Gene Expression Analysis of Aggressive Adult Xp11.2 Translocation Renal Cell Carcinoma at Clinical Stage T1N0M0 to Identify Potential Prognostic and Therapeutic Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Tissues
2.2. Tissue Preparation
2.3. RNA Extraction and Sequencing
2.4. RNA Sequencing (RNA-Seq) Analysis and Identification of Differentially Expressed Genes
2.5. Fusion Type Analysis
2.6. KEGG Analysis
2.7. Xp11.2 tRCC Cell Lines
2.8. Treatment Regimens
2.9. Cell Proliferation Assay
2.10. Reverse Transcription-qPCR Analysis
2.11. Wound-Healing Assay
2.12. Invasion Assay
2.13. Western Blot Analysis
2.14. Statistical Analysis
3. Results
3.1. Baseline Characteristics
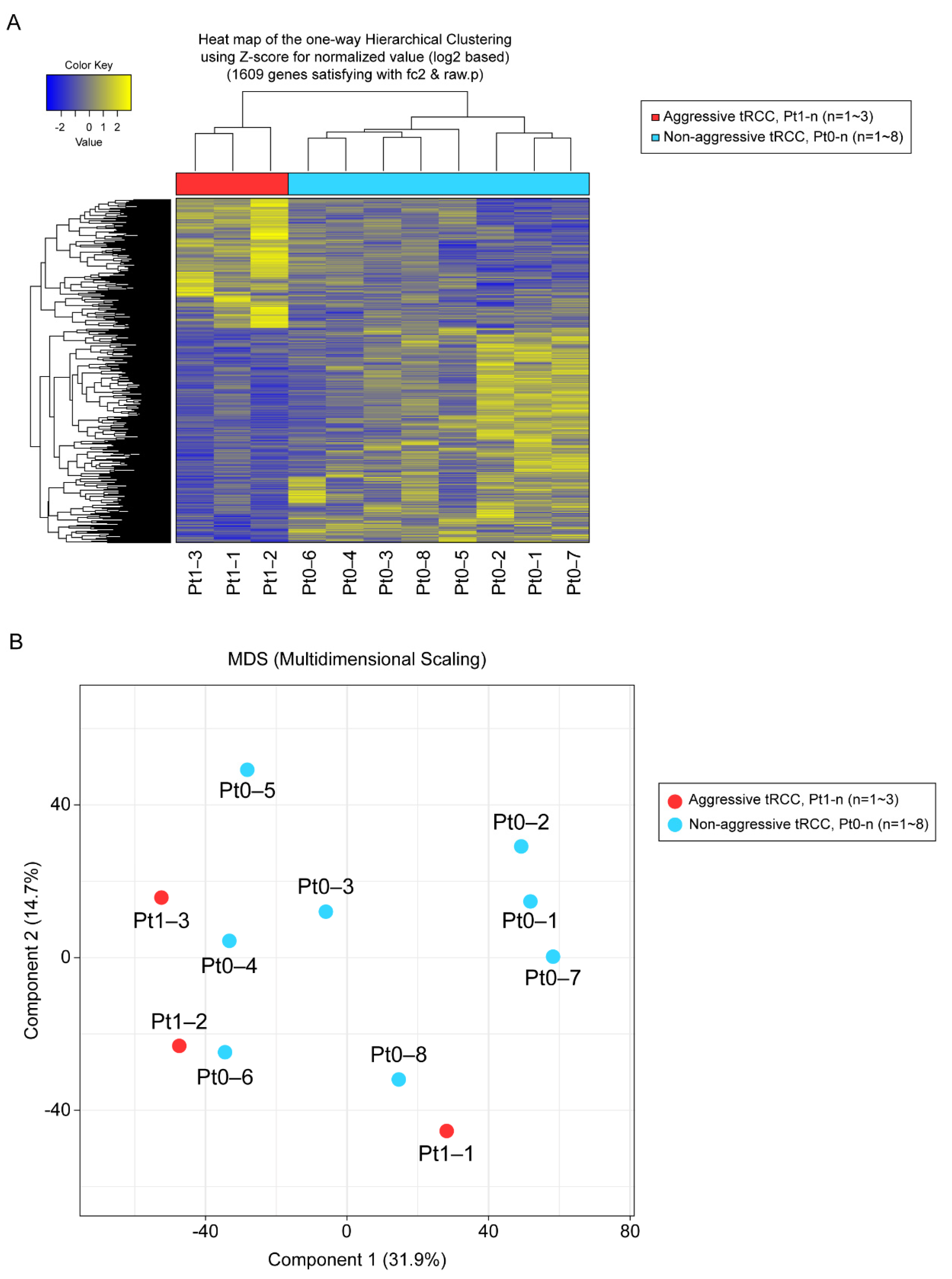
3.2. RNA-Seq Analysis
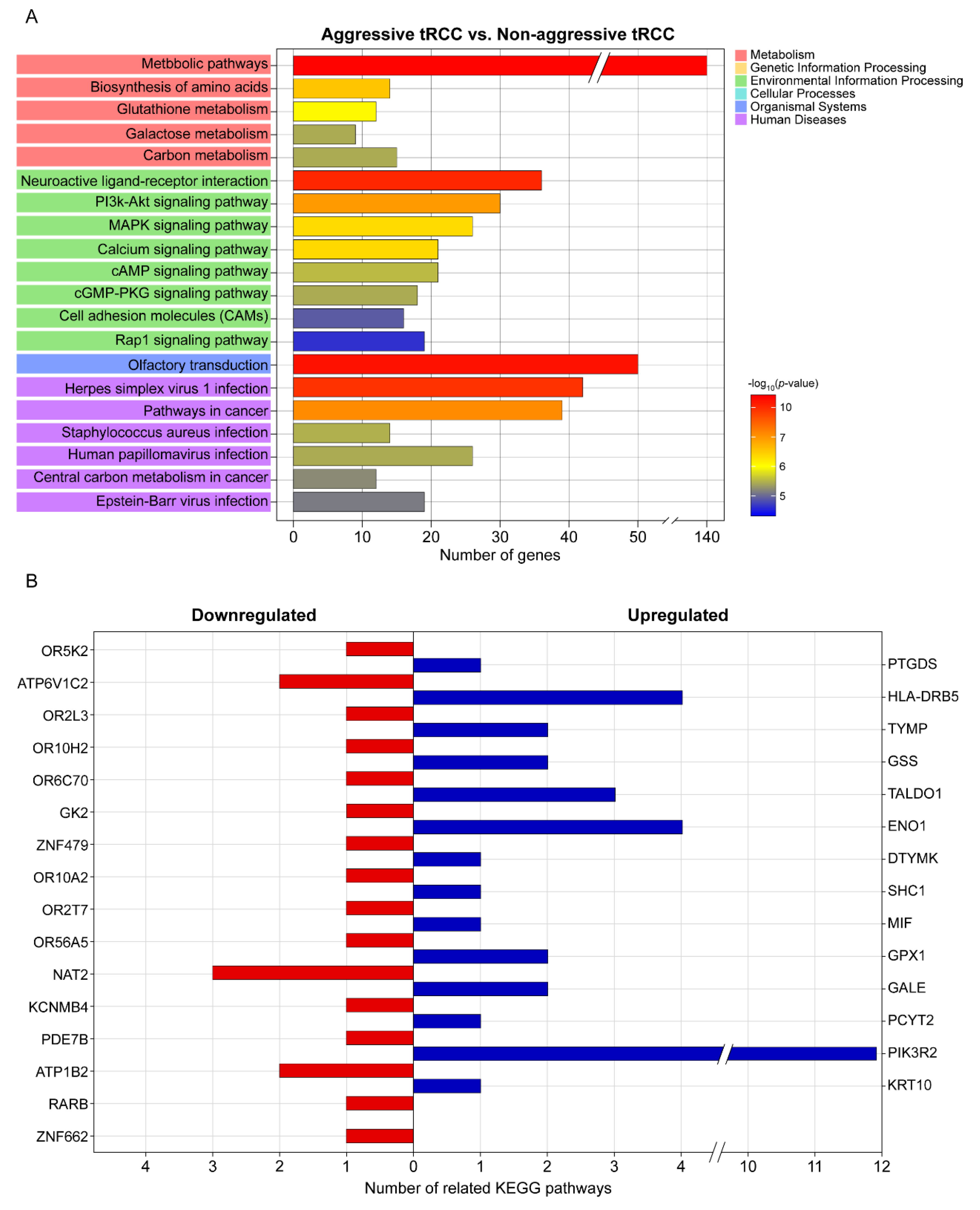
3.3. DEGs, Fusion Types, and KEGG Pathway Enrichment
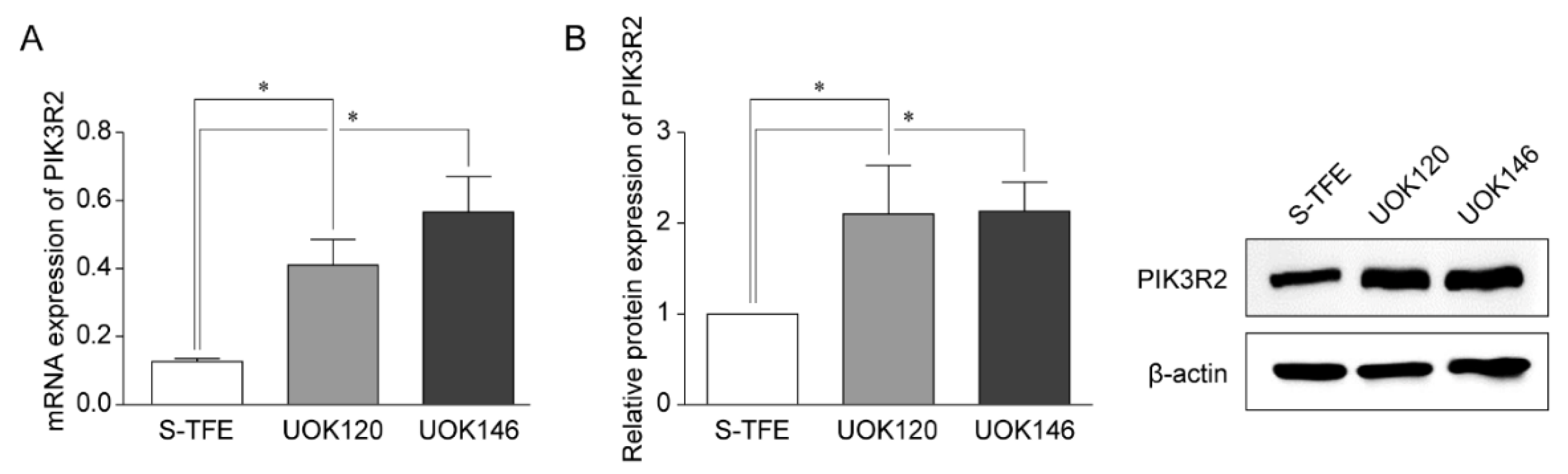
3.4. PIK3R2 Expression in tRCC Cell Lines
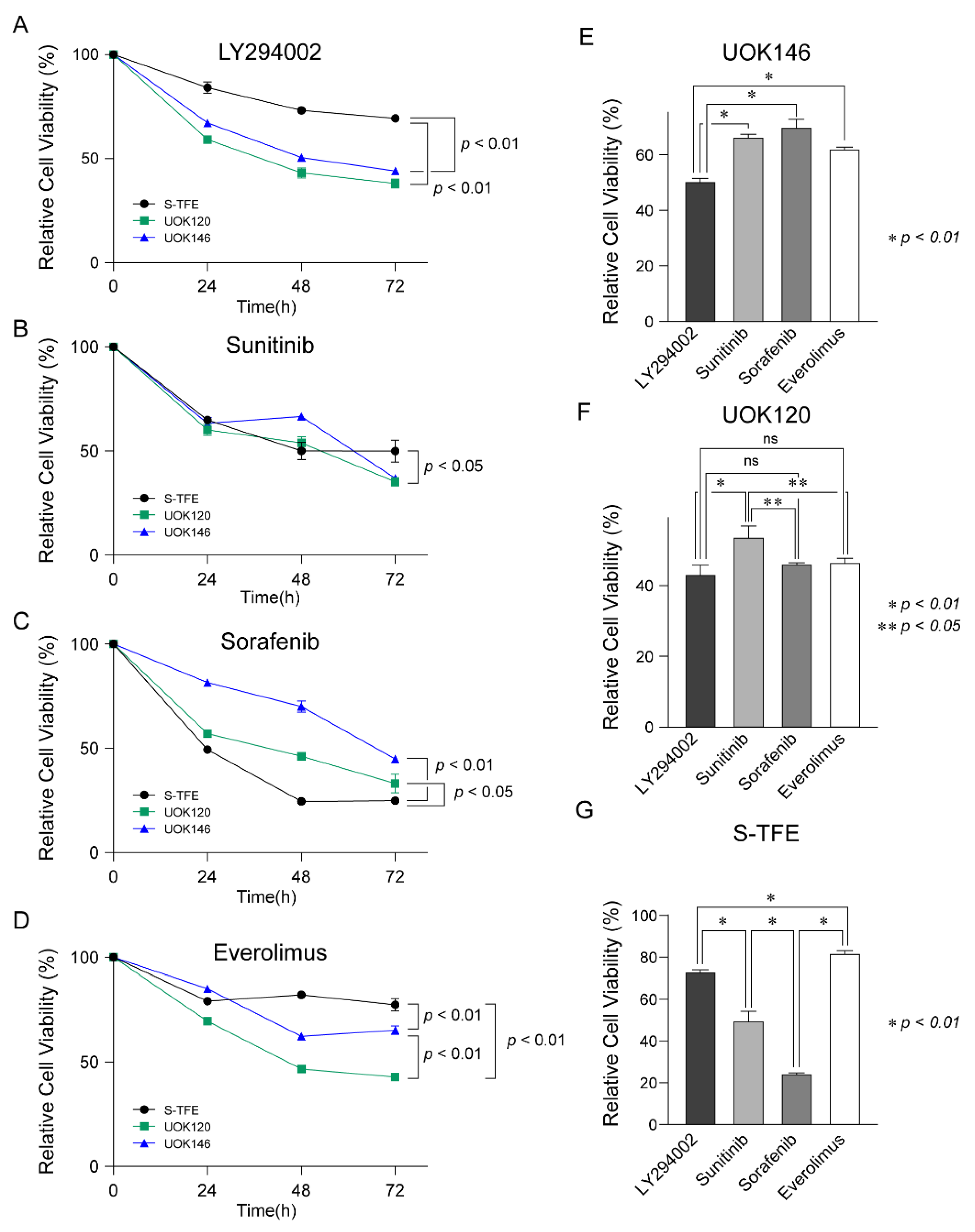
3.5. Effects of Various Treatments on tRCC Cell Proliferation
3.6. Effects of LY294002 on the Migration and Invasion Ability of tRCC Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solano, C.; Thapa, S.; Chisti, M.M. Adult Xp11.2 translocation renal cell carcinoma managed effectively with pazopanib. BMJ Case Rep. CP 2021, 14, e243058. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Beltran, A.; Scarpelli, M.; Montironi, R.; Kirkali, Z. 2004 WHO classification of the renal tumors of the adults. Eur. Urol. 2006, 49, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: Renal, penile, and testicular tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Argani, P.; Ladanyi, M. Renal carcinomas associated with Xp11.2 translocations/TFE3 gene fusions. In World Health Organization Classification of Tumours; Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs; Eble, J.N., Sauter, G., Epstein, J.I., Sesterhenn, I., Eds.; IARC Press: Lyon, France, 2004; pp. 37–38. [Google Scholar]
