Exploring New Drug Targets for Type 2 Diabetes: Success, Challenges and Opportunities
Abstract
:1. Epidemiology
2. Classification of Diabetes Mellitus
2.1. Type 1 Diabetes Mellitus
2.2. Type 2 Diabetes Mellitus
2.3. Gestational Diabetes Mellitus (GDM)
3. Common Targets for Anti-Diabetic Drug
3.1. Insulin Secretagogues
3.2. Insulin Mimickers and Sensitizers
3.3. Starch Blockers
4. Emerging Targets for the Treatment of T2DM
4.1. 11β-Hydroxysteroid Dehydrogenase
4.2. Glutamine Fructose-6-Phosphate Amido Transferase
4.3. Protein Tyrosine Phosphatase 1B
4.4. SLC16A11
4.5. Nephroblastoma Overexpressed (CCN3/NOV)
4.6. FoxO1
4.7. FFA2/FFA3
4.8. Epoxyeicosatrienoic Acids (EETs)
4.9. Peroxisome Proliferator-Activated Receptor Gamma Co-Activator Alpha (PGC-1α)
4.10. Peroxisome Proliferator-Activated Receptor Gama (PPAR©)
4.11. Glucocorticoid Receptor
4.12. Nuclear Factor (Erythroid-Derived 2)-Like 2 (NRF2)
4.13. Neprilysin
5. Drugs Targeting Molecular Pathways Implicated in Diabetes and CVD
6. Diabetes and the Gut Microbiota: Dietary Influence
7. Crosstalk between Diabetes and Fatty Liver Disease-NASH and Prospective Therapeutic Drug Targets
8. Challenges and Opportunities
9. Summary
10. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shrivastava, S.R.; Shrivastava, P.S.; Ramasamy, J. Role of self-care in management of diabetes mellitus. J. Diabetes Metab. Disord. 2013, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, C.N.J.; Mbanya, C.J. IDF Diabetes Atlas: Global, regional, and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2021, 109119. [Google Scholar] [CrossRef]
- Cassels, S. Overweight in the Pacific: Links between foreign dependence, global food trade, and obesity in the Federated States of Micronesia. Glob. Health 2006, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Chan, R.S.; Woo, J. Prevention of overweight and obesity: How effective is the current public health approach. Int. J. Environ. Res. Public Health 2010, 7, 765–783. [Google Scholar] [CrossRef] [Green Version]
- Ichiho, H.M.; Demei, Y.; Kuartei, S.; Aitaoto, N. An assessment of non-communicable diseases, diabetes, and related risk factors in the Republic of Palau: A systems perspective. Hawaii J. Med. Public Health 2013, 72 (Suppl. S1), 98–105. [Google Scholar]
- Kaveeshwar, S.A.; Cornwall, J. The current state of diabetes mellitus in India. Australas. Med. J. 2014, 7, 45–48. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Magdalena, P.; Ropero, A.B.; Soriano, S.; Quesada, I.; Nadal, A. Bisphenol-A: A new diabetogenic factor? Hormones 2010, 9, 118–126. [Google Scholar] [CrossRef]
- Lakhtakia, R. The History of Diabetes Mellitus. Sultan Qaboos Univ. Med. J. 2013, 13, 368–370. [Google Scholar] [CrossRef]
- Nejabat, M.; Maleki, B.; Nimrouzi, M.; Mahbodi, A.; Salehi, A. Avicenna and Cataracts: A New Analysis of Contributions to Diagnosis and Treatment from the Canon. Iran. Red Crescent Med. J. 2012, 14, 265–270. [Google Scholar]
- Tan, S.Y.; Merchant, J. Frederick Banting (1891–1941): Discoverer of insulin. Singap. Med. J. 2017, 58, 2–3. [Google Scholar] [CrossRef]
- WH Organization. Classification of Diabetes Mellitus. 2019. Available online: https://www.who.int/publications/i/item/classification-of-diabetes-mellitus (accessed on 15 October 2021).
