Cell and Tissue Nanomechanics: From Early Development to Carcinogenesis
Abstract
:1. Introduction
2. Molecular Mechanisms and Predictors of Cell and Tissue Nanomechanics
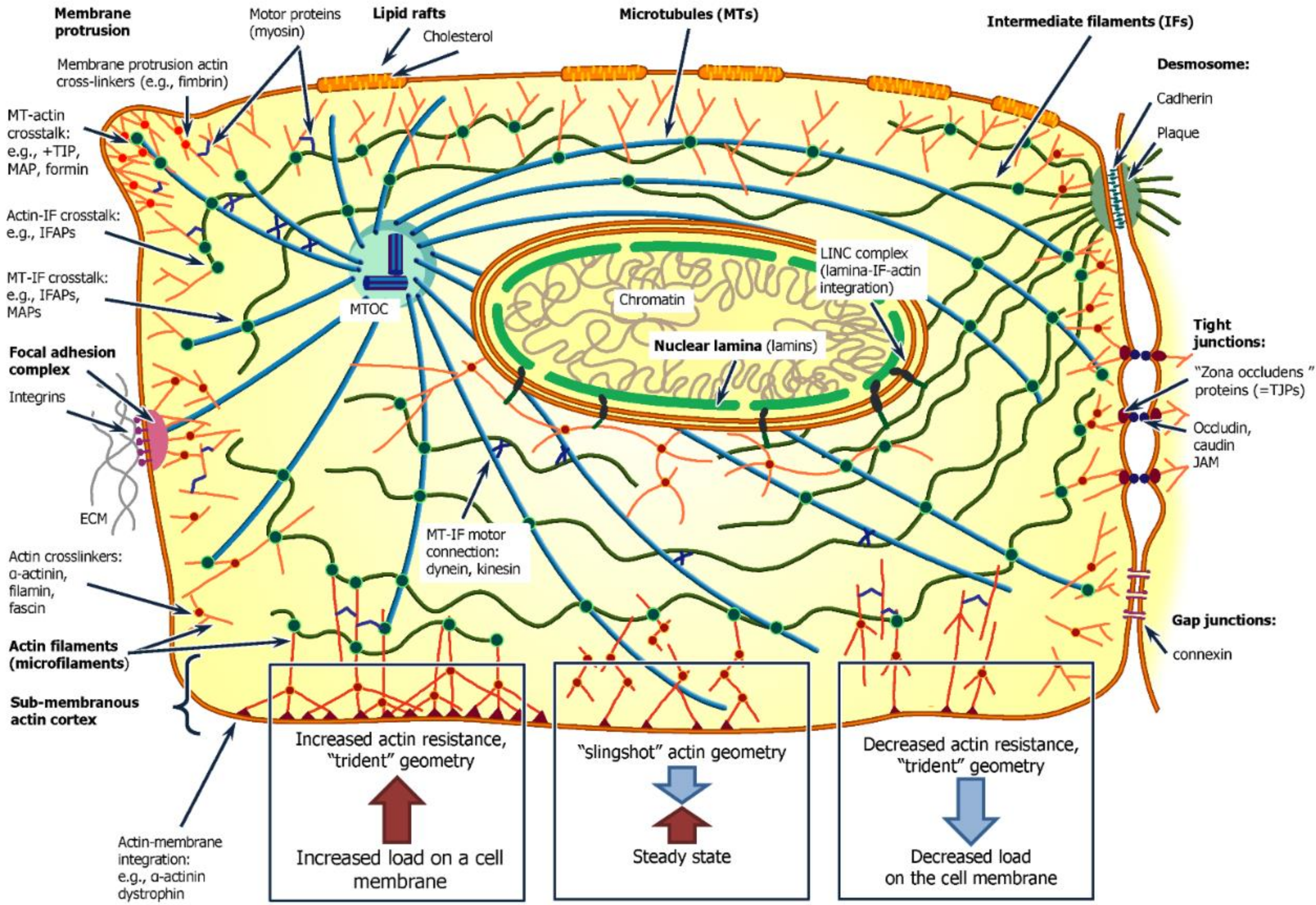
2.1. Cytoskeleton Role
2.2. Lipid Membrane Organization
2.3. Nuclear Organization
3. Cell Mechanoreception and Behavior
3.1. Cell Mechanoreception and Responding to Mechanical Signals
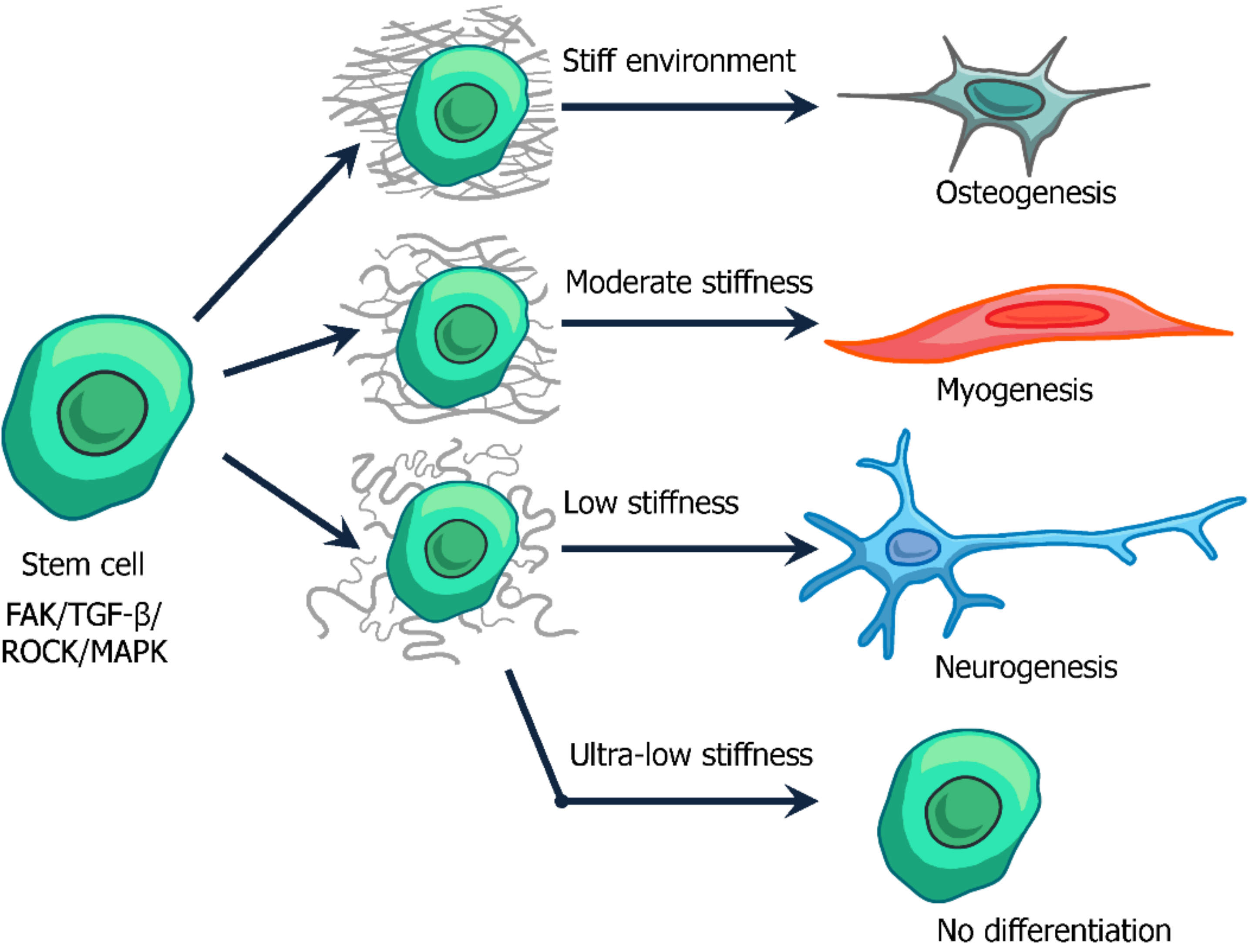
3.2. The Role of ECM Physical Properties on Cell Functions
4. Nanomechanical-Based Differentiation and Specification of the Cells during Ontogenesis
4.1. Blastocyst
4.2. Primary Organogenesis and Tissue Genesis
4.3. Secondary Organogenesis
5. Nanomechanical Features That Provide Cancer Aggression and Invasion
5.1. Cellular Component
5.2. Extracellular Component
5.3. Tumor Stem Cells Niche ECM and Its Effect on Tumor Progression
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Burridge, K.; Monaghan-Benson, E.; Graham, D.M. Mechanotransduction: From the Cell Surface to the Nucleus via RhoA. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2019, 374, 20180229. [Google Scholar] [CrossRef] [PubMed]
- Fels, B.; Kusche-Vihrog, K. It Takes More than Two to Tango: Mechanosignaling of the Endothelial Surface. Pflugers Arch.-Eur. J. Physiol. 2020, 472, 419–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radke, M.H.; Polack, C.; Methawasin, M.; Fink, C.; Granzier, H.L.; Gotthardt, M. Deleting Full Length Titin Versus the Titin M-Band Region Leads to Differential Mechanosignaling and Cardiac Phenotypes. Circulation 2019, 139, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Ridge, S.M.; Sullivan, F.J.; Glynn, S.A. Mesenchymal Stem Cells: Key Players in Cancer Progression. Mol. Cancer 2017, 16, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The Role of Microenvironment in Tumor Angiogenesis. J. Exp. Clin. Cancer Res. CR 2020, 39, 204. [Google Scholar] [CrossRef]
- Shi, F.; Wang, Y.-C.; Hu, Z.-B.; Xu, H.-Y.; Sun, J.; Gao, Y.; Li, X.-T.; Yang, C.-B.; Xie, C.; Li, C.-F.; et al. Simulated Microgravity Promotes Angiogenesis through RhoA-Dependent Rearrangement of the Actin Cytoskeleton. Cell. Physiol. Biochem. 2017, 41, 227–238. [Google Scholar] [CrossRef]
- Lincz, L.F.; Buret, A.; Burns, G.F. Formation of Spheroid Structures in a Human Colon Carcinoma Cell Line Involves a Complex Series of Intercellular Rearrangements. Differ. Res. Biol. Divers. 1997, 61, 261–274. [Google Scholar] [CrossRef]
