---|
| Young, 2013 | 20 | rCBV mean | 2.75 | 1.50 | 0.009 |
| Prager, 2015 | 68 | rCBV mean | 1.81 | 1.015 | 0.003 |
| Boxerman, 2017 | 19 | rCBV mean | 2.17 | 2.35 | 0.67 |
| Wang, 2018 | 68 | rCBV mean | 3.39 | 1.39 | <0.001 |
| Rowe, 2018 | 67 | Increase rCBV | 73.7% | 93.3% | - |
| Study | N | Type of MRS | Parameter | TP | PsP | p |
|---|---|---|---|---|---|---|
| Smith, 2009 | 33 | 2D CSI | Median Cho/NAA | 3.2 | 1.43 | <0.001 |
| Median Cho/NAA | 2.56 | 1.57 | <0.001 | |||
| Median NAA/Cr | 0.85 | 1.14 | 0.018 | |||
| Elias, 2011 | 25 | 2D CSI | Mean Cho/NAA | 2.81 | 1.39 | 0.0004 |
| Mean Cho/Cr | 2.23 | 1.84 | 0.24 | |||
| Mean NAA/Cr | 0.85 | 1.36 | 0.0033 | |||
| Ambarloui, 2015 | 33 | SV | Median Cho/NAA | 2.72 | 1.46 | 0.01 |
| Median NAA/Cr | 2.46 | 0.6 | 0.01 | |||
| Bulik, 2015 | 24 | 2D CSI | Median CHO/NAA | 2 | 0.77 | <0.001 |
| Median Cho/Cr | 0.45 | 0.99 | <0.01 | |||
| Kazda, 2016 | 39 | 2D CSI | Median Cho/NAA | 2.13 | 0.74 | <0.001 |
| Median Cho/Cr | 0.89 | 0.64 | 0.013 | |||
| Median NAA/Cr | 0.99 | 0.41 | <0.001 | |||
| Verma, 2018 | 27 | 3D EPSI | Cho/NAA | 2.69 | 1.56 | 0.003 |
| Cho/Cr | 1.74 | 1.34 | 0.023 |
| Study | Patients (N) | Imaging | Preprocessing | Segmentation | Feature Classification | Main Features or Parameters Found | Results | External Validation |
|---|---|---|---|---|---|---|---|---|
| Ismail, 2018 | 59:21 PsP and 38 TP | T1CE, T2, FLAIR | Skull stripping Intensity normalization | Manual | SVM 4-fold cross-validation | Mean of KT roundness, eccentricity Median of C, elongation shape factor | Accuracy: 91.5% | Yes Accuracy: 90.2% |
| Kim, 2019 | 61:26 PsP and 35 TP | T1CE, FLAIR, DSC DWI | hybrid white-stripe normalization excluding outliers inside the region of interes | Semi-automated | LASSO 10-fold cross-validation | 14 features | Accuracy: 90% Se: 91.4% Sp: 76.9% | Yes Accuracy: 85% Se: 71.4% Sp: 90% |
| Elshafeey, 2019 | 98:76 TP and 22 PsP | T1CE, DSC | NA | Semi-automated | MMR SVM C5.0 LOOCV 10-fold cross-validation | Ktrans rCBV | Accuracy: 90.82% Se: 91.36% Sp: 88.2% | No |
| Bani-Sadr, 2019 | 76:53 TP and 23 PsP | FLAIR, T1CE | NA | Manual | SCPA 10-fold cross-validation | 11 radiomic features | Accuracy: 75% Se: 81.6% Sp: 50% | Yes Accuracy: 76% Se: 94% Sp: 37.5% |
| Sun, 2021 | 77:51 TP and 26 PsP | T1CE | Normalization | Semi-automated | Random forest classification (SMOTE) 5-fold cross-validation | 50 radiomic features | Accuracy: 72.78% Se: 78.36% Sp: 61.33% | No |
| Baine, 2021 | 35:27 TP and 8 PSP | T1CE | N4 Bias field correction Histogram matching normalization | Manual | ANOVA analysis 1000-time 3-fold cross-validations, | Wavelet_HHL_firstorder_Mean Original_firstorder_Minimum WaveLet LHL_glszm_SizeZoneNonUniformityNormalized | Mean AUC = 0.82 for the radiomic model | No |
| Akbari et al., 2020 | 63:35 TP, 10 Psp, 18 mixed response | T1CE, FLAIR, DSC DTI | Smoothed Correction of magnetic inhomogeneities Skull stripped | Manual | SVM LOOCV | 1040 radiomics features analysed and 2 classifiers | Accuracy 87% to predict PSP, interinstitutional cohort accuracy 75% | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidibe, I.; Tensaouti, F.; Roques, M.; Cohen-Jonathan-Moyal, E.; Laprie, A. Pseudoprogression in Glioblastoma: Role of Metabolic and Functional MRI-Systematic Review. Biomedicines 2022, 10, 285. https://doi.org/10.3390/biomedicines10020285
Sidibe I, Tensaouti F, Roques M, Cohen-Jonathan-Moyal E, Laprie A. Pseudoprogression in Glioblastoma: Role of Metabolic and Functional MRI-Systematic Review. Biomedicines. 2022; 10(2):285. https://doi.org/10.3390/biomedicines10020285
Chicago/Turabian StyleSidibe, Ingrid, Fatima Tensaouti, Margaux Roques, Elizabeth Cohen-Jonathan-Moyal, and Anne Laprie. 2022. "Pseudoprogression in Glioblastoma: Role of Metabolic and Functional MRI-Systematic Review" Biomedicines 10, no. 2: 285. https://doi.org/10.3390/biomedicines10020285
APA StyleSidibe, I., Tensaouti, F., Roques, M., Cohen-Jonathan-Moyal, E., & Laprie, A. (2022). Pseudoprogression in Glioblastoma: Role of Metabolic and Functional MRI-Systematic Review. Biomedicines, 10(2), 285. https://doi.org/10.3390/biomedicines10020285