- Quinn, L.M.; Wong, F.S.; Narendran, P. Environmental Determinants of Type 1 Diabetes: From Association to Proving Causality. Front. Immunol. 2021, 12, 737964. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.S.; Kang, L.; Wasserman, D.H. The extracellular matrix and insulin resistance. Trends Endocrinol. Metab. 2015, 26, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Herath, H.; Herath, R.; Wickremasinghe, R. Gestational diabetes mellitus and risk of type 2 diabetes 10 years after the index pregnancy in Sri Lankan women-A community based retrospective cohort study. PLoS ONE 2017, 12, e0179647. [Google Scholar] [CrossRef] [Green Version]
- Pilacinski, S.; Zozulinska-Ziolkiewicz, D.A. Influence of lifestyle on the course of type 1 diabetes mellitus. Arch. Med. Sci. 2014, 10, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Moller David, E. Metabolic Disease Drug Discovery—“Hitting the Target” Is Easier Said Than Done. Cell Metab. 2012, 15, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Ngoc Doan Trang, N.; Ly Thi, L. Targeted proteins for diabetes drug design. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 013001. [Google Scholar] [CrossRef]
- Kunhiraman, B.P.; Jawa, A.; Fonseca, V.A. Potential cardiovascular benefits of insulin sensitizers. Endocrinol. Metab. Clin. N. Am. 2005, 34, 117–135. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J. Acarbose, lente carbohydrate, and prebiotics promote metabolic health and longevity by stimulating intestinal production of GLP-1. Open Heart 2015, 2, e000205. [Google Scholar] [CrossRef]
- Nauck, M. Incretin therapies: Highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes. Metab. 2016, 18, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Mikhail, N. Place of sodium-glucose co-transporter type 2 inhibitors for treatment of type 2 diabetes. World J. Diabetes 2014, 5, 854–859. [Google Scholar] [CrossRef]
- Cooper, M.S.; Stewart, P.M. 11Beta-hydroxysteroid dehydrogenase type 1 and its role in the hypothalamus-pituitary-adrenal axis, metabolic syndrome, and inflammation. J. Clin. Endocrinol. Metab. 2009, 94, 4645–4654. [Google Scholar] [CrossRef] [Green Version]
- Schleicher, E.D.; Weigert, C. Role of the hexosamine biosynthetic pathway in diabetic nephropathy. Kidney Int. 2000, 58, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- Stull, A.J.; Wang, Z.Q.; Zhang, X.H.; Yu, Y.; Johnson, W.D.; Cefalu, W.T. Skeletal muscle protein tyrosine phosphatase 1B regulates insulin sensitivity in African Americans. Diabetes 2012, 61, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Ruiz, R.; Vieira, E.; Garcia-Roves, P.M.; Gomis, R. Protein tyrosine phosphatase-1B modulates pancreatic beta-cell mass. PLoS ONE 2014, 9, e90344. [Google Scholar] [CrossRef] [Green Version]
- Qian, S.; Zhang, M.; He, Y.; Wang, W.; Liu, S. Recent advances in the development of protein tyrosine phosphatase 1B inhibitors for Type 2 diabetes. Future Med. Chem. 2016, 8, 1239–1258. [Google Scholar] [CrossRef]
