The Road to Personalized Medicine in Alzheimer’s Disease: The Use of Artificial Intelligence
Abstract
:1. Introduction
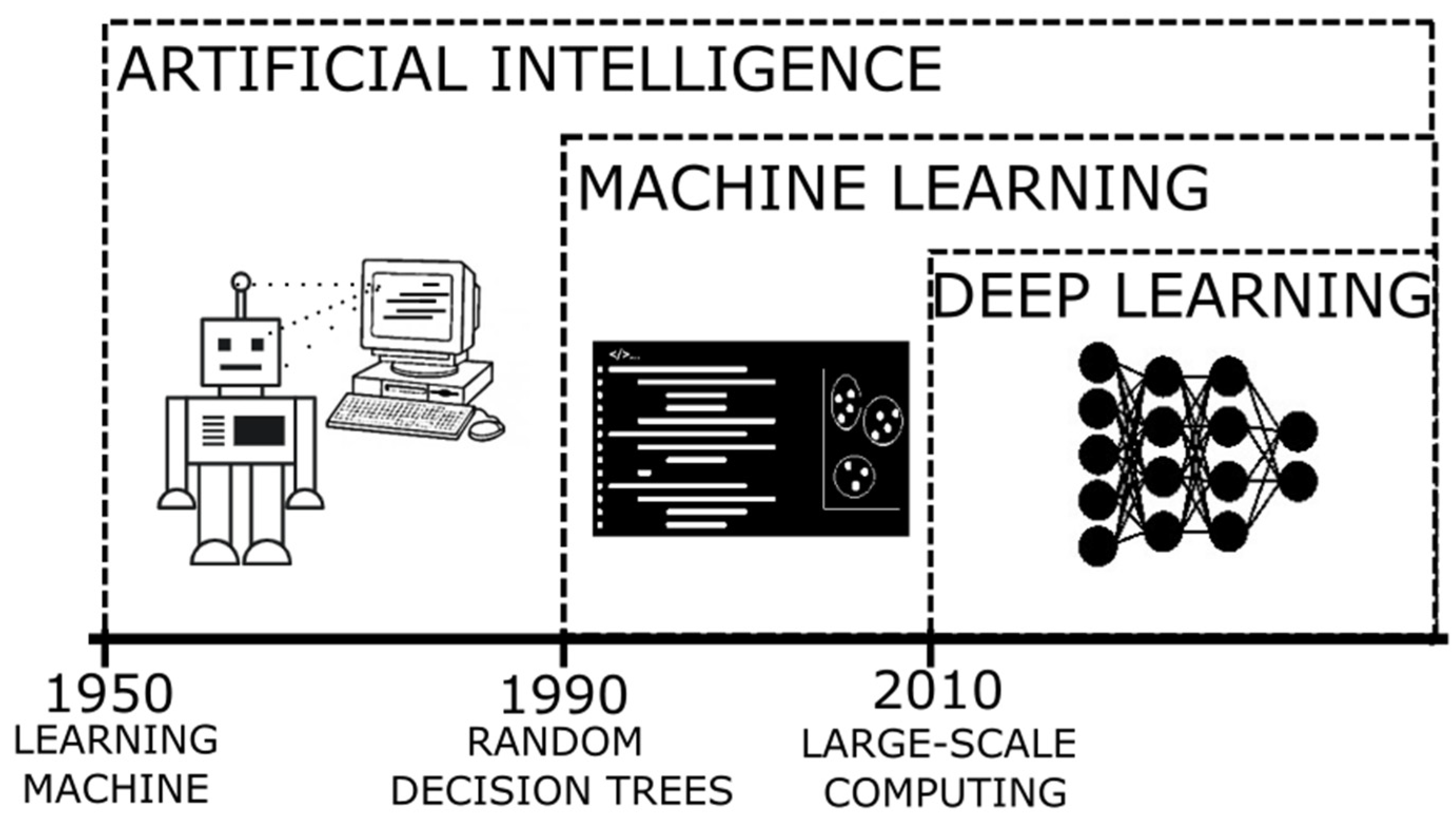
2. Approaches for Developing ML Models in AD Research
2.1. Supervised Training
2.2. Unsupervised Training
2.3. Deep Learning
3. Main Applications of AI in AD Research
3.1. Neuroimaging
3.2. Multimodal Biomarker-Based Studies
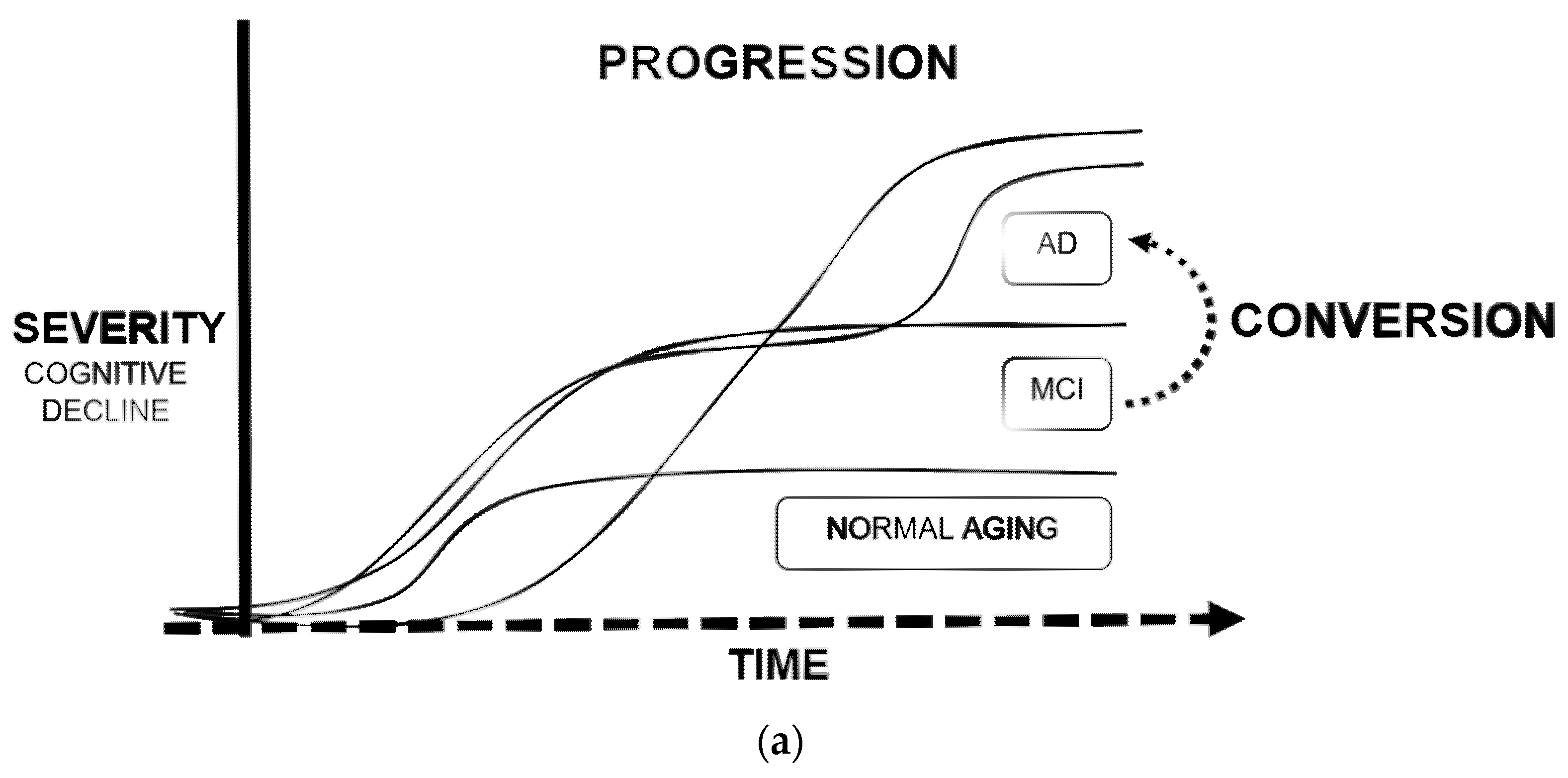
3.3. Conversion and Progression
3.4. Drug Discovery
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Baldeiras, I.; Santana, I.; Leitão, M.J.; Gens, H.; Pascoal, R.; Tábuas-Pereira, M.; Beato-Coelho, J.; Duro, D.; Almeida, M.R.; Oliveira, C.R. Addition of the Aβ42/40 ratio to the cerebrospinal fluid biomarker profile increases the predictive value for underlying Alzheimer’s disease dementia in mild cognitive impairment. Alzheimers Res. Ther. 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia, a systematic review and metaanalysis. Alzheimers Dement. 2013, 9, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Herukka, S.K.; Simonsen, A.H.; Andreasen, N.; Baldeiras, I.; Bjerke, M.; Blennow, K.; Engelborghs, S.; Frisoni, G.B.; Gabryelewicz, T.; Galluzzi, S.; et al. Recommendations for cerebrospinal fluid Alzheimer’s disease biomarkers in the diagnostic evaluation of mild cognitive impairment. Alzheimers Dement. 2017, 13, 285–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellazzi, G.; Cuzzoni, M.G.; Cotta Ramusino, M.; Martinelli, D.; Denaro, F.; Ricciardi, A.; Vitali, P.; Anzalone, N.; Bernini, S.; Palesi, F.; et al. A Machine Learning Approach for the Differential Diagnosis of Alzheimer and Vascular Dementia Fed by MRI Selected Features. Front. Neuroinform. 2020, 14, 25. [Google Scholar] [CrossRef]
- Battaglia, S.; Garofalo, S.; di Pellegrino, G. Context-dependent extinction of threat memories, influences of healthy aging. Sci. Rep. 2018, 8, 12592. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework, Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Pirzada, R.H.; Javaid, N.; Choi, S. The Roles of the NLRP3 Inflammasome in Neurodegenerative and Metabolic Diseases and in Relevant Advanced Therapeutic Interventions. Genes 2020, 11, 131. [Google Scholar] [CrossRef] [Green Version]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories are not written in stone, Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef]
- Martí-Juan, G.; Sanroma-Guell, G.; Piella, G. A survey on machine and statistical learning for longitudinal analysis of neuroimaging data in Alzheimer’s disease. Comput. Methods Programs Biomed. 2020, 189, 105348. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.K.; Anwar, A.; Saleem, S.; Malik, P.; Rasul, B.; Patel, K.; Yao, R.; Seshadri, A.; Yousufuddin, M.; Arumaithurai, K. Artificial intelligence as an emerging technology in the current care of neurological disorders. J. Neurol. 2021, 268, 1623–1642. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Martin, M.; Nevado, A.; Carro, B. Detection of early stages of Alzheimer’s disease based on MEG activity with a randomized convolutional neural network. Artif. Intell. Med. 2020, 107, 101924. [Google Scholar] [CrossRef] [PubMed]