- Selle, B.; Furtwängler, R.; Graf, N.; Kaatsch, P.; Bruder, E.; Leuschner, I. Population-based study of renal cell carcinoma in children in Germany, 1980–2005: More frequently localized tumors and underlying disorders compared with adult counterparts. Cancer 2006, 107, 2906–2914. [Google Scholar] [CrossRef] [PubMed]
- Komai, Y.; Fujiwara, M.; Fujii, Y.; Mukai, H.; Yonese, J.; Kawakami, S.; Yamamoto, S.; Migita, T.; Ishikawa, Y.; Kurata, M.; et al. Adult Xp11 translocation renal cell carcinoma diagnosed by cytogenetics and immunohistochemistry. Clin. Cancer Res. 2009, 15, 1170–1176. [Google Scholar] [CrossRef] [Green Version]
- Zhong, M.; De Angelo, P.; Osborne, L.; Paniz-Mondolfi, A.E.; Geller, M.; Yang, Y.; Linehan, W.M.; Merino, M.J.; Cordon-Cardo, C.; Cai, D. Translocation renal cell carcinomas in adults: A single-institution experience. Am. J. Surg. Pathol. 2012, 36, 654–662. [Google Scholar] [CrossRef] [Green Version]
- Argani, P.; Olgac, S.; Tickoo, S.K.; Goldfischer, M.; Moch, H.; Chan, D.Y.; Eble, J.N.; Bonsib, S.M.; Jimeno, M.; Lloreta, J.; et al. Xp11 translocation renal cell carcinoma in adults: Expanded clinical, pathologic, and genetic spectrum. Am. J. Surg. Pathol. 2007, 31, 1149–1160. [Google Scholar] [CrossRef]
- Wang, X.T.; Xia, Q.Y.; Ye, S.B.; Wang, X.; Li, R.; Fang, R.; Shi, S.S.; Zhang, R.S.; Tan, X.; Chen, J.Y.; et al. RNA sequencing of Xp11 translocation-associated cancers reveals novel gene fusions and distinctive clinicopathologic correlations. Mod. Pathol. 2018, 31, 1346–1360. [Google Scholar] [CrossRef]
- Ye, C.; Wang, J.; Li, W.; Yuan, M.; Chai, Y. Effective treatment of advanced alveolar soft part sarcoma with sunitinib: A case report. Medicine 2018, 97, e13584. [Google Scholar] [CrossRef]