- Rusu, V.; Hoch, E.; Mercader, J.M.; Tenen, D.E.; Gymrek, M.; Hartigan, C.R.; DeRan, M.; von Grotthuss, M.; Fontanillas, P.; Spooner, A.; et al. Type 2 Diabetes Variants Disrupt Function of SLC16A11 through Two Distinct Mechanisms. Cell 2017, 170, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Rhee, E.P.; Cheng, S.; Larson, M.G.; Walford, G.A.; Lewis, G.D.; McCabe, E.; Yang, E.; Farrell, L.; Fox, C.S.; O’Donnell, C.J.; et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J. Clin. Investig. 2011, 121, 1402–1411. [Google Scholar] [CrossRef] [Green Version]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Natesan, V.; Shi, H.; Hamik, A.; Kawanami, D.; Hao, C.; Mahabaleshwar, G.H.; Wang, W.; Jin, Z.G.; Atkins, G.B.; et al. A novel role of CCN3 in regulating endothelial inflammation. J. Cell Commun. Signal. 2010, 4, 141–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakradouni, J.; Le Goff, W.; Calmel, C.; Antoine, B.; Villard, E.; Frisdal, E.; Abifadel, M.; Tordjman, J.; Poitou, C.; Bonnefont-Rousselot, D.; et al. Plasma NOV/CCN3 levels are closely associated with obesity in patients with metabolic disorders. PLoS ONE 2013, 8, e66788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinerie, C.; Garcia, M.; Do, T.T.; Antoine, B.; Moldes, M.; Dorothee, G.; Kazazian, C.; Auclair, M.; Buyse, M.; Ledent, T.; et al. NOV/CCN3: A New Adipocytokine Involved in Obesity-Associated Insulin Resistance. Diabetes 2016, 65, 2502–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.Y.; Wang, Y.D.; Qi, X.Y.; Ran, L.; Hong, T.; Yang, J.; Yan, B.; Liao, Z.Z.; Liu, J.H.; Xiao, X.H. Serum CCN3 levels are increased in type 2 diabetes mellitus and associated with obesity, insulin resistance and inflammation. Clin. Chim. Acta 2019, 494, 52–57. [Google Scholar] [CrossRef]
- Kang, S.; Tsai, L.T.; Rosen, E.D. Nuclear Mechanisms of Insulin Resistance. Trends Cell Biol. 2016, 26, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, N.E.; Kotarsky, K.; Owman, C.; Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003, 303, 1047–1052. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Kebede, M.A.; Alquier, T.; Latour, M.G.; Poitout, V. Lipid receptors and islet function: Therapeutic implications? Diabetes Obes. Metab. 2009, 11 (Suppl. S4), 10–20. [Google Scholar] [CrossRef] [Green Version]
- Bahar Halpern, K.; Veprik, A.; Rubins, N.; Naaman, O.; Walker, M.D. GPR41 gene expression is mediated by internal ribosome entry site (IRES)-dependent translation of bicistronic mRNA encoding GPR40 and GPR41 proteins. J. Biol. Chem. 2012, 287, 20154–20163. [Google Scholar] [CrossRef] [Green Version]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Ahmed, K.; Gille, A.; Lu, S.; Gröne, H.J.; Tunaru, S.; Offermanns, S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat. Med. 2015, 21, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Karamanou, M.; Protogerou, A.; Tsoucalas, G.; Androutsos, G.; Poulakou-Rebelakou, E. Milestones in the history of diabetes mellitus: The main contributors. World J. Diabetes 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.H.; Falck, J.R.; Harris, R.C. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J. Lipid Res. 2000, 41, 163–181. [Google Scholar] [CrossRef]
- Waldman, M.; Bellner, L.; Vanella, L.; Schragenheim, J.; Sodhi, K.; Singh, S.P.; Lin, D.; Lakhkar, A.; Li, J.; Hochhauser, E.; et al. Epoxyeicosatrienoic Acids Regulate Adipocyte Differentiation of Mouse 3T3 Cells, Via PGC-1alpha Activation, Which Is Required for HO-1 Expression and Increased Mitochondrial Function. Stem Cells Dev. 2016, 25, 1084–1094. [Google Scholar] [CrossRef] [Green Version]