- Mundt, J.C.; Freed, D.M.; Greist, J.H. Lay person-based screening for early detection of Alzheimer’s disease: Development and validation of an instrument. J. Gerontol. B Psychol. Sci. Soc. Sci. 2000, 55, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzmeier, N.; Koutsouleris, N.; Benzinger, T.; Goate, A.; Karch, C.M.; Fagan, A.M.; McDade, E.; Duering, M.; Dichgans, M.; Levin, J.; et al. Predicting sporadic Alzheimer’s disease progression via inherited Alzheimer’s disease-informed machine-learning. Alzheimers Dement. 2020, 16, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, R. Prediction of Alzheimer’s diagnosis using semi-supervised distance metric learning with label propagation. Comput. Biol. Chem. 2008, 32, 438–441. [Google Scholar] [CrossRef]
- Lee, J.; Kumar, S.; Lee, S.Y.; Park, S.J.; Kim, M.H. Development of Predictive Models for Identifying Potential S100A9 Inhibitors Based on Machine Learning Methods. Front Chem. 2019, 7, 779. [Google Scholar] [CrossRef]
- Gao, Y.; Sengupta, A.; Li, M.; Zu, Z.; Rogers, B.P.; Anderson, A.W.; Ding, Z.; Gore, J.C.; Alzheimer’s Disease Neuroimaging Initiative. Functional connectivity of white matter as a biomarker of cognitive decline in Alzheimer’s disease. PLoS ONE 2020, 15, e0240513. [Google Scholar] [CrossRef]
- Capriotti, E.; Calabrese, R.; Casadio, R. Predicting the insurgence of human genetic diseases associated to single point protein mutations with support vector machines and evolutionary information. Bioinformatics 2006, 22, 2729–2734. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Shi, F.; Pu, F.; Li, X.; Jiang, T.; Xie, S.; Wang, Y. Hippocampal shape analysis of Alzheimer disease based on machine learning methods. AJNR Am. J. Neuroradiol. 2007, 28, 1339–1345. [Google Scholar] [CrossRef] [Green Version]
- Noble, W.S. What is a support vector machine? Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.R.; Oommen, A.M.; Varma, S.; Casanova, R.; An, Y.; Andrews, R.M.; O’Brien, R.; Pletnikova, O.; Troncoso, J.C.; Toledo, J.; et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study. PLoS Med. 2018, 15, e1002482. [Google Scholar] [CrossRef]
- Achalia, R.; Sinha, A.; Jacob, A.; Achalia, G.; Kaginalkar, V.; Venkatasubramanian, G.; Rao, N.P. A proof-of-concept machine learning analysis using multimodal neuroimaging and neurocognitive measures as predictive biomarker in bipolar disorder. Asian J. Psychiatr. 2020, 50, 101984. [Google Scholar] [CrossRef]
- Royall, D.R.; Palmer, R.; Mulroy, A.R.; Polk, M.J.; Román, G.C.; David, J.P.; Delacourte, A. Pathological determinants of the transition to clinical dementia in Alzheimer’s disease. Exp. Aging Res. 2002, 28, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, P.; Lou, R.; Zhang, M.Q.; Wu, G.; Qiang, B.; Zhang, Z.; Shen, Y. Gene expression profiling in developing human hippocampus. J. Neurosci. Res. 2002, 70, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Guess, M.J.; Wilson, S.B. Introduction to hierarchical clustering. J. Clin. Neurophysiol. 2002, 19, 144–151. [Google Scholar] [CrossRef]
- Gottfries, J.; Blennow, K.; Lehmann, M.W.; Regland, B.; Gottfries, C.G. One-carbon metabolism and other biochemical correlates of cognitive impairment as visualized by principal component analysis. J. Geriatr. Psychiatry Neurol. 2001, 14, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Growth, D.; Hartmann, S.; Klie, S.; Selbig, J. Principal components analysis. Methods Mol. Biol. 2013, 930, 527–547. [Google Scholar]