- Sidhar, S.K.; Clark, J.; Gill, S.; Hamoudi, R.; Crew, A.J.; Gwilliam, R.; Ross, M.; Linehan, W.M.; Birdsall, S.; Shipley, J.; et al. The t(X;1)(P11.2;Q21.2) translocation in papillary renal cell carcinoma fuses a novel gene PRCC to the TFE3 transcription factor gene. Hum. Mol. Genet. 1996, 5, 1333–1338. [Google Scholar] [CrossRef]
- Hirobe, M.; Masumori, N.; Tanaka, T.; Kitamura, H.; Tsukamoto, T. Establishment of an ASPL-TFE3 renal cell carcinoma cell line (S-TFE). Cancer Biol. Ther. 2013, 14, 502–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Liu, N.; Gan, W.; Li, X.; Zhang, G.; Li, D.; Guo, H. Postoperative recurrence of adult renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion. J. Int. Med. Res. 2017, 45, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Sukov, W.R.; Hodge, J.C.; Lohse, C.M.; Leibovich, B.C.; Thompson, R.H.; Pearce, K.E.; Wiktor, A.E.; Cheville, J.C. TFE3 rearrangements in adult renal cell carcinoma: Clinical and pathologic features with outcome in a large series of consecutively treated patients. Am. J. Surg. Pathol. 2012, 36, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Geller, J.I.; Argani, P.; Adeniran, A.; Hampton, E.; De Marzo, A.; Hicks, J.; Collins, M.H. Translocation renal cell carcinoma: Lack of negative impact due to lymph node spread. Cancer 2008, 112, 1607–1616. [Google Scholar] [CrossRef]
- Asaki, H.E.; Moshero, G.; Stanton, M.L.; Humphreys, M.R. Xp11.2 translocation tumor: A rare cause of gross hematuria. J. Am. Acad. Physician Assist. 2014, 27, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, N.; Mikami, S.; Pan, C.C.; Cohen, R.J.; Hes, O.; Michal, M.; Nagashima, Y.; Tanaka, Y.; Inoue, K.; Shuin, T.; et al. Review of renal carcinoma associated with Xp11.2 translocations/TFE3 gene fusions with focus on pathobiological aspect. Histol. Histopathol 2012, 27, 133–140. [Google Scholar] [CrossRef]