- Zeldin, D.C. Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 2001, 276, 36059–36062. [Google Scholar] [CrossRef] [Green Version]
- Node, K.; Huo, Y.; Ruan, X.; Yang, B.; Spiecker, M.; Ley, K.; Zeldin, D.C.; Liao, J.K. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 1999, 285, 1276–1279. [Google Scholar] [CrossRef] [Green Version]
- Spiecker, M.; Liao, J.K. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch. Biochem. Biophys. 2005, 433, 413–420. [Google Scholar] [CrossRef]
- Zha, W.; Edin, M.L.; Vendrov, K.C.; Schuck, R.N.; Lih, F.B.; Jat, J.L.; Bradbury, J.A.; DeGraff, L.M.; Hua, K.; Tomer, K.B.; et al. Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity. J. Lipid Res. 2014, 55, 2124–2136. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Wenz, T.; Rossi, S.G.; Rotundo, R.L.; Spiegelman, B.M.; Moraes, C.T. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc. Natl. Acad. Sci. USA 2009, 106, 20405–20410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, S.; Mepani, R.J.; Laznik, D.; Ye, L.; Jurczak, M.J.; Jornayvaz, F.R.; Estall, J.L.; Chatterjee Bhowmick, D.; Shulman, G.I.; Spiegelman, B.M.; et al. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc. Natl. Acad. Sci. USA 2012, 109, 9635–9640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraitis, A.G.; Block, T.; Nguyen, D.; Belanoff, J.K. The role of glucocorticoid receptors in metabolic syndrome and psychiatric illness. J. Steroid Biochem. Mol. Biol. 2017, 165 Pt A, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharm. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guglielmi, V.; Sbraccia, P. GLP-1 receptor independent pathways: Emerging beneficial effects of GLP-1 breakdown products. Eat. Weight Disord. 2017, 22, 231–240. [Google Scholar] [CrossRef]
- Hamilton, B.S.; Himmelsbach, F.; Nar, H.; Schuler-Metz, A.; Krosky, P.; Guo, J.; Guo, R.; Meng, S.; Zhao, Y.; Lala, D.S.; et al. Pharmacological characterization of the selective 11β-hydroxysteroid dehydrogenase 1 inhibitor, BI 135585, a clinical candidate for the treatment of type 2 diabetes. Eur. J. Pharmacol. 2015, 746, 50–55. [Google Scholar] [CrossRef]
- Martocchia, A.; Stefanelli, M.; Falaschi, G.M.; Toussan, L.; Ferri, C.; Falaschi, P. Recent advances in the role of cortisol and metabolic syndrome in age-related degenerative diseases. Aging Clin. Exp. Res. 2016, 28, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.D.; Sousa, K.M.; Mattern, D.L.; Wagner, J.; Fu, X.; Vaidehi, N.; Forman, B.M.; Huang, W. Stereoselective synthesis, biological evaluation, and modeling of novel bile acid-derived G-protein coupled Bile acid receptor 1 (GP-BAR1, TGR5) agonists. Bio Organ. Med. Chem. 2015, 23, 1613–1628. [Google Scholar] [CrossRef]
- Ido, Y. Diabetic complications within the context of ageing: NADH/NAD+ redox, insulin C-peptide, SIRT1-LKB1-AMPK positive feedback and FOXO3. J. Diabetes Investig. 2016, 7, 448–458. [Google Scholar] [CrossRef]
- Kanwal, A.; Banerjee, S.K. SGLT inhibitors: A novel target for diabetes. Pharm. Pat. Anal. 2013, 2, 77–91. [Google Scholar] [CrossRef]
- Bailey, C.J.; Tahrani, A.A.; Barnett, A.H. Future glucose-lowering drugs for type 2 diabetes. Lancet Diabetes Endocrinol. 2016, 4, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Malek, R.; Davis, S.N. Tyrosine kinase inhibitors under investigation for the treatment of type II diabetes. Expert Opin. Investig. Drugs 2016, 25, 287–296. [Google Scholar] [CrossRef]
