Nicotinamide Adenine Dinucleotide Phosphate Oxidase 2 Expression and Effects of Alpha Lipoic Acid on Recovery in a Rat Model of Facial Nerve Injury
Abstract
:1. Introduction
2. Methods
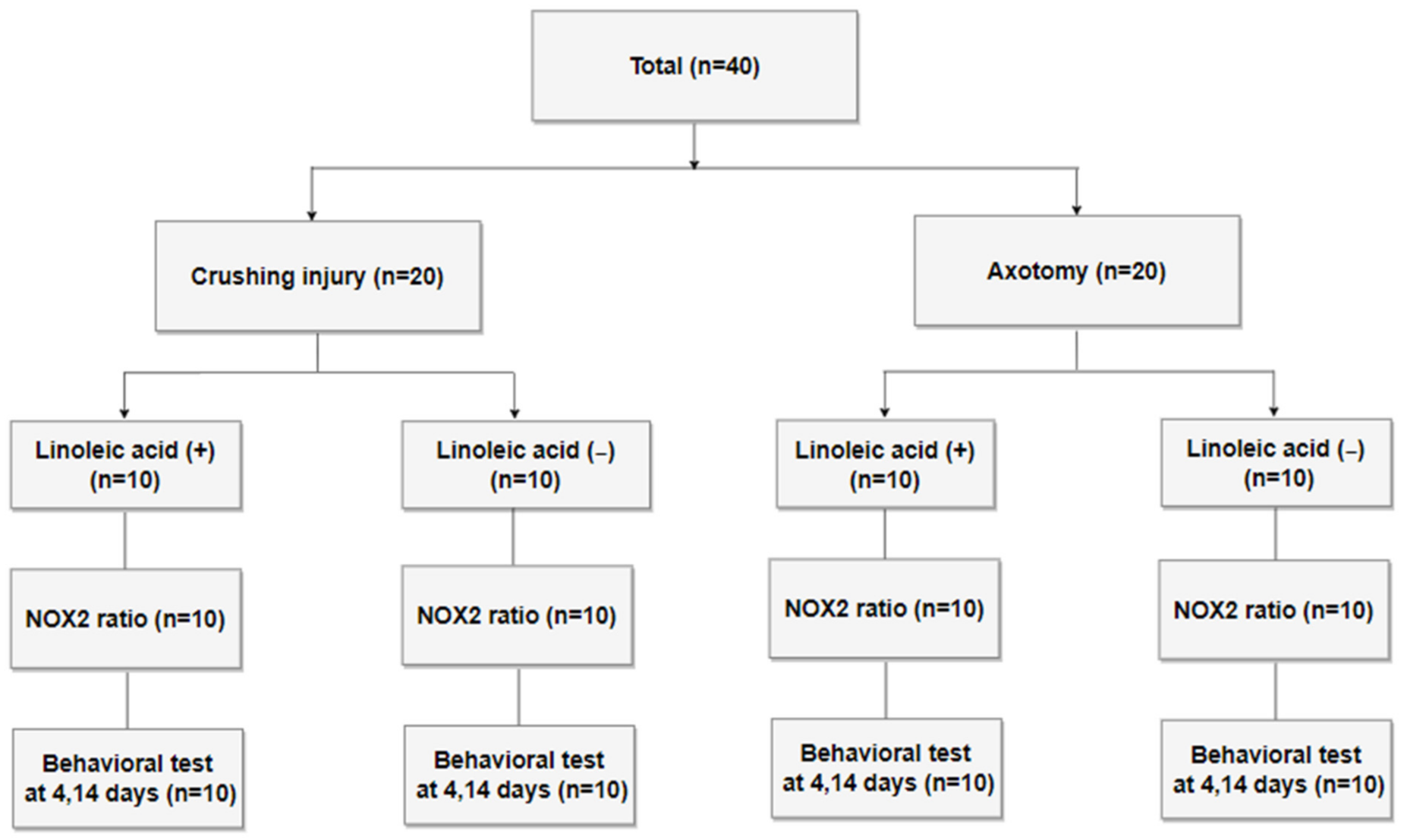
2.1. Study Design and Preparation of the Nerve Injury Model
2.2. Behavioral Tests
2.3. Immunohistochemical Analysis of NOX2 Expression
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hohman, M.H.; Hadlock, T.A. Etiology, diagnosis, and management of facial palsy: 2000 patients at a facial nerve center. Laryngoscope 2014, 124, E283–E293. [Google Scholar] [CrossRef] [PubMed]
- Roob, G.; Fazekas, F.; Hartung, H.-P. Peripheral facial palsy: Etiology, diagnosis and treatment. Eur. Neurol. 1999, 41, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jung, J.; Lee, J.H.; Byun, J.Y.; Park, M.S.; Yeo, S.G. Delayed facial nerve decompression for Bell’s palsy. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Tekdemir, E.; Tatlipinar, A.; Özbeyli, D.; Tekdemir, Ö.; Kınal, E. The effects of lipoic acid and methylprednisolone on nerve healing in rats with facial paralysis. Acta Oto-Laryngol. 2018, 138, 537–541. [Google Scholar] [CrossRef]
- Yegiyants, S.; Dayicioglu, D.; Kardashian, G.; Panthaki, Z.J. Traumatic peripheral nerve injury: A wartime review. J. Craniofac. Surg. 2010, 21, 998–1001. [Google Scholar] [CrossRef]
- Feng, X.; Yuan, W. Dexamethasone enhanced functional recovery after sciatic nerve crush injury in rats. BioMed Res. Int. 2015, 2015, 627923. [Google Scholar] [CrossRef]
- Subbanna, P.K.T.; Prasanna, C.; Gunale, B.K.; Tyagi, M.G. Acetyl salicylic acid augments functional recovery following sciatic nerve crush in mice. J. Brachial Plex. Peripher. Nerve Inj. 2007, 2, e91–e94. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, L.; Yannas, I.; Hsu, H.P.; Strichartz, G.; Spector, M. Near-terminus axonal structure and function following rat sciatic nerve regeneration through a collagen-GAG matrix in a ten-millimeter gap. J. Neurosci. Res. 2000, 60, 666–677. [Google Scholar] [CrossRef]