- Nickels, J.D.; Smith, M.D.; Alsop, R.J.; Himbert, S.; Yahya, A.; Cordner, D.; Zolnierczuk, P.; Stanley, C.B.; Katsaras, J.; Cheng, X.; et al. Lipid Rafts: Buffers of Cell Membrane Physical Properties. J. Phys. Chem. B 2019, 123, 2050–2056. [Google Scholar] [CrossRef]
- Khrustalev, V.V.; Khrustaleva, T.A.; Poboinev, V.V.; Yurchenko, K.V. Mutational Pressure and Natural Selection in Epidermal Growth Factor Receptor Gene during Germline and Somatic Mutagenesis in Cancer Cells. Mutat. Res. 2019, 815, 1–9. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Steps in Metastasis: An Updated Review. Med. Oncol. 2021, 38, 3. [Google Scholar] [CrossRef]
- Parker, T.; Madan, E.; Gupta, K.; Moreno, E.; Gogna, R. Cell Competition Spurs Selection of Aggressive Cancer Cells. Trends Cancer 2020, 6, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sharma, S.; Rao, E.; Rowat, A.C.; Gimzewski, J.K.; Han, D.; Rao, J. Cancer Cell Mechanobiology: A New Frontier for Cancer Research. J. Natl. Cancer Cent. 2021, in press. [Google Scholar] [CrossRef]
- Amirouche, A.; Esteves, J.; Lavoignat, A.; Picot, S.; Ferrigno, R.; Faivre, M. Dual Shape Recovery of Red Blood Cells Flowing out of a Microfluidic Constriction. Biomicrofluidics 2020, 14, 024116. [Google Scholar] [CrossRef] [PubMed]
- Fregin, B.; Czerwinski, F.; Biedenweg, D.; Girardo, S.; Gross, S.; Aurich, K.; Otto, O. High-Throughput Single-Cell Rheology in Complex Samples by Dynamic Real-Time Deformability Cytometry. Nat. Commun. 2019, 10, 415. [Google Scholar] [CrossRef] [Green Version]
- Lazarides, E. Intermediate Filaments as Mechanical Integrators of Cellular Space. Nature 1980, 283, 249–256. [Google Scholar] [CrossRef]
- Goldman, R.D.; Milsted, A.; Schloss, J.A.; Starger, J.; Yerna, M.J. Cytoplasmic Fibers in Mammalian Cells: Cytoskeletal and Contractile Elements. Annu. Rev. Physiol. 1979, 41, 703–722. [Google Scholar] [CrossRef]
- Osmanagic-Myers, S.; Dechat, T.; Foisner, R. Lamins at the Crossroads of Mechanosignaling. Genes Dev. 2015, 29, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Gruenbaum, Y.; Foisner, R. Lamins: Nuclear Intermediate Filament Proteins with Fundamental Functions in Nuclear Mechanics and Genome Regulation. Annu. Rev. Biochem. 2015, 84, 131–164. [Google Scholar] [CrossRef]
- Reye, G.; Huang, X.; Haupt, L.M.; Murphy, R.J.; Northey, J.J.; Thompson, E.W.; Momot, K.I.; Hugo, H.J. Mechanical Pressure Driving Proteoglycan Expression in Mammographic Density: A Self-Perpetuating Cycle? J. Mammary Gland. Biol. Neoplasia 2021, 26, 277–296. [Google Scholar] [CrossRef]