- Tang, W.J. Targeting Insulin-Degrading Enzyme to Treat Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2016, 27, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosqvist, F.; Iggman, D.; Kullberg, J.; Cedernaes, J.; Johansson, H.E.; Larsson, A.; Johansson, L.; Ahlström, H.; Arner, P.; Dahlman, I.; et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, R.; Xiong, X.; Liangpunsakul, S.; Dong, X.C. Sestrin 3 Protein Enhances Hepatic Insulin Sensitivity by Direct Activation of the mTORC2-Akt Signaling. Diabetes 2014, 64, 1211–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.I.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M.; et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493–499. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, L.; Wang, D.; Zhang, X.; Li, J.; Ali, S.; Lu, J.; Zong, H.; Xu, X. Staurosporine as an agonist for induction of GLUT4 translocation, identified by a pH-sensitive fluorescent IRAP-mOrange2 probe. Biochem. Biophys. Res. Commun. 2016, 480, 534–538. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Yao, X.G.; Shen, H.; Chen, J.; Li, C.; Chen, L.; Zheng, M.; Ye, J.; Hu, L.; et al. (+)-Rutamarin as a dual inducer of both GLUT4 translocation and expression efficiently ameliorates glucose homeostasis in insulin-resistant mice. PLoS ONE 2012, 7, e31811. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- Woundman, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Materna, A.; Friedman, J.; Campos-Baptista, M.; Blackburn, M.; Perrotta, A.; Erdman, S.E.; Alm, E.J. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014, 15, R89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez-Sainz, E.; Milagro, F.; Riezu-Boj, J.; Lorente-Cebrián, S. Effects of gut microbiota–derived extracellular vesicles on obesity and diabetes and their potential modulation through diet. J. Physiol. Biochem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Leeming, E.; Johnson, A.; Spector, T.; Le Roy, C. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.; Keilbaugh, S.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Hu, F.; Xiang, D.; Lu, H.; Li, W.; Zhao, A.; Huang, L.; Wang, R. The metabolic effect of gut microbiota on drugs. Drug Metab. Rev. 2020, 52, 139–156. [Google Scholar] [CrossRef]
- Maliehe, A.; Ghahremani, S.; Kharghani, S.; Masumeh, G.; Maliehe, A.; Ghahremani, S.; Kharghani, S.; Ghazanfarpour, M.; Shariati, K.; Kazemi, M.; et al. Effect of Isoflavones and Genistein on Glucose Metabolism in Peri- and Post-Menopausal Women: An Overview of Meta-Analysis. J. Menopausal Med. 2019, 25, 69–73. [Google Scholar] [CrossRef]
- Squadrito, F.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; D’Anna, R.; Arcoraci, V.; Burnett, B.P.; Minutoli, L.; et al. Genistein in the Metabolic Syndrome: Results of a Randomized Clinical Trial. J. Clin. Endocrinol. Metab. 2013, 98, 3366–3374. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Liu, G.; Ding, M.; Zong, G.; Hu, F.B.; Willett, W.C.; Rimm, E.B.; Manson, J.E.; Sun, Q. Isoflavone Intake and the Risk of Coronary Heart Disease in US Men and Women. Circulation 2020, 141, 1127–1137. [Google Scholar] [CrossRef]
- Omoruyi, F.; Stennett, D.; Foster, S.; Dilworth, L. New Frontiers for the Use of IP6 and Inositol Combination in Treating Diabetes Mellitus: A Review. Molecules 2020, 25, 1720. [Google Scholar] [CrossRef] [Green Version]