- Aoyagi, T.; Shinohara, N.; Kubota-Chikai, K.; Kuroda, N.; Nonomura, K. Long-term survival in a patient with node-positive adult-onset Xp11.2 translocation renal cell carcinoma. Urol. Int. 2011, 86, 487–490. [Google Scholar] [CrossRef] [Green Version]
- Choueiri, T.K.; Lim, Z.D.; Hirsch, M.S.; Tamboli, P.; Jonasch, E.; McDermott, D.F.; Dal Cin, P.; Corn, P.; Vaishampayan, U.; Heng, D.Y.; et al. Vascular endothelial growth factor-targeted therapy for the treatment of adult metastatic Xp11.2 translocation renal cell carcinoma. Cancer 2010, 116, 5219–5225. [Google Scholar] [CrossRef] [PubMed]
- Rua Fernández, O.R.; Escala Cornejo, R.; Navarro Martín, M.; García Muñoz, M.; Antunez Plaza, P.; García Dominguez, A.R.; Cruz Hernández, J.J. Renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene-fusion: A long response to mammalian target of rapamycin (mTOR) inhibitors. Urology 2018, 117, 41–43. [Google Scholar] [CrossRef]
- Malouf, G.G.; Camparo, P.; Oudard, S.; Schleiermacher, G.; Theodore, C.; Rustine, A.; Dutcher, J.; Billemont, B.; Rixe, O.; Bompas, E.; et al. Targeted agents in metastatic Xp11 translocation/TFE3 gene fusion renal cell carcinoma (RCC): A report from the juvenile RCC network. Ann. Oncol. 2010, 21, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szczylik, C.; Kim, S.T.; et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Liu, Q.; Wu, F.; Liu, N.; Qu, H.; Yuan, Y.; Li, Y.; Gao, H.; Ge, J.; Xu, Y.; et al. Prognostic significance of PI3K/AKT/mTOR signaling pathway members in clear cell renal cell carcinoma. PeerJ 2020, 8, e9261. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhao, R.; Wang, H.; Chang, K.; Yang, X.; Zhou, X.; Dai, B.; Zhu, Y.; Shi, G.; Zhang, H.; et al. Phosphorylated 4EBP1 is associated with tumor progression and poor prognosis in Xp11.2 translocation renal cell carcinoma. Sci. Rep. 2016, 6, 