Mitochondrial Transplantation Enhances Phagocytic Function and Decreases Lipid Accumulation in Foam Cell Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Oil Red O Staining (ORO)
2.4. Phagocytosis Assay
2.5. Quantitative Real Time Polymerase Reaction (qPCR)
2.6. Isolation of Mitochondrial Fraction
2.7. Mitochondrial Transfer
2.8. Electron Microscopy
2.9. Statistical Analysis
3. Results
3.1. Efficiently Transferred Mitochondria between Macrophages
3.2. Mitochondrial Transfer between Macrophages Shifts Macrohage Phenotype
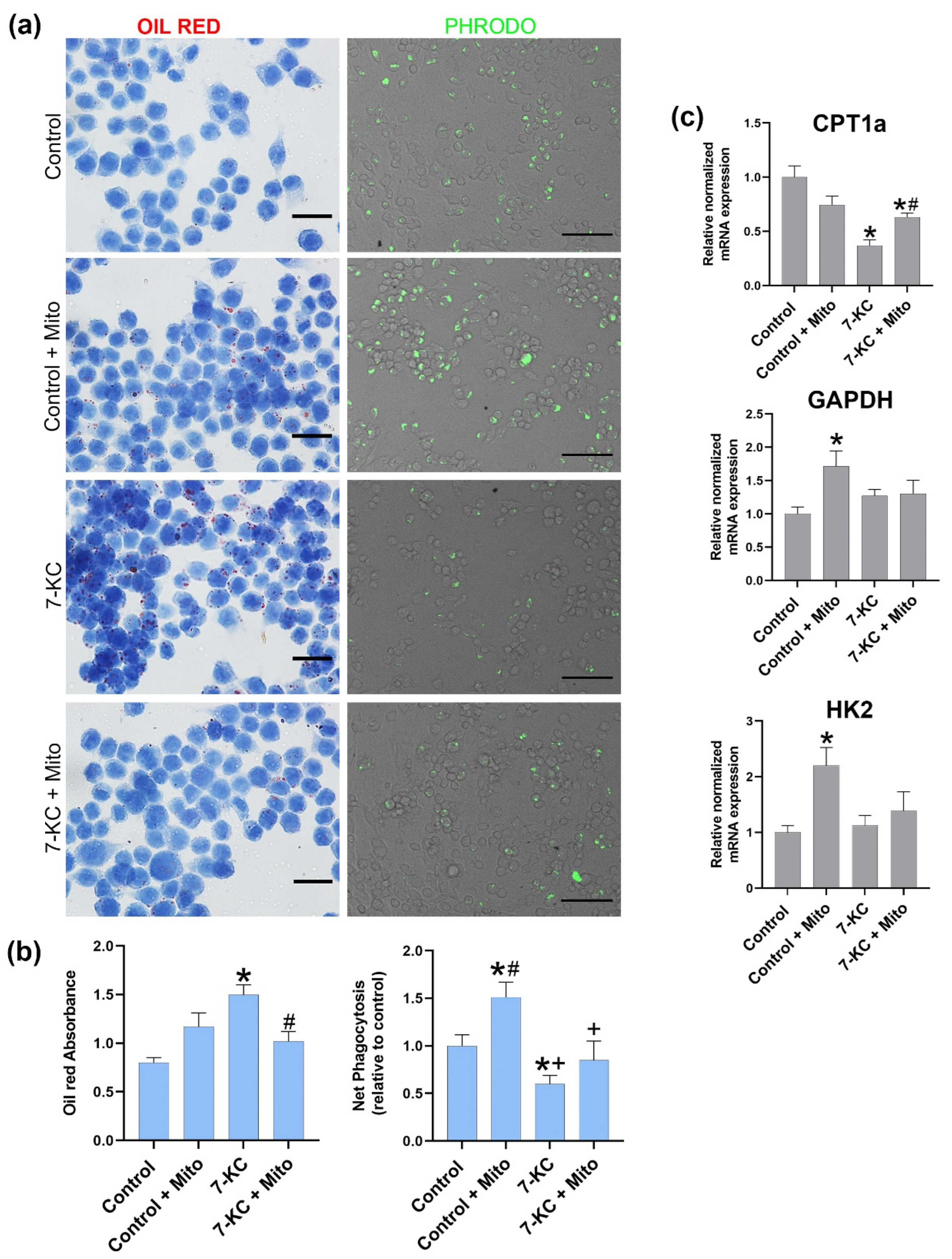
3.3. Mitochondrial Tranfer between Macrophages Modulates Cell Lipid Content, Phagocytosis and CPT1a Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sinha, P.; Islam, M.N.; Bhattacharya, S.; Bhattacharya, J. Intercellular mitochondrial transfer: Bioenergetic crosstalk between cells. Curr. Opin. Genet. Dev. 2016, 38, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsu, K.; Das, S.; Houser, S.D.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood 2009, 113, 4197–4205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, M.; Krasnodembskaya, A. Analysis of Mitochondrial Transfer in Direct Co-cultures of Human Monocyte-derived Macrophages (MDM) and Mesenchymal Stem Cells (MSC). Bio-Protocol 2017, 7, e2255. [Google Scholar] [PubMed] [Green Version]
- Luz-Crawford, P.; Hernandez, J.; Djouad, F.; Luque-Campos, N.; Caicedo, A.; Carrère-Kremer, S.; Brondello, J.M.; Vignais, M.L.; Pène, J.; Jorgensen, C. Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer. Stem Cell Res. Ther. 2019, 10, 232. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef]
- Yu, X.-H.; Fu, Y.-C.; Zhang, D.-W.; Yin, K.; Tang, C.-K. Foam cells in atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Macrophage-mediated cholesterol handling in atherosclerosis. J. Cell. Mol. Med. 2016, 20, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Yao, Q.; Xu, S.; Wang, H.; Qu, P. Inhibition of the NLRP3 inflammasome attenuates foam cell formation of THP-1 macrophages by suppressing ox-LDL uptake and promoting cholesterol efflux. Biochem. Biophys. Res. Commun. 2018, 495, 382–387. [Google Scholar] [CrossRef]
- Kagan, J.C.; Horng, T. NLRP3 inflammasome activation: CD36 serves double duty. Nat. Immunol. 2013, 14, 772–774. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.C.; Everts, B.; Ivanova, Y.; O’sullivan, D.; Nascimento, M.; Smith, A.M.; Beatty, W.; Love-Gregory, L.; Lam, W.Y.; O’neill, C.M.; et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014, 15, 846–855. [Google Scholar] [CrossRef] [Green Version]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, J.; Hooi, S.C.; Jiang, Y.-M.; Lu, G.-D. Fatty acid activation in carcinogenesis and cancer development: Essential roles of long-chain acyl-CoA synthetases (Review). Oncol. Lett. 2018, 16, 1390–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szeto, H.H. Pharmacologic Approaches to Improve Mitochondrial Function in AKI and CKD. J. Am. Soc. Nephrol. 2017, 28, 2856–2865. [Google Scholar] [CrossRef]