- Yki-Jarvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and nonalcoholic steatohepatitis in adults. Aliment. Pharm. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Gastaldelli, A.; Vanni, E.; Gambino, R.; Cassader, M.; Baldi, S.; Ponti, V.; Pagano, G.; Ferrannini, E.; Rizzetto, M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: Sites and mechanisms. Diabetologia 2005, 48, 634–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gastaldelli, A.; Cusi, K.; Pettiti, M.; Hardies, J.; Miyazaki, Y.; Berria, R.; Buzzigoli, E.; Sironi, A.M.; Cersosimo, E.; Ferrannini, E.; et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007, 133, 496–506. [Google Scholar] [CrossRef]
- Bril, F.; Lomonaco, R.; Orsak, B.; Ortiz-Lopez, C.; Webb, A.; Tio, F.; Hecht, J.; Cusi, K. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 2178–2187. [Google Scholar] [CrossRef]
- Cersosimo, E.; Gastaldelli, A.; Cervera, A.; Wajcberg, E.; Sriwijilkamol, A.; Fernandez, M.; Zuo, P. Effect of exenatide on splanchnic and peripheral glucose metabolism in type 2 diabetic subjects. J. Clin. Endocrinol. Metab. 2011, 96, 1763–1770. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Hull, D.; Guo, K.; Barton, D.; Hazlehurst, J.M.; Gathercole, L.L.; Nasiri, M.; Yu, J.; Gough, S.C.; Newsome, P.N.; et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J. Hepatol. 2016, 64, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Scheen, A. The safety of gliptins: Updated data in 2018. Expert Opin. Drug Saf. 2018, 17, 387–405. [Google Scholar] [CrossRef]
- Cusi, K.; Sanyal, A.J.; Zhang, S.; Hartman, M.L.; Bue-Valleskey, J.M.; Hoogwerf, B.J.; Haupt, A. Nonalcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 1630–1634. [Google Scholar] [CrossRef]
- Andreux, P.A.; Houtkooper, R.H.; Auwerx, J. Pharmacological approaches to restore mitochondrial function. Review. Nat. Rev. Drug Discov. 2013, 12, 465–483. [Google Scholar] [CrossRef] [Green Version]

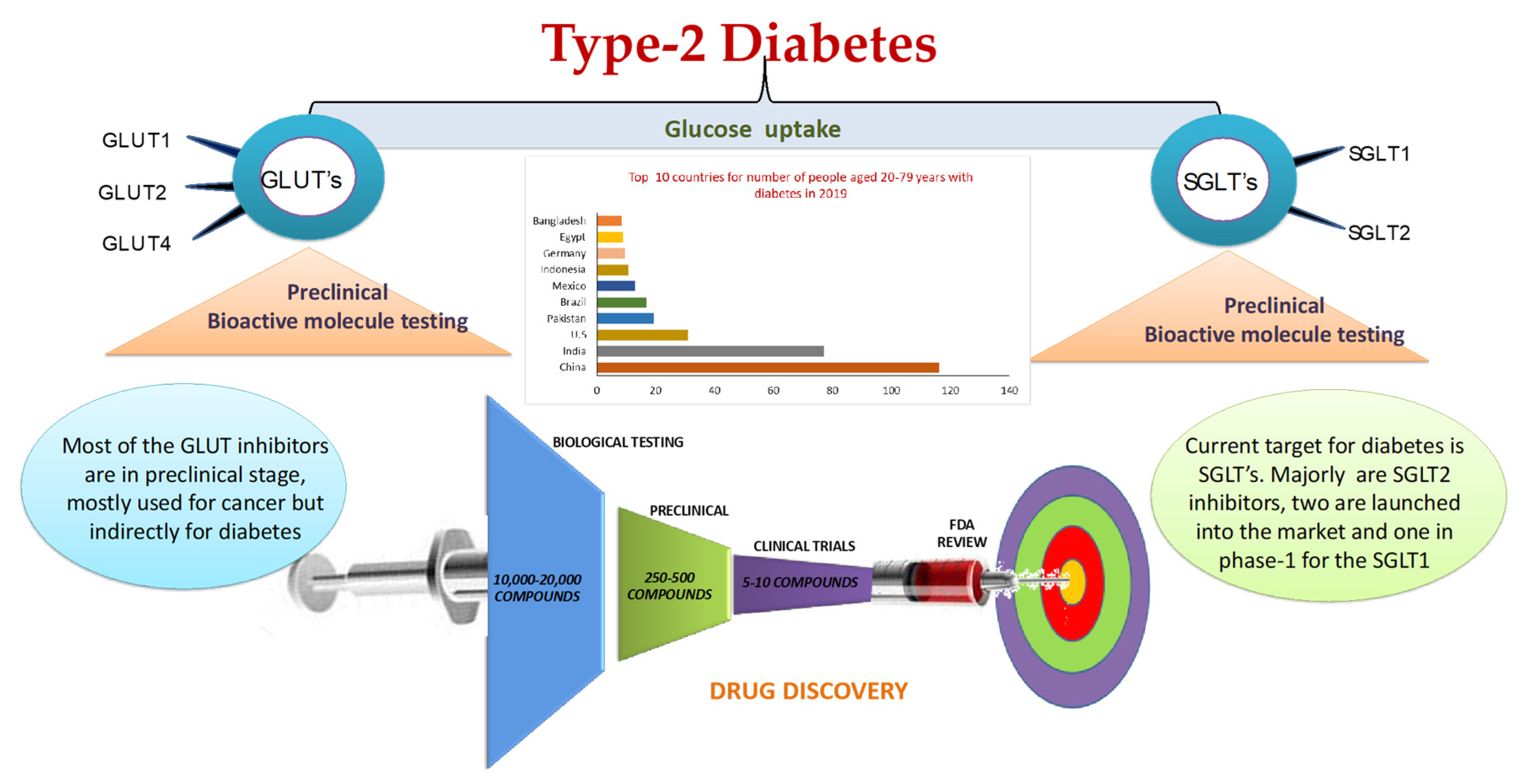
| 2019 Rank | Country/Territory | 2019 (Millions) | Country/Territory | 2045 (Millions) |
|---|---|---|---|---|
| 1 | China | 116.4 | India | 134.3 |
| 2 | India | 77 | China | 119.8 |
| 3 | U. S. A. | 31 | U. S. A. | 35.6 |
| 4 | Pakistan | 19.4 | Mexico | 21.8 |
| 5 | Brazil | 16.8 | Brazil | 20.3 |
| 6 | Mexico | 12.8 | Egypt | 16.7 |
| 7 | Indonesia | 10.7 | Indonesia | 16.7 |
| 8 | Germany | 9.5 | Pakistan | 16.1 |
| 9 | Egypt | 8.9 | Bangladesh | 13.7 |
| 10 | Bangladesh | 8.4 | Turkey | 11.2 |
| Name | Sponsor/Developer | Mechanism of Action | Indication |
|---|---|---|---|
| Afrezza | MannKind | Ultra-rapid-acting mealtime insulin therapy | Adults with type 1 or type 2 diabetes |
| Albiglutide | GlaxoSmithKline | Glucagon-like peptide (GLP) 1 agonist | Once weekly for adults with type 2 diabetes |
| Aleglitazar | Roche | Dual peroxisome proliferator-activated receptor (PPAR) α/γ activation | Cardiovascular risk reduction in type 2 diabetes |
| Alogliptin, Alogliptin and pioglitazone, Alogliptin and metformin | Takeda Pharmaceuticals and Furiex Pharmaceuticals | DPP-4 inhibitor | Oral treatment of type 2 diabetes, individually and in two fixed-dose combinations |
| Atrasentan | AbbVie | Selective endothelin-A receptor antagonist | Oral once-daily treatment for diabetic nephropathy |