23594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Li, D.; Gu, Y.; Wen, Z.M.; Jie, J.; Zhao, D.; Peng, L.P. MicroRNA-126 Targeting PIK3R2 inhibits NSCLC A549 cell proliferation, migration, and invasion by regulation of PTEN/PI3K/AKT pathway. Clin. Lung Cancer 2016, 17, e65–e75. [Google Scholar] [CrossRef]
- Vallejo-Díaz, J.; Chagoyen, M.; Olazabal-Morán, M.; González-García, A.; Carrera, A.C. The opposing roles of PIK3R1/p85α and PIK3R2/p85β in cancer. Trends Cancer 2019, 5, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Kato, I.; Furuya, M.; Baba, M.; Kameda, Y.; Yasuda, M.; Nishimoto, K.; Oyama, M.; Yamasaki, T.; Ogawa, O.; Niino, H.; et al. RBM10-TFE3 renal cell carcinoma characterised by paracentric inversion with consistent closely split signals in break-apart fluorescence in-situ hybridisation: Study of 10 cases and a literature review. Histopathology 2019, 75, 254–265. [Google Scholar] [CrossRef]
- Argani, P.; Zhong, M.; Reuter, V.E.; Fallon, J.T.; Epstein, J.I.; Netto, G.J.; Antonescu, C.R. TFE3-fusion variant analysis defines specific clinicopathologic associations among Xp11 translocation cancers. Am. J. Surg. Pathol. 2016, 40, 723–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caliò, A.; Segala, D.; Munari, E.; Brunelli, M.; Martignoni, G. MiT family translocation renal cell carcinoma: From the early descriptions to the current knowledge. Cancers 2019, 11, 1110. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Gu, C.; Wang, H.; Chang, K.; Yang, X.; Zhou, X.; Dai, B.; Zhu, Y.; Shi, G.; Zhang, H.; et al. Diagnosis of adults Xp11.2 translocation renal cell carcinoma by immunohistochemistry and FISH assays: Clinicopathological data from ethnic Chinese population. Sci. Rep. 2016, 6, 21677. [Google Scholar] [CrossRef] [Green Version]