- Capaci, V.; Bascetta, L.; Fantuz, M.; Beznoussenko, G.V.; Sommaggio, R.; Cancila, V.; Bisso, A.; Campaner, E.; Mironov, A.A.; Wiśniewski, J.R.; et al. Mutant P53 Induces Golgi Tubulo-Vesiculation Driving a Prometastatic Secretome. Nat. Commun. 2020, 11, 3945. [Google Scholar] [CrossRef]
- Belousov, A.; Titov, S.; Shved, N.; Garbuz, M.; Malykin, G.; Gulaia, V.; Kagansky, A.; Kumeiko, V. The Extracellular Matrix and Biocompatible Materials in Glioblastoma Treatment. Front. Bioeng. Biotechnol. 2019, 7, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebata, T.; Hirata, H.; Kawauchi, K. Functions of the Tumor Suppressors P53 and Rb in Actin Cytoskeleton Remodeling. BioMed. Res. Int. 2016, 2016, 9231057. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Ebata, T.; Guo, A.K.; Tobiume, K.; Wolf, S.J.; Kawauchi, K. P53 Regulates Cytoskeleton Remodeling to Suppress Tumor Progression. Cell. Mol. Life Sci. 2015, 72, 4077–4094. [Google Scholar] [CrossRef] [PubMed]
- Salazar, G.; Cullen, A.; Huang, J.; Zhao, Y.; Serino, A.; Hilenski, L.; Patrushev, N.; Forouzandeh, F.; Hwang, H.S. SQSTM1/P62 and PPARGC1A/PGC-1alpha at the Interface of Autophagy and Vascular Senescence. Autophagy 2020, 16, 1092–1110. [Google Scholar] [CrossRef]
- Homberg, M.; Magin, T.M. Chapter Six—Beyond Expectations: Novel Insights into Epidermal Keratin Function and Regulation. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 311, pp. 265–306. [Google Scholar]
- Liu, Z.; Yu, S.; Ye, S.; Shen, Z.; Gao, L.; Han, Z.; Zhang, P.; Luo, F.; Chen, S.; Kang, M. Keratin 17 Activates AKT Signalling and Induces Epithelial-Mesenchymal Transition in Oesophageal Squamous Cell Carcinoma. J. Proteom. 2020, 211, 103557. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Deng, J.-L.; Wang, L.-P.; Zhang, H.-B.; Tang, L.; Huang, Y.; Tang, J.; Wang, S.-M.; Wang, G. Identification of Candidate Genes Associated with Breast Cancer Prognosis. DNA Cell Biol. 2020, 39, 1205–1227. [Google Scholar] [CrossRef]
- Abashev, T.M.; Metzler, M.A.; Wright, D.M.; Sandell, L.L. Retinoic Acid Signaling Regulates Krt5 and Krt14 Independently of Stem Cell Markers in Submandibular Salivary Gland Epithelium. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2017, 246, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Chamcheu, J.C.; Lorié, E.P.; Akgul, B.; Bannbers, E.; Virtanen, M.; Gammon, L.; Moustakas, A.; Navsaria, H.; Vahlquist, A.; Törmä, H. Characterization of Immortalized Human Epidermolysis Bullosa Simplex (KRT5) Cell Lines: Trimethylamine N-Oxide Protects the Keratin Cytoskeleton against Disruptive Stress Condition. J. Dermatol. Sci. 2009, 53, 198–206. [Google Scholar] [CrossRef]