- Lombardi, A.; Amoroso, N.; Diacono, D.; Monaco, A.; Logroscino, G.; De Blasi, R.; Bellotti, R.; Tangaro, S. Association between Structural Connectivity and Generalized Cognitive Spectrum in Alzheimer’s Disease. Brain Sci. 2020, 10, 879. [Google Scholar] [CrossRef]
- Ezzati, A.; Zammit, A.R.; Harvey, D.J.; Habeck, C.; Hall, C.B.; Lipton, R.B.; Alzheimer’s Disease Neuroimaging Initiative. Optimizing Machine Learning Methods to Improve Predictive Models of Alzheimer’s Disease. J. Alzheimers Dis. 2019, 71, 1027–1036. [Google Scholar] [CrossRef]
- Geirhos, R.; Jacobsen, J.H.; Michaelis, C.; Michaelis, C.; Zemel, R.; Brendel, W.; Bethge, M.; Wichmann, F.A. Shortcut learning in deep neural networks. Nat. Mach Intell. 2020, 2, 665–673. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Ernesto, C.; Schafer, K.; Coats, M.; Leon, S.; Sano, M.; Thal, L.J.; Woodbury, P. Clinical dementia rating training and reliability in multicenter studies, the Alzheimer’s Disease Cooperative Study experience. Neurology 1997, 48, 1508–1510. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.W.; Veitch, D.P.; Aisen, P.S.; Beckett, L.A.; Cairns, N.J.; Green, R.C.; Harvey, D.; Jack, C.R., Jr.; Jagust, W.; Morris, J.C.; et al. Alzheimer’s Disease Neuroimaging Initiative. The Alzheimer’s Disease Neuroimaging Initiative 3, Continued innovation for clinical trial improvement. Alzheimers Dement. 2017, 13, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Yan, J.; Yao, X.; Kim, S.; Nho, K.; Risacher, S.L.; Saykin, A.J.; Shen, L.; Huang, H. Longitudinal Genotype-Phenotype Association Study through Temporal Structure Auto-Learning Predictive Model. J. Comput. Biol. 2018, 25, 809–824. [Google Scholar] [CrossRef]
- Yang, J.; Feng, X.; Laine, A.F.; Angelini, E.D. Characterizing Alzheimer’s Disease with Image and Genetic Biomarkers Using Supervised Topic Models. IEEE J. Biomed. Health Inform. 2020, 24, 1180–1187. [Google Scholar] [CrossRef]
- Tam, A.; Dansereau, C.; Iturria-Medina, Y.; Urchs, S.; Orban, P.; Sharmarke, H.; Breitner, J.; Bellec, P.; Alzheimer’s Disease Neuroimaging Initiative. A highly predictive signature of cognition and brain atrophy for progression to Alzheimer’s dementia. Gigascience 2019, 8, 055. [Google Scholar] [CrossRef] [Green Version]
- Platero, C.; Lin, L.; Tobar, M.C. Longitudinal Neuroimaging Hippocampal Markers for Diagnosing Alzheimer’s Disease. Neuroinformatics 2019, 17, 43–61. [Google Scholar] [CrossRef]
- Zhou, T.; Thung, K.H.; Zhu, X.; Shen, D. Effective feature learning and fusion of multimodality data using stage-wise deep neural network for dementia diagnosis. Hum. Brain Mapp. 2019, 40, 1001–1016. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Suk, H.I.; Gao, Y.; Lee, S.W.; Shen, D. Leveraging Coupled Interaction for Multimodal Alzheimer’s Disease Diagnosis. IEEE Trans. Neural Netw. Learn. Syst. 2020, 31, 186–200. [Google Scholar] [CrossRef]