- Babior, B.M.; Kipnes, R.S. Superoxide-forming enzyme from human neutrophils: Evidence for a flavin requirement. Blood 1977, 50, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Sorescu, D.; Szöcs, K.; Griendling, K.K. NAD (P) H oxidases and their relevance to atherosclerosis. Trends Cardiovasc. Med. 2001, 11, 124–131. [Google Scholar] [CrossRef]
- Senoglu, M.; Nacitarhan, V.; Kurutas, E.B.; Senoglu, N.; Altun, I.; Atli, Y.; Ozbag, D. Intraperitoneal Alpha-Lipoic Acid to prevent neural damage after crush injury to the rat sciatic nerve. J. Brachial Plex. Peripher. Nerve Inj. 2009, 4, e109–e114. [Google Scholar] [CrossRef] [Green Version]
- Mitsui, Y.; Schmelzer, J.D.; Zollman, P.J.; Mitsui, M.; Tritschler, H.J.; Low, P.A. Alpha-lipoic acid provides neuroprotection from ischemia-reperfusion injury of peripheral nerve. J. Neurol. Sci. 1999, 163, 11–16. [Google Scholar] [CrossRef]
- Turamanlar, O.; Songur, A.; Özen, O.A.; Yağmurca, M.; Aktaş, C.; Akçer, S.; Mollaoğlu, H. Protective effect of alpha lipoic acid on rat sciatic nerve ischemia reperfusion damage. Balk. Med. J. 2015, 32, 196–202. [Google Scholar] [CrossRef]
- Kocaoğlu, S.; Aktaş, Ö.; Zengi, O.; Tufan, A.; Karagöz, F. Effects of alpha lipoic acid on motor function and antioxidant enzyme activity of nerve tissue after sciatic nerve crush injury in rats. Turk. Neurosurg. 2017, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- de Faria, S.D.; Testa, J.R.G.; Borin, A.; Toledo, R.N. Standardization of techniques used in facial nerve section and facial movement evaluation in rats. Braz. J. Otorhinolaryngol. 2006, 72, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Heaton, J.T.; Kowaleski, J.; Edwards, C.; Smitson, C.; Hadlock, T.A. Evidence for Facial Nerve—Independent Mechanisms of Blinking in the Rat. Investig. Ophthalmol. Vis. Sci. 2010, 51, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Devasagayam, T.; Tilak, J.; Boloor, K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R. Free radicals and antioxidants in human health: Current status and future prospects. JAPI 2004, 52, 4. [Google Scholar]
- Edreva, A. Generation and scavenging of reactive oxygen species in chloroplasts: A submolecular approach. Agric. Ecosyst. Environ. 2005, 106, 119–133. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [Green Version]