- Kuthi, L.; Somorácz, Á.; Micsik, T.; Jenei, A.; Hajdu, A.; Sejben, I.; Imre, D.; Pósfai, B.; Kóczián, K.; Semjén, D.; et al. Clinicopathological findings on 28 cases with Xp11.2 renal cell carcinoma. Pathol. Oncol. Res. 2020, 26, 2123–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S.; Pierorazio, P.M.; Lee, J.H.; Lee, H.J.; Lim, Y.S.; Jang, W.S.; Kim, J.; Lee, S.H.; Rha, K.H.; Cho, N.H.; et al. Gene expression analysis of aggressive clinical T1 stage clear cell renal cell carcinoma for identifying potential diagnostic and prognostic biomarkers. Cancers 2020, 12, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakoki, K.; Miyata, Y.; Mochizuki, Y.; Iwata, T.; Obatake, M.; Abe, K.; Nagayasu, T.; Sakai, H. Long-term treatment with sequential molecular targeted therapy for Xp11.2 translocation renal cell carcinoma: A case report and review of the literature. Clin. Genitourin. Cancer 2017, 15, e503–e506. [Google Scholar] [CrossRef] [PubMed]
- Heyer, E.E.; Deveson, I.W.; Wooi, D.; Selinger, C.I.; Lyons, R.J.; Hayes, V.M.; O’Toole, S.A.; Ballinger, M.L.; Gill, D.; Thomas, D.M.; et al. Diagnosis of fusion genes using targeted RNA sequencing. Nat. Commun. 2019, 10, 1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massari, F.; Mollica, V.; Rizzo, A.; Cosmai, L.; Rizzo, M.; Porta, C. Safety evaluation of immune-based combinations in patients with advanced renal cell carcinoma: A systematic review and meta-analysis. Expert Opin. Drug Saf. 2020, 19, 1329–1338. [Google Scholar] [CrossRef]
- Massari, F.; Rizzo, A.; Mollica, V.; Rosellini, M.; Marchetti, A.; Ardizzoni, A.; Santoni, M. Immune-based combinations for the treatment of metastatic renal cell carcinoma: A meta-analysis of randomised clinical trials. Eur. J. Cancer 2021, 154, 120–127. [Google Scholar] [CrossRef]

| Aggressive (Y/N) | Patient ID | Sex | Age (year) | Tumor Size (cm) | WHO/ISUP Grade | Outcome a | Recurrence-Free Time b (m) | Recurrence Site | Death (Y/N) | Survival Time c (m) |