- Malandrino, M.I.; Fucho, R.; Weber, M.; Calderon-Dominguez, M.; Mir, J.F.; Valcarcel, L.; Escoté, X.; Gómez-Serrano, M.; Peral, B.; Salvadó, L.; et al. Enhanced fatty acid oxidation in adipocytes and macrophages reduces lipid-induced triglyceride accumulation and inflammation. Am. J. Physiol.-Endocrinol. Metab. 2015, 308, E756–E769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calle, P.; Muñoz, A.; Sola, A.; Hotter, G. CPT1a gene expression reverses the inflammatory and anti-phagocytic effect of 7-ketocholesterol in RAW264.7 macrophages. Lipids Health Dis. 2019, 18, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabas, I.; Bornfeldt, K.E. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ. Res. 2016, 118, 653–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Q.; Zeng, F.; Liu, X.; Wang, Q.J.; Deng, F. Fatty acid oxidation and carnitine palmitoyltransferase I: Emerging therapeutic targets in cancer. Cell Death Dis. 2016, 7, e2226. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.; Jolliet, P.; Morin, C.; Simon, N.; Jolliet, P.; Morin, C.; Zini, R.; Urien, S.; Tillement, J.P. Glucocorticoids decrease cytochrome c oxidase activity of isolated rat kidney mitochondria. FEBS Lett. 1998, 435, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Gibson, M.S.; Domingues, N.; Vieira, O.V. Lipid and non-lipid factors affecting macrophage dysfunction and inflammation in atherosclerosis. Front. Physiol. 2018, 9, 654. [Google Scholar] [CrossRef]
- Testa, G.; Rossin, D.; Poli, G.; Biasi, F.; Leonarduzzi, G. Implication of oxysterols in chronic inflammatory human diseases. Biochimie 2018, 153, 220–231. [Google Scholar] [CrossRef]
- Nury, T.; Sghaier, R.; Zarrouk, A.; Ménétrier, F.; Uzun, T.; Leoni, V.; Caccia, C.; Meddeb, W.; Namsi, A.; Sassi, K.; et al. Induction of peroxisomal changes in oligodendrocytes treated with 7-ketocholesterol: Attenuation by α-tocopherol. Biochimie 2018, 153, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, F.R.; Tabas, I. Role of cholesterol and lipid organization in disease. Nature 2005, 438, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Solsona-Vilarrasa, E.; Fucho, R.; Torres, S.; Nuñez, S.; Nuño-Lámbarri, N.; Enrich, C.; García-Ruiz, C.; Fernández-Checa, J.C. Cholesterol enrichment in liver mitochondria impairs oxidative phosphorylation and disrupts the assembly of respiratory supercomplexes. Redox Biol. 2019, 24, 101214. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zheng, X.; Wang, X.; Jin, T.; Cui, L.; Chen, Z. Mesenchymal Stem/Stromal Cell-Mediated Mitochondrial Transfer and the Therapeutic Potential in Treatment of Neurological Diseases. Stem Cells Int. 2020, 2020, 8838046. [Google Scholar] [CrossRef]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef]