- Tarpey, M.M.; Fridovich, I. Methods of detection of vascular reactive species: Nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ. Res. 2001, 89, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, K.; Martin, D.P.; Schmelzer, J.D.; Mitsui, Y.; Low, P.A. Pro-and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp. Neurol. 2001, 169, 386–391. [Google Scholar] [CrossRef]
- Li, M.; Zhang, P.; Li, H.; Zhu, Y.; Cui, S.; Yao, D. TGF-β1 is critical for Wallerian degeneration after rat sciatic nerve injury. Neuroscience 2015, 284, 759–767. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, A.-P.; Duan, X.-M.; Hu, G.-H.; Song, G.-L.; Zuo, M.-L.; Yang, Z.-B. Upregulation of NOX2 and NOX4 mediated by TGF-β signaling pathway exacerbates cerebral ischemia/reperfusion oxidative stress injury. Cell. Physiol. Biochem. 2018, 46, 2103–2113. [Google Scholar] [CrossRef]
- Krstić, J.; Trivanović, D.; Mojsilović, S.; Santibanez, J.F. Transforming growth factor-beta and oxidative stress interplay: Implications in tumorigenesis and cancer progression. Oxidative Med. Cell. Longev. 2015, 2015, 654594. [Google Scholar] [CrossRef]
- Ziegler, D.; Reljanovic, M.; Mehnert, H.; Gries, F. α-Lipoic acid in the treatment of diabetic polyneuropathy in Germany: Current evidence from clinical trials. Exp. Clin. Endocrinol. Diabetes 1999, 107, 421–430. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, H.; Chen, Z. Alpha-lipoic acid attenuates cerebral ischemia and reperfusion injury via insulin receptor and PI3K/Akt-dependent inhibition of NADPH oxidase. Int. J. Endocrinol. 2015, 2015, 903186. [Google Scholar] [CrossRef] [Green Version]
- Maritim, A.; Sanders, R.; Watkins, J.W., III. Effects of α-lipoic acid on biomarkers of oxidative stress in streptozotocin-induced diabetic rats. J. Nutr. Biochem. 2003, 14, 288–294. [Google Scholar] [CrossRef]
- Odabasoglu, F.; Halici, Z.; Aygun, H.; Halici, M.; Atalay, F.; Cakir, A.; Cadirci, E.; Bayir, Y.; Suleyman, H. α-Lipoic acid has anti-inflammatory and anti-oxidative properties: An experimental study in rats with carrageenan-induced acute and cotton pellet-induced chronic inflammations. Br. J. Nutr. 2011, 105, 31–43. [Google Scholar] [CrossRef] [Green Version]