- Yao, D.; Calhoun, V.D.; Fu, Z.; Du, Y.; Sui, J. An ensemble learning system for a 4-way classification of Alzheimer’s disease and mild cognitive impairment. J. Neurosci. Methods 2018, 302, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, A.; Harvey, D.J.; Habeck, C.; Golzar, A.; Qureshi, I.A.; Zammit, A.R.; Hyun, J.; Truelove-Hill, M.; Hall, C.B.; Davatzikos, C.; et al. Alzheimer’s Disease Neuroimaging Initiative. Predicting Amyloid-β Levels in Amnestic Mild Cognitive Impairment Using Machine Learning Techniques. J. Alzheimers Dis. 2020, 73, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Lanka, P.; Rangaprakash, D.; Dretsch, M.N.; Katz, J.S.; Denney, T.S., Jr.; Deshpande, G. Supervised machine learning for diagnostic classification from large-scale neuroimaging datasets. Brain Imaging. Behav. 2020, 14, 2378–2416. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Ryu, S.; Qureshi, M.N.I.; Choi, M.; Lee, K.H.; Lee, B. Hybrid multivariate pattern analysis combined with extreme learning machine for Alzheimer’s dementia diagnosis using multi-measure rs-fMRI spatial patterns. PLoS ONE 2019, 14, e0212582. [Google Scholar] [CrossRef]
- Ding, Y.; Sohn, J.H.; Kawczynski, M.G.; Trivedi, H.; Harnish, R.; Jenkins, N.W.; Lituiev, D.; Copeland, T.P.; Aboian, M.S.; Aparici, C.M.; et al. A Deep Learning Model to Predict a Diagnosis of Alzheimer Disease by Using 18F-FDG PET of the Brain. Radiology 2019, 290, 456–464. [Google Scholar] [CrossRef]
- Luckett, P.H.; McCullough, A.; Gordon, B.A.; Strain, J.; Flores, S.; Dincer, A.; McCarthy, J.; Kuffner, T.; Stern, A.; Meeker, K.L.; et al. Dominantly Inherited Alzheimer Network (DIAN). Modeling autosomal dominant Alzheimer’s disease with machine learning. Alzheimers Dement. 2021, 17, 1005–1016. [Google Scholar] [CrossRef]
- Jo, T.; Nho, K.; Risacher, S.L.; Saykin, A.J.; Alzheimer’s Neuroimaging Initiative. Deep learning detection of informative features in tau PET for Alzheimer’s disease classification. BMC Bioinform. 2020, 21, 496. [Google Scholar] [CrossRef]
- Ezzati, A.; Lipton, R.B.; Alzheimer’s Disease Neuroimaging Initiative. Machine Learning Predictive Models Can Improve Efficacy of Clinical Trials for Alzheimer’s Disease. J. Alzheimers Dis. 2020, 74, 55–63. [Google Scholar] [CrossRef]
- van Maurik, I.S.; Vos, S.J.; Bos, I.; Bouwman, F.H.; Teunissen, C.E.; Scheltens, P.; Barkhof, F.; Frolich, L.; Kornhuber, J.; Wiltfang, J.; et al. Alzheimer’s Disease Neuroimaging Initiative. Biomarker-based prognosis for people with mild cognitive impairment (ABIDE), a modelling study. Lancet Neurol. 2019, 18, 1034–1044. [Google Scholar] [CrossRef]
- Li, H.; Habes, M.; Wolk, D.A.; Fan, Y.; Alzheimer’s Disease Neuroimaging Initiative and the Australian Imaging Biomarkers and Lifestyle Study of Aging. A deep learning model for early prediction of Alzheimer’s disease dementia based on hippocampal magnetic resonance imaging data. Alzheimers Dement. 2019, 15, 1059–1070. [Google Scholar] [CrossRef]
- Amoroso, N.; Diacono, D.; Fanizzi, A.; La Rocca, M.; Monaco, A.; Lombardi, A.; Guaragnella, C.; Bellotti, R.; Tangaro, S.; Alzheimer’s Disease Neuroimaging Initiative. Deep learning reveals Alzheimer’s disease onset in MCI subjects, Results from an international challenge. J. Neurosci. Methods 2018, 302, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, X.; Li, S.; Xiao, B.; Li, Y.; Wang, G.; Ma, X. Computer aided Alzheimer’s disease diagnosis by an unsupervised deep learning technology. Neurocomputing 2019, 392, 296–304. [Google Scholar] [CrossRef]