- Kim, S.; Coulombe, P.A. Intermediate Filament Scaffolds Fulfill Mechanical, Organizational, and Signaling Functions in the Cytoplasm. Genes Dev. 2007, 21, 1581–1597. [Google Scholar] [CrossRef] [Green Version]
- Grimm-Günter, E.-M.S.; Revenu, C.; Ramos, S.; Hurbain, I.; Smyth, N.; Ferrary, E.; Louvard, D.; Robine, S.; Rivero, F. Plastin 1 Binds to Keratin and Is Required for Terminal Web Assembly in the Intestinal Epithelium. Mol. Biol. Cell 2009, 20, 2549–2562. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Yamada, S.; Wirtz, D.; Coulombe, P.A. A “hot-Spot” Mutation Alters the Mechanical Properties of Keratin Filament Networks. Nat. Cell Biol. 2001, 3, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Werner, N.S.; Windoffer, R.; Strnad, P.; Grund, C.; Leube, R.E.; Magin, T.M. Epidermolysis Bullosa Simplex-Type Mutations Alter the Dynamics of the Keratin Cytoskeleton and Reveal a Contribution of Actin to the Transport of Keratin Subunits. Mol. Biol. Cell 2004, 15, 990–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial Fibrillary Acidic Protein: GFAP-Thirty-One Years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Sereika, M.; Urbanaviciute, R.; Tamasauskas, A.; Skiriute, D.; Vaitkiene, P. GFAP Expression Is Influenced by Astrocytoma Grade and Rs2070935 Polymorphism. J. Cancer 2018, 9, 4496–4502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Chen, G.; Dang, Y.; Chen, L.-H. Overexpression of DcR3 and Its Significance on Tumor Cell Differentiation and Proliferation in Glioma. Sci. World, J. 2014, 2014, 605236. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, F.; Peterson, M.K.; Caldeira Araújo, H.; Lautenschläger, F.; Gad, A.K.B. Vimentin Diversity in Health and Disease. Cells 2018, 7, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Z.; Kreplak, L.; Buehler, M.J. Hierarchical Structure Controls Nanomechanical Properties of Vimentin Intermediate Filaments. PLoS ONE 2009, 4, e7294. [Google Scholar] [CrossRef]
- Li, Z.; Mericskay, M.; Agbulut, O.; Butler-Browne, G.; Carlsson, L.; Thornell, L.E.; Babinet, C.; Paulin, D. Desmin Is Essential for the Tensile Strength and Integrity of Myofibrils but Not for Myogenic Commitment, Differentiation, and Fusion of Skeletal Muscle. J. Cell Biol. 1997, 139, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.; Joumaa, V.; Stevens, L.; Neagoe, C.; Li, Z.; Mounier, Y.; Linke, W.; Goubel, F. Passive Stiffness Changes in Soleus Muscles from Desmin Knockout Mice Are Not Due to Titin Modifications. Pflüg. Arch. 2002, 444, 771–776. [Google Scholar] [CrossRef]