| Dulaglutide (LY2189265) | Eli Lilly | GLP-1 analog | Once weekly for type 2 diabetes |
| Empagliflozin (BI10773) | Boehringer Ingelheim and Eli Lilly | Sodium dependent glucose transporter 2 (SGLT2) inhibitor | Oral treatment for adults with type 2 diabetes |
| Ertugliflozin (MK-8835; PF-04971729) | Merck & Co., licensed from Pfizer | SGLT2 inhibitor | Type 2 diabetes |
| Fasiglifam (TAK-875) | Takeda | G-protein-coupled receptor (GPCR) 40 agonist | Type 2 diabetes |
| FIAsp (NN1218) | Novo Nordisk | Faster-acting formulation of insulin aspart | Type 1 and 2 diabetes |
| Forxiga™ (dapagliflozin) | Bristol Myers Squibb and AstraZeneca | SGLT2 inhibitor | Once-daily tablets for adults with type 2 diabetes |
| IDegLira (NN9068) | Novo Nordisk | Combination drug therapy | Type 2 diabetes |
| Invokana (canagliflozin) | Johnson & Johnson | SGLT2 inhibitor | Once-daily tablets for adults with type 2 diabetes |
| Ipragliflozin L-proline (ASP1941) | Astellas, MSD, and Kotobuki Pharmaceutical | SGLT2 inhibitor | Type 2 diabetes |
| Luseogliflozin hydrate (TS-071) | Taisho Pharmaceutical | SGLT2 inhibitor | Once-daily for type 2 diabetes |
| LixiLan (lixisenatide+ insulin glargine) | Sanofi; lixisenatide | Combination drug therapy | Type 2 diabetes |
| Lyxumia® (lixisenatide) | Sanofi; licensed from Zealand Pharma | GLP-1 agonist | Once-daily for type 2 diabetes |
| LY2605541 (basal insulin peglispro) | Eli Lilly | Basal insulin analog | Type 1 and 2 diabetes |
| LY2963016 (new insulin glargine product) | Eli Lilly and Boehringer Ingelheim | Basal insulin | Type 1 and 2 diabetes |
| Omarigliptin (MK-3102) | Merck & Co. | DPP-4 inhibitor | Once-weekly for adults with type 2 diabetes |
| Ryzodeg® (insulin degludec + insulin aspart) | Novo Nordisk | Soluble fixed combination of basal insulin with bolus insulin aspart | Once-daily for type 1 and 2 diabetes |
| Semaglutide (NN9535) | Novo Nordisk | GLP-1 analog | Once-weekly for type 2 diabetes |
| SYR-472 (trelagliptin succinate) | Takeda Pharmaceuticals and Furiex Pharmaceuticals | DPP-4 inhibitor | Once-weekly oral treatment for type 2 diabetes |
| Tresiba® (Insulin degludec) | Novo Nordisk | Once-daily basal insulin | Type 1 and 2 diabetes |
| U300 | Sanofi | Insulin glargine | Type 1 and 2 diabetes |
| Targets | Mechanism of Action | Leads | Ref. |
|---|---|---|---|
| 11β-HSD1 (hydroxysteroid dehydrogenase) | Blocking cortisol | 1. INCB13739, MK-0916, 2. BI 135585 | [57,58] |
| G protein-coupled receptor (GPR119) | Increases cAMP signaling | APD5979, MBX-2982 | |
| TGR5 (bile-acid activated GPCR) | cAMP accumulation and enhances GLP-1 secretion (intestine), anti-inflammatory effect (liver) | INT-777 | [59] |
| SIRT1 | Improves insulin sensitivity, increases glucose homeostasis, increases mitochondrial capacity | SRT2104, resveratrol | [60] |
| SGLT-2 | Increases kidney-dependent glucose homeostasis | Dapagliflozin, canagliflozin, sergliflozin, remogliflozin, ipragliflozin, and empagliflozin, etc. | [61] |
| GPR40 | Increases incretin secretion, improves glucose tolerance | Modulators | [62] |
| PPAR-γ | Improves serum lipid profile, glucose homeostasis, insulin sensitivity, reduces inflammation and weight gain | Piolitazone, aleglitazar, glitazones, GFT505 | |