- Vos, S.J.; Verhey, F.; Frölich, L.; Kornhuber, J.; Wiltfang, J.; Maier, W.; Peters, O.; Rüther, E.; Nobili, F.; Morbelli, S.; et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain 2015, 138, 1327–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abate, G.; Vezzoli, M.; Polito, L.; Guaita, A.; Albani, D.; Marizzoni, M.; Garrafa, E.; Marengoni, A.; Forloni, G.; Frisoni, G.B.; et al. A Conformation Variant of p53 Combined with Machine Learning Identifies Alzheimer Disease in Preclinical and Prodromal Stages. J. Pers. Med. 2020, 26, 14. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chiu, S.I.; Chen, T.F.; Jang, J.R.; Chiu, M.J. Classifications of Neurodegenerative Disorders Using a Multiplex Blood Biomarkers-Based Machine Learning Model. Int. J. Mol. Sci 2020, 21, 6914. [Google Scholar] [CrossRef]
- Palmqvist, S.; Tideman, P.; Cullen, N.; Zetterberg, H.; Blennow, K.; Alzheimer’s Disease Neuroimaging Initiative; Dage, J.L.; Stomrud, E.; Janelidze, S.; Mattsson-Carlgren, N.; et al. Prediction of future Alzheimer’s disease dementia using plasma phospho-tau combined with other accessible measures. Nat. Med. 2021, 27, 1034–1042. [Google Scholar] [CrossRef]
- Ashton, N.J.; Janelidze, S.; Al Khleifat, A.; Leuzy, A.; van der Ende, E.L.; Karikari, T.K.; Benedet, A.L.; Pascoal, T.A.; Lleó, A.; Parnetti, L.; et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat. Commun. 2021, 12, 3400. [Google Scholar] [CrossRef]
- Beltrán, J.F.; Wahba, B.M.; Hose, N.; Shasha, D.; Kline, R.P.; Alzheimer’s Disease Neuroimaging Initiative. Inexpensive, non-invasive biomarkers predict Alzheimer transition using machine learning analysis of the Alzheimer’s Disease Neuroimaging (ADNI) database. PLoS ONE 2020, 15, e0235663. [Google Scholar] [CrossRef]
- Peng, B.; Yao, X.; Risacher, S.L.; Saykin, A.J.; Shen, L.; Ning, X. Cognitive biomarker prioritization in Alzheimer’s Disease using brain morphometric data. BMC Med. Inform. Decis Mak. 2020, 20, 319. [Google Scholar] [CrossRef]
- Yao, T.; Sweeney, E.; Nagorski, J.; Shulman, J.M.; Allen, G.I. Quantifying cognitive resilience in Alzheimer’s Disease, The Alzheimer’s Disease Cognitive Resilience Score. PLoS ONE 2020, 15, e0241707. [Google Scholar] [CrossRef]
- Nunes, A.; Silva, G.; Duque, C.; Januário, C.; Santana, I.; Ambrósio, A.F.; Castelo-Branco, M.; Bernardes, R. Retinal texture biomarkers may help to discriminate between Alzheimer’s; Parkinson’s; and healthy controls. PLoS ONE 2019, 14, e0218826. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.; Perna, G.; Caldirola, D.; Schruers, K.; Duara, R.; Loewenstein, D.A. A Clinically-Translatable Machine Learning Algorithm for the Prediction of Alzheimer’s Disease Conversion in Individuals with Mild and Premild Cognitive Impairment. J. Alzheimers Dis. 2018, 61, 1555–1573. [Google Scholar] [CrossRef]
- Grassi, M.; Loewenstein, D.A.; Caldirola, D.; Schruers, K.; Duara, R.; Perna, G. A clinically-translatable machine learning algorithm for the prediction of Alzheimer’s disease conversion, further evidence of its accuracy via a transfer learning approach. Int. Psychogeriatr. 2019, 31, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.; Rouleaux, N.; Caldirola, D.; Loewenstein, D.; Schruers, K.; Perna, G.; Dumontier, M.; Alzheimer’s Disease Neuroimaging Initiative. A Novel Ensemble-Based Machine Learning Algorithm to Predict the Conversion from Mild Cognitive Impairment to Alzheimer’s Disease Using Socio-Demographic Characteristics; Clinical Information; and Neuropsychological Measures. Front. Neurol. 