- Kiss, B.; Karsai, Á.; Kellermayer, M.S.Z. Nanomechanical Properties of Desmin Intermediate Filaments. J. Struct. Biol. 2006, 155, 327–339. [Google Scholar] [CrossRef]
- Zhao, J.; Liem, R.K.H. Chapter Seventeen—α-Internexin and Peripherin: Expression, Assembly, Functions, and Roles in Disease. In Methods in Enzymology. 2016, 568, 477–507. [Google Scholar]
- Dechat, T.; Adam, S.A.; Taimen, P.; Shimi, T.; Goldman, R.D. Nuclear Lamins. Cold Spring Harb. Perspect. Biol. 2010, 2, a000547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butin-Israeli, V.; Adam, S.A.; Goldman, A.E.; Goldman, R.D. Nuclear Lamin Functions and Disease. Trends Genet. 2012, 28, 464–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Harush, K.; Wiesel, N.; Frenkiel-Krispin, D.; Moeller, D.; Soreq, E.; Aebi, U.; Herrmann, H.; Gruenbaum, Y.; Medalia, O. The Supramolecular Organization of the C. Elegans Nuclear Lamin Filament. J. Mol. Biol. 2009, 386, 1392–1402. [Google Scholar] [CrossRef]
- Yan, S.; Li, P.; Wang, Y.; Yu, W.; Qin, A.; Liu, M.; Xiang, A.P.; Zhang, W.; Li, W. Nestin Regulates Neural Stem Cell Migration via Controlling the Cell Contractility. Int. J. Biochem. Cell Biol. 2016, 78, 349–360. [Google Scholar] [CrossRef]
- Perrin, B.J.; Ervasti, J.M. The Actin Gene Family: Function Follows Isoform. Cytoskeleton 2010, 67, 630–634. [Google Scholar] [CrossRef]
- Köster, D.V.; Husain, K.; Iljazi, E.; Bhat, A.; Bieling, P.; Mullins, R.D.; Rao, M.; Mayor, S. Actomyosin Dynamics Drive Local Membrane Component Organization in an in Vitro Active Composite Layer. Proc. Natl. Acad. Sci. USA 2016, 113, E1645–E1654. [Google Scholar] [CrossRef] [Green Version]
- Svitkina, T.M. Ultrastructure of the actin cytoskeleton. Curr. Opin. Cell Biol. 2018, 54, 1–8. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, D. The Hippo Signaling Pathway in Development and Disease. Dev. Cell 2019, 50, 264–282. [Google Scholar] [CrossRef]
- Carlier, M.F.; Pantaloni, D. Kinetic Analysis of Guanosine 5′-Triphosphate Hydrolysis Associated with Tubulin Polymerization. Biochemistry 1981, 20, 1918–1924. [Google Scholar] [CrossRef]