| Tyrosine Kinase | Reduces beta cell apoptosis, enhances insulin secretion, increases beta cell survival, reduces insulin resistance, | Imatinib, Sunitinib, Dasatinib, Sorafenib, Erlotinib | [63] |
| PPLR | Improves JAK2/STAT5 pathway for glucose uptake | Bromocriptine | |
| Insulin degrading enzyme (IDE) | Thiol zinc-metalloendopeptidase that cleaves small proteins of diverse sequence | BDM44768, 6bK, NTE-1 | [64] |
| FATP5 | Enhances the uptake of long-chain and very long-chain fatty acids into the cells | Chenodiol and Ursodiol | [65] |
| Sestrin | Enhances hepatic insulin sensitivity | 3. Enhances hepatic insulin sensitivity | [66] |
| Statin | For diabetic dyslipidemia | ||
| Adiponectin | Decreased adiponectin levels are thought to play a central role in the development of type 2 diabetes and obesity | AdipoRon is a novel orally-active small molecule that serves as a potent selective agonist of the AdipoR1 and AdipoR2 adiponectin receptors | [67,68] |
| Glut4 | Triggering the canonical PI3K–AKT pathway is essential and ample to activate exocytosis of GLUT4 storage vesicles to the plasma membrane | Staurosporine is used to promotes GSVs translocation and glucose uptake through the AMPK pathway Rutamarin as a dual inducer of both GLUT4 translocation and expression efficiently ameliorates glucose homeostasis in insulin-resistant mice | [69,70] |
| PGC-1α | Reduction in PGC-1α triggers insulin resistance and ultimately causes diabetes | Still waiting to synthesize agonist | [71,72] |
| Trend Box |
|---|
| Diabetes mellitus is a complex metabolic disruption characterized by chronic hyperglycemia due to deficiencies in insulin production, its systemic release and action, and resistance. |
| Incident diabetes is also due to changes in performance of multiple key enzymes. Therefore, identifying and understanding key targets at structure-function-dynamics levels and their potential leads and/or drugs, shortcomings, and associated challenges all together can provide a roadmap for the discovery of new selective modulators. |
| We listed promising therapeutic targets for treatment of T2DM, most of them involving improvements in glucose tolerance. |
| Novel drugs from various targets have numerous locales of activity and each focus on a few components of the metabolic disorder. These may help pave the way for future type 2 diabetes treatments. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanwal, A.; Kanwar, N.; Bharati, S.; Srivastava, P.; Singh, S.P.; Amar, S. Exploring New Drug Targets for Type 2 Diabetes: Success, Challenges and Opportunities. Biomedicines 2022, 10, 331. https://doi.org/10.3390/biomedicines10020331
Kanwal A, Kanwar N, Bharati S, Srivastava P, Singh SP, Amar S. Exploring New Drug Targets for Type 2 Diabetes: Success, Challenges and Opportunities. Biomedicines. 2022; 10(2):331. https://doi.org/10.3390/biomedicines10020331
Chicago/Turabian StyleKanwal, Abhinav, Navjot Kanwar, Sanjay Bharati, Prateek Srivastava, Shailendra P. Singh, and Salomon Amar. 2022. "Exploring New Drug Targets for Type 2 Diabetes: Success, Challenges and Opportunities" Biomedicines 10, no. 2: 331. https://doi.org/10.3390/biomedicines10020331
APA StyleKanwal, A., Kanwar, N., Bharati, S., Srivastava, P., Singh, S. P., & Amar, S. (2022). Exploring New Drug Targets for Type 2 Diabetes: Success, Challenges and Opportunities. Biomedicines, 10(2), 331. https://doi.org/10.3390/biomedicines10020331