2019, 10, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, S.; Domingo-Fernández, D.; Iyappan, A.; Emon, M.A.; Hofmann-Apitius, M.; Fröhlich, H. Using Multi-Scale Genetic; Neuroimaging and Clinical Data for Predicting Alzheimer’s Disease and Reconstruction of Relevant Biological Mechanisms. Sci. Rep. 2018, 8, 11173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moscoso, A.; Silva-Rodríguez, J.; Aldrey, J.M.; Cortés, J.; Fernández-Ferreiro, A.; Gómez-Lado, N.; Ruibal, Á.; Aguiar, P.; Alzheimer’s Disease Neuroimaging Initiative. Prediction of Alzheimer’s disease dementia with MRI beyond the short-term, Implications for the design of predictive models. Neuroimage Clin. 2019, 23, 101837. [Google Scholar] [CrossRef]
- Pusil, S.; Dimitriadis, S.I.; López, M.E.; Pereda, E.; Maestú, F. Aberrant MEG multi-frequency phase temporal synchronization predicts conversion from mild cognitive impairment-to-Alzheimer’s disease. Neuroimage Clin. 2019, 24, 101972. [Google Scholar] [CrossRef]
- Spasov, S.; Passamonti, L.; Duggento, A.; Liò, P.; Toschi, N.; Alzheimer’s Disease Neuroimaging Initiative. A parameter-efficient deep learning approach to predict conversion from mild cognitive impairment to Alzheimer’s disease. Neuroimage 2019, 189, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Somer, E.; Grau, V. Classification of amyloid PET images using novel features for early diagnosis of Alzheimer’s disease and mild cognitive impairment conversion. Nucl. Med. Commun. 2019, 40, 242–248. [Google Scholar] [CrossRef]
- Skolariki, K.; Terrera, G.M.; Danso, S. Multivariate Data Analysis and Machine Learning for Prediction of MCI-to-AD Conversion. Adv. Exp. Med. Biol. 2020, 1194, 81–103. [Google Scholar]
- Bhagwat, N.; Viviano, J.D.; Voineskos, A.N.; Chakravarty, M.M.; Alzheimer’s Disease Neuroimaging Initiative. Modeling and prediction of clinical symptom trajectories in Alzheimer’s disease using longitudinal data. PLoS Comput. Biol. 2018, 14, e1006376. [Google Scholar] [CrossRef] [PubMed]
- Geifman, N.; Kennedy, R.E.; Schneider, L.S.; Buchan, I.; Brinton, R.D. Data-driven identification of endophenotypes of Alzheimer’s disease progression, implications for clinical trials and therapeutic interventions. Alzheimers Res. Ther. 2018, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, J.W.; Vachon-Presseau, E.; Pichet Binette, A.; Tam, A.; Orban, P.; La Joie, R.; Savard, M.; Picard, C.; Poirier, J.; Bellec, P.; et al. Alzheimer’s Disease Neuroimaging Initiative* and the PREVENT-AD Research Group. Brain properties predict proximity to symptom onset in sporadic Alzheimer’s disease. Brain 2018, 141, 1871–1883. [Google Scholar] [CrossRef]
- Fisher, C.K.; Smith, A.M.; Walsh, J.R. Machine learning for comprehensive forecasting of Alzheimer’s Disease progression. Sci. Rep. 2019, 9, 13622. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhang, D.; Shen, D.; Liu, M. Multi-task exclusive relationship learning for Alzheimer’s disease progression prediction with longitudinal data. Med. Image Anal. 2019, 53, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Albright, J. Forecasting the progression of Alzheimer’s disease using neural networks and a novel preprocessing algorithm. Alzheimers Dement. 2019, 5, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, J.; Landau, S.M.; Jagust, W.J.; Tino, P.; Kourtzi, Z.; Alzheimer’s Disease Neuroimaging Initiative. Modelling prognostic trajectories of cognitive decline due to Alzheimer’s disease. Neuroimage Clin. 2020, 26, 102199. [Google Scholar] [CrossRef]