|---|---|---|---|---|---|---|---|---|---|---|
| N | Pt0-1 | F | 21 | 2.5 | 2 | ned | N | 111 | ||
| N | Pt0-2 | F | 41 | 3.9 | 3 | ned | N | 112 | ||
| N | Pt0-3 | M | 56 | 2.2 | 3 | ned | N | 97 | ||
| N | Pt0-4 | F | 73 | 4.9 | 2 | ned | N | 63 | ||
| N | Pt0-5 | F | 29 | 5.3 | 3 | ned | N | 67 | ||
| N | Pt0-6 | M | 54 | 3.2 | 2 | ned | N | 54 | ||
| N | Pt0-7 | F | 30 | 6.0 | 3 | ned | N | 58 | ||
| N | Pt0-8 | F | 46 | 7.0 | 3 | ned | N | 25 | ||
| Y | Pt1-1 | F | 68 | 5.7 | 3 | pd | 42 | Lung | N | 93 |
| Y | Pt1-2 | M | 21 | 6.5 | 3 | pd | 2 | Lung, Lymph nodes, liver | Y | 11 |
| Y | Pt1-3 | M | 57 | 6.8 | 3 | pd | 18 | Lung, Lymph nodes, liver | N | 40 |
| Upregulated | Downregulated | ||||||
|---|---|---|---|---|---|---|---|
| Gene Symbol | Gene Name | Log2 Fold Change (Aggressive/Non-Aggressive) | # Of Related Pathways | Gene Symbol | Gene Name | Log2 Fold Change (Aggressive/Non-Aggressive) | # Of Related Pathways |
| KRT10 | Keratin 10 | 2.09 | 1 | ZNF662 | Zinc Finger Protein 662 | −2.56 | 1 |
| PIK3R2 | Phosphoinositide-3-Kinase Regulatory Subunit 2 | 2.22 | 11 | RARB | Retinoic Acid Receptor Beta | −2.76 | 1 |
| PCYT2 | Phosphate Cytidylyltransferase 2, Ethanolamine | 2.37 | 1 | ATP1B2 | ATPase Na+/K+ Transporting Subunit Beta 2 | −3.36 | 2 |
| GALE | UDP-Galactose-4-Epimerase | 2.48 | 2 | PDE7B | Phosphodiesterase 7B | −3.73 | 1 |
| GPX1 | Glutathione Peroxidase 1 | 2.55 | 2 | KCNMB4 | Potassium Calcium-Activated Channel Subfamily M Regulatory Beta Subunit 4 | −5.54 | 1 |
| MIF | Macrophage Migration Inhibitory Factor | 2.64 | 1 | NAT2 | N-Acetyltransferase 2 | −6.45 | 3 |
| SHC1 | SHC Adaptor Protein 1 | 2.66 | 1 | OR56A5 | Olfactory Receptor Family 56 Subfamily A Member 5 | −6.91 | 1 |
| DTYMK | Deoxythymidylate Kinase | 2.71 | 1 | OR2T7 | Olfactory Receptor Family 2 Subfamily T Member 7 | −7.47 | 1 |