- Jackson, M.V.; Morrison, T.J.; Doherty, D.F.; McAuley, D.F.; Matthay, M.A.; Kissenpfennig, A.; O’Kane, C.M.; Krasnodembskaya, A.D. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells 2016, 34, 2210–2223. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Hematti, P. Mesenchymal stem cell–educated macrophages: A novel type of alternatively activated macrophages. Exp. Hematol. 2009, 37, 1445–1453. [Google Scholar] [CrossRef] [Green Version]
- Ménégaut, L.; Thomas, C.; Lagrost, L.; Masson, D. Fatty acid metabolism in macrophages: A target in cardio-metabolic diseases. Curr. Opin. Lipidol. 2017, 28, 19–26. [Google Scholar] [CrossRef]
- Nomura, M.; Liu, J.; Rovira, I.I.; Gonzalez-Hurtado, E.; Lee, J.; Wolfgang, M.J.; Finkel, T. Fatty acid oxidation in macrophage polarization. Nat. Immunol. 2016, 17, 216–217. [Google Scholar] [CrossRef]
- Diskin, C.; Pålsson-McDermott, E.M. Metabolic Modulation in Macrophage Effector Function. Front. Immunol. 2018, 9, 270. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Zhang, C.; Gao, J. Macrophages as Emerging Key Players in Mitochondrial Transfers. Front. Cell Dev. Biol. 2021, 9, 747377. [Google Scholar] [CrossRef] [PubMed]



| Genes | Forward | Reverse | Efficiency (%) |
|---|---|---|---|
| 18s RNA | CCTGCGGCTTAATTTGACTC | GACAAATCGCTCCACCAACT | 97 |
| Arg 1 | AGACCACAGTCTGGCAGTTG | TGTCAGTGTGAGCATCCACC | 102 |
| Genes | Unique Assay ID | Chromosome Location | Efficiency (%) |
|---|---|---|---|
| TNF | qMmuCED0004141 | 17:35201717-35201865 | 98 |
| CPT1a | qMmuCED0045595 | 19:3385535-3385661 | 98 |
| IL-10 | qMmuCED0044967 | 1:131024363-131024476 | 100 |
| HK2 | qMmuCED0045344 | 6:82749208-82749307 | 101 |
| GAPDH | qMmuCED0027497 | 6:125162278-125162382 | 101 |
| NLRP3 | qMmuCID0010647 | 11:59558525-59565111 | 101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Játiva, S.; Calle, P.; Torrico, S.; Muñoz, Á.; García, M.; Martinez, I.; Sola, A.; Hotter, G. Mitochondrial Transplantation Enhances Phagocytic Function and Decreases Lipid Accumulation in Foam Cell Macrophages. Biomedicines 2022, 10, 329. https://doi.org/10.3390/biomedicines10020329
Játiva S, Calle P, Torrico S, Muñoz Á, García M, Martinez I, Sola A, Hotter G. Mitochondrial Transplantation Enhances Phagocytic Function and Decreases Lipid Accumulation in Foam Cell Macrophages. Biomedicines. 2022; 10(2):329. https://doi.org/10.3390/biomedicines10020329
Chicago/Turabian StyleJátiva, Soraya, Priscila Calle, Selene Torrico, Ángeles Muñoz, Miriam García, Ivet Martinez, Anna Sola, and Georgina Hotter. 2022. "Mitochondrial Transplantation Enhances Phagocytic Function and Decreases Lipid Accumulation in Foam Cell Macrophages" Biomedicines 10, no. 2: 329. https://doi.org/10.3390/biomedicines10020329
APA StyleJátiva, S., Calle, P., Torrico, S., Muñoz, Á., García, M., Martinez, I., Sola, A., & Hotter, G. (2022). Mitochondrial Transplantation Enhances Phagocytic Function and Decreases Lipid Accumulation in Foam Cell Macrophages. Biomedicines, 10(2), 329. https://doi.org/10.3390/biomedicines10020329