- Tabarestani, S.; Aghili, M.; Eslami, M.; Cabrerizo, M.; Barreto, A.; Rishe, N.; Curiel, R.E.; Loewenstein, D.; Duara, R.; Adjouadi, M. A distributed multitask multimodal approach for the prediction of Alzheimer’s disease in a longitudinal study. Neuroimage 2020, 206, 116317. [Google Scholar] [CrossRef]
- Thabtah, F.; Spencer, R.; Ye, Y. The correlation of everyday cognition test scores and the progression of Alzheimer’s disease, a data analytics study. Health Inf. Sci. Syst. 2020, 8, 24. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Ren, F.; Kong, J. Group Guided Fused Laplacian Sparse Group Lasso for Modeling Alzheimer’s Disease Progression. Comput. Math. Methods Med. 2020, 2020, 4036560. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Z.; Zhu, Y.; Pettigrew, C.; Soldan, A.; Gross, A.; Albert, M. AD risk score for the early phases of disease based on unsupervised machine learning. Alzheimers Dement. 2020, 16, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Saif, N.; Yan, P.; Niotis, K.; Scheyer, O.; Rahman, A.; Berkowitz, M.; Krikorian, R.; Hristov, H.; Sadek, G.; Bellara, S.; et al. Feasibility of Using a Wearable Biosensor Device in Patients at Risk for Alzheimer’s Disease Dementia. J. Prev. Alzheimers Dis. 2020, 7, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.B.; Jenkins, N.; Dolan, C.; Pullen, H.; Ritchie, C.; Muniz-Terrera, G. Reliability of Telephone and Videoconference Methods of Cognitive Assessment in Older Adults with and without Dementia. J. Alzheimers Dis. 2021, 81, 1625–1647. [Google Scholar] [CrossRef] [PubMed]
- Muurling, M.; de Boer, C.; Kozak, R.; Religa, D.; Koychev, I.; Verheij, H.; Nies, V.J.M.; Duyndam, A.; Sood, M.; Fröhlich, H.; et al. RADAR-AD Consortium. Remote monitoring technologies in Alzheimer’s disease, design of the RADAR-AD study. Alzheimers Res. Ther. 2021, 13, 89. [Google Scholar] [CrossRef]
- van Oudenhoven, F.M.; Swinkels, S.H.N.; Soininen, H.; Kivipelto, M.; Hartmann, T.; Rizopoulos, D.; LipiDiDiet Clinical Study Group. A competing risk joint model for dealing with different types of missing data in an intervention trial in prodromal Alzheimer’s disease. Alzheimers Res. Ther. 2021, 13, 63. [Google Scholar] [CrossRef]
- Kovacs, G.G. Clinical stratification of subtypes of Alzheimer’s disease. Lancet Neurol. 2012, 11, 839–841. [Google Scholar] [CrossRef]
- Schultz, K.J.; Colby, S.M.; Yesiltepe, Y.; Nuñez, J.R.; McGrady, M.Y.; Renslow, R.S. Application and assessment of deep learning for the generation of potential NMDA receptor antagonists. Phys. Chem. Chem. Phys. 2021, 23, 1197–1214. [Google Scholar] [CrossRef]
- Vignaux, P.A.; Minerali, E.; Foil, D.H.; Puhl, A.C.; Ekins, S. Machine Learning for Discovery of GSK3β Inhibitors. ACS Omega 2020, 5, 26551–26561. [Google Scholar] [CrossRef]
- Vignaux, P.A.; Minerali, E.; Lane, T.R.; Foil, D.H.; Madrid, P.B.; Puhl, A.C.; Ekins, S. The Antiviral Drug Tilorone Is a Potent and Selective Inhibitor of Acetylcholinesterase. Chem. Res. Toxicol. 2021, 34, 1296–1307. [Google Scholar] [CrossRef]