| ENO1 | Enolase 1 | 2.93 | 4 | OR10A2 | Olfactory Receptor Family 10 Subfamily A Member 2 | −8.75 | 1 |
| TALDO1 | Transaldolase 1 | 3.00 | 3 | ZNF479 | Zinc Finger Protein 479 | −9.17 | 1 |
| GSS | Glutathione Synthetase | 3.30 | 2 | GK2 | Glycerol Kinase 2 | −9.30 | 1 |
| TYMP | Thymidine Phosphorylase | 3.42 | 2 | OR6C70 | Olfactory Receptor Family 6 Subfamily C Member 70 | −9.67 | 1 |
| HLA-DRB5 | Major Histocompatibility Complex, Class II, DR Beta 5 | 9.81 | 4 | OR10H2 | Olfactory Receptor Family 10 Subfamily H Member 2 | −9.89 | 1 |
| PTGDS | Prostaglandin D2 Synthase | 23.73 | 1 | OR2L3 | Olfactory Receptor Family 2 Subfamily L Member 3 | −12.53 | 1 |
| ATP6V1C2 | ATPase H+ Transporting V1 Subunit C2 | −12.56 | 2 | ||||
| OR5K2 | Olfactory Receptor Family 5 Subfamily K Member 2 | −16.55 | 1 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.S.; Lee, M.E.; Jang, W.S.; Kim, J.; Park, S.M.; Ham, W.S. Gene Expression Analysis of Aggressive Adult Xp11.2 Translocation Renal Cell Carcinoma at Clinical Stage T1N0M0 to Identify Potential Prognostic and Therapeutic Biomarkers. Biomedicines 2022, 10, 321. https://doi.org/10.3390/biomedicines10020321
Park JS, Lee ME, Jang WS, Kim J, Park SM, Ham WS. Gene Expression Analysis of Aggressive Adult Xp11.2 Translocation Renal Cell Carcinoma at Clinical Stage T1N0M0 to Identify Potential Prognostic and Therapeutic Biomarkers. Biomedicines. 2022; 10(2):321. https://doi.org/10.3390/biomedicines10020321
Chicago/Turabian StylePark, Jee Soo, Myung Eun Lee, Won Sik Jang, Jongchan Kim, Se Mi Park, and Won Sik Ham. 2022. "Gene Expression Analysis of Aggressive Adult Xp11.2 Translocation Renal Cell Carcinoma at Clinical Stage T1N0M0 to Identify Potential Prognostic and Therapeutic Biomarkers" Biomedicines 10, no. 2: 321. https://doi.org/10.3390/biomedicines10020321