- In, H.S.; Kim, D.W.; Park, Y.M.; Kim, B. Experimental intraperitoneal injection of alcohol in rats: Peritoneal findings and histopathology. Toxicol. Rep. 2014, 1, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Schechter, M.D.; Lovano, D.M. Ethanol-chlordiazepoxide interactions in the rat. Pharmacol. Biochem. Behav. 1985, 23, 927–930. [Google Scholar] [CrossRef]
- Wiberg, G.S.; Trenholm, H.L.; Coldwell, B.B. Increased ethanol toxicity in old rats: Changes in LD50, in vivo and in vitro metabolism, and liver alcohol dehydrogenase activity. Toxicol. Appl. Pharmacol. 1970, 16, 718–727. [Google Scholar] [CrossRef]
- Hadlock, T.A.; Heaton, J.; Cheney, M.; Mackinnon, S.E. Functional recovery after facial and sciatic nerve crush injury in the rat. Arch. Facial Plast. Surg. 2005, 7, 17–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigoni, M.; Negro, S. Signals orchestrating peripheral nerve repair. Cells 2020, 9, 1768. [Google Scholar] [CrossRef] [PubMed]
- Hervera, A.; De Virgiliis, F.; Palmisano, I.; Zhou, L.; Tantardini, E.; Kong, G.; Hutson, T.; Danzi, M.C.; Perry, R.B.-T.; Santos, C.X. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 2018, 20, 307–319. [Google Scholar] [CrossRef]
- Krämer-Albers, E.-M. Exosomes deliver ROS for regeneration. Nat. Cell Biol. 2018, 20, 225–226. [Google Scholar] [CrossRef]
- Giridharan, S.S.P.; Caplan, S. MICAL-family proteins: Complex regulators of the actin cytoskeleton. Antioxid. Redox Signal. 2014, 20, 2059–2073. [Google Scholar] [CrossRef] [Green Version]
- Kallenborn-Gerhardt, W.; Hohmann, S.W.; Syhr, K.M.; Schröder, K.; Sisignano, M.; Weigert, A.; Lorenz, J.E.; Lu, R.; Brüne, B.; Brandes, R.P.; et al. Nox2-dependent signaling between macrophages and sensory neurons contributes to neuropathic pain hypersensitivity. Pain 2014, 155, 2161–2170. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Yao, Y.; Li, J.; Zhang, X.; Li, C.; Cheng, Y.; Ding, G.; Liu, L.; Ding, Z. Essential role of ERK activation in neurite outgrowth induced by α-lipoic acid. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2011, 1813, 827–838. [Google Scholar] [CrossRef] [Green Version]