- Zeng, X.; Zhu, S.; Liu, X.; Zhou, Y.; Nussinov, R.; Cheng, F. deepDR, a network-based deep learning approach to in silico drug repositioning. Bioinformatics 2019, 35, 5191–5198. [Google Scholar] [CrossRef]
- Fernández-Martínez, J.L.; Álvarez-Machancoses, Ó.; de Andrés-Galiana, E.J.; Bea, G.; Kloczkowski, A. Robust Sampling of Defective Pathways in Alzheimer’s Disease. Implications in Drug Repositioning. Int. J. Mol. Sci. 2020, 21, 3594. [Google Scholar] [CrossRef] [PubMed]
- Anastasio, T.J. Exploring the Correlation between the Cognitive Benefits of Drug Combinations in a Clinical Database and the Efficacies of the Same Drug Combinations Predicted from a Computational Model. J. Alzheimers Dis. 2019, 70, 287–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Xie, L. TranSynergy, Mechanism-driven interpretable deep neural network for the synergistic prediction and pathway deconvolution of drug combinations. PLoS Comput. Biol. 2021, 17, e1008653. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Hase, T.; Yachie-Kinoshita, A.; Nishino, T.; Ghosh, S.; Kikuchi, M.; Shimokawa, K.; Aburatani, H.; Kitano, H.; Tanaka, H. Artificial intelligence-based computational framework for drug-target prioritization and inference of novel repositionable drugs for Alzheimer’s disease. Alzheimers Res. Ther. 2021, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Tian, L.P.; Ruan, J.; Wu, F.X. Disease Gene Prediction by Integrating PPI Networks; Clinical RNA-Seq Data and OMIM Data. IEEE/ACM Trans Comput. Biol. Bioinform. 2019, 16, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, G.; Comi, G.P.; Corti, S. Advancing Drug Discovery for Neurological Disorders Using iPSC-Derived Neural Organoids. Int. J. Mol. Sci. 2021, 22, 2659. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Salloway, S.; Farlow, M.; McDade, E.; Clifford, D.B.; Wang, G.; Llibre-Guerra, J.J.; Hitchcock, J.M.; Mills, S.L.; Santacruz, A.M.; Aschenbrenner, A.J.; et al. Dominantly Inherited Alzheimer Network–Trials Unit. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nat. Med. 2021, 27, 1187–1196. [Google Scholar] [CrossRef]
- Bruce, P.; Bruce, A.; Gedeck, P. Practical Statistics for Data Scientists, 2nd ed.; O’Reilly: Sebastopol, CA, USA, 2020; pp. 195–229. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Spínola, A.; Baldeiras, I.; Arrais, J.P.; Santana, I. The Road to Personalized Medicine in Alzheimer’s Disease: The Use of Artificial Intelligence. Biomedicines 2022, 10, 315. https://doi.org/10.3390/biomedicines10020315
Silva-Spínola A, Baldeiras I, Arrais JP, Santana I. The Road to Personalized Medicine in Alzheimer’s Disease: The Use of Artificial Intelligence. Biomedicines. 2022; 10(2):315. https://doi.org/10.3390/biomedicines10020315
Chicago/Turabian StyleSilva-Spínola, Anuschka, Inês Baldeiras, Joel P. Arrais, and Isabel Santana. 2022. "The Road to Personalized Medicine in Alzheimer’s Disease: The Use of Artificial Intelligence" Biomedicines 10, no. 2: 315. https://doi.org/10.3390/biomedicines10020315
APA StyleSilva-Spínola, A., Baldeiras, I., Arrais, J. P., & Santana, I. (2022). The Road to Personalized Medicine in Alzheimer’s Disease: The Use of Artificial Intelligence. Biomedicines, 10(2), 315. https://doi.org/10.3390/biomedicines10020315