| Variable | β Estimate | 95% CI | p-Value | |
|---|---|---|---|---|
| Injury type | ||||
| Crushing injury | 0.21 | −0.45 | 0.88 | 0.520 |
| Axotomy | 0.00 | |||
| Time after injury | ||||
| Day 4 | −0.60 | −1.27 | 0.07 | 0.076 |
| Day 14 | 0.00 | |||
| ALA treatment | ||||
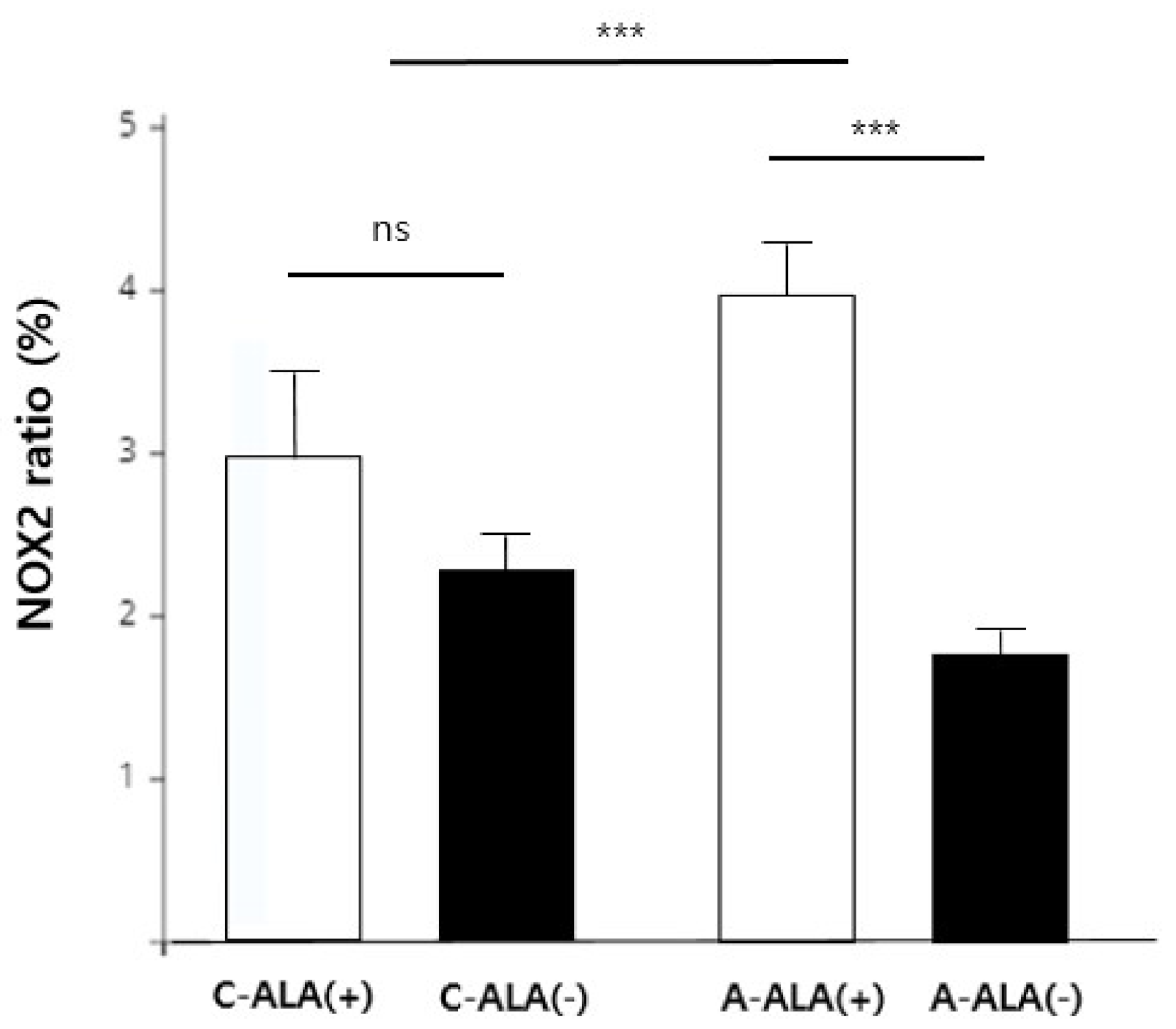
| ALA (−) | −1.57 | −2.24 | −0.90 | <0.001 * |
| ALA (+) | 0.00 | |||
| Time after Injury | Vibrissae Eye Closing | |||||
|---|---|---|---|---|---|---|
| Crushing | Axotomy | p | Crushing | Axotomy | p | |
| Day 4 | 1.30 ± 0.14 | 1.20 ± 0.13 | 0.586 | 1.60 ± 0.17 | 1.20 ± 0.15 | 0.274 |
| Day 14 | 1.95 ± 0.14 | 1.20 ± 0.13 | <0.001 * | 2.30 ± 0.17 | 1.30 ± 0.15 | <0.001 * |
| Time after injury | Vibrissae Eye closing | |||||
| C-ALA (+) | C-ALA (−) | p | C-ALA (+) | C-ALA (−) | p | |
| Day 4 | 1.30 ± 0.20 | 1.20 ± 0.20 | 0.374 | 1.90 ± 0.24 | 1.20 ± 0.20 | 0.031 † |
| Day 14 | 2.00 ± 0.20 | 1.90 ± 0.20 | 0.478 | 2.20 ± 0.25 | 2.00 ± 0.20 | 0.374 |
| Time after injury | Vibrissae Eye closing | |||||
| A-ALA (+) | A-ALA (−) | p | A-ALA (+) | A-ALA (−) | p | |
| Day 4 | 1.50 ± 0.15 | 1.70 ± 0.15 | 0.524 | 1.50 ± 0.15 | 1.50 ± 0.15 | 1.000 |
| Day 14 | 1.40 ± 0.15 | 1.60 ± 0.15 | 0.580 | 1.30 ± 0.15 | 1.40 ± 0.15 | 0.450 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, M.C.; Ryu, I.Y.; Choi, J.W.; Lee, J.M.; Byun, J.Y.; Yeo, S.G. Nicotinamide Adenine Dinucleotide Phosphate Oxidase 2 Expression and Effects of Alpha Lipoic Acid on Recovery in a Rat Model of Facial Nerve Injury. Biomedicines 2022, 10, 291. https://doi.org/10.3390/biomedicines10020291
Yoo MC, Ryu IY, Choi JW, Lee JM, Byun JY, Yeo SG. Nicotinamide Adenine Dinucleotide Phosphate Oxidase 2 Expression and Effects of Alpha Lipoic Acid on Recovery in a Rat Model of Facial Nerve Injury. Biomedicines. 2022; 10(2):291. https://doi.org/10.3390/biomedicines10020291
Chicago/Turabian StyleYoo, Myung Chul, In Yong Ryu, Jin Woo Choi, Jae Min Lee, Jae Yong Byun, and Seung Geun Yeo. 2022. "Nicotinamide Adenine Dinucleotide Phosphate Oxidase 2 Expression and Effects of Alpha Lipoic Acid on Recovery in a Rat Model of Facial Nerve Injury" Biomedicines 10, no. 2: 291. https://doi.org/10.3390/biomedicines10020291