- Brouhard, G.J.; Rice, L.M. Microtubule Dynamics: An Interplay of Biochemistry and Mechanics. Nat. Rev. Mol. Cell Biol. 2018, 19, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Gardel, M.L.; Kasza, K.E.; Brangwynne, C.P.; Liu, J.; Weitz, D.A. Mechanical Response of Cytoskeletal Networks. Methods Cell Biol. 2008, 89, 487–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, P.; Wang, J.; He, Y.; Zhang, S.; Hu, B.; Xue, X.; Miao, L.; Ren, H. AtFH14 Crosslinks Actin Filaments and Microtubules in Different Manners. Biol. Cell 2021, 113, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, S.V.; Pasapera, A.M.; Sabass, B.; Waterman, C.M. Force Fluctuations within Focal Adhesions Mediate ECM-Rigidity Sensing to Guide Directed Cell Migration. Cell 2012, 151, 1513–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Beyrouthy, J.; Makhoul-Mansour, M.M.; Taylor, G.; Sarles, S.A.; Freeman, E.C. A New Approach for Investigating the Response of Lipid Membranes to Electrocompression by Coupling Droplet Mechanics and Membrane Biophysics. J. R. Soc. Interface 2019, 16, 20190652. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, T.; Capraro, B.R.; Zhu, C.; Das, S.L. Thermodynamics and Mechanics of Membrane Curvature Generation and Sensing by Proteins and Lipids. Annu. Rev. Phys. Chem. 2011, 62, 483–506. [Google Scholar] [CrossRef] [Green Version]
- Sierra-Valdez, F.J.; Forero-Quintero, L.S.; Zapata-Morin, P.A.; Costas, M.; Chavez-Reyes, A.; Ruiz-Suárez, J.C. The Influence of Non Polar and Polar Molecules in Mouse Motile Cells Membranes and Pure Lipid Bilayers. PLoS ONE 2013, 8, e59364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudreau, N.; Myers, C.; Bissell, M.J. From Laminin to Lamin: Regulation of Tissue-Specific Gene Expression by the ECM. Trends Cell Biol. 1995, 5, 1–4. [Google Scholar] [CrossRef]
- Muncie, J.M.; Weaver, V.M. Chapter One—The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. Current Topics in Developmental Biology 2018, 130, 1–37. [Google Scholar]
- Kim, D.-H.; Hah, J.; Wirtz, D. Mechanics of the Cell Nucleus. Adv. Exp. Med. Biol. 2018, 1092, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Fracchia, A.; Asraf, T.; Salmon-Divon, M.; Gerlitz, G. Increased Lamin B1 Levels Promote Cell Migration by Altering Perinuclear Actin Organization. Cells 2020, 9, E2161. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Spinler, K.R.; Swift, J.; Chasis, J.A.; Mohandas, N.; Discher, D.E. Lamins Regulate Cell Trafficking and Lineage Maturation of Adult Human Hematopoietic Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 18892–18897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, K.; Te Lindert, M.; Krause, M.; Alexander, S.; Te Riet, J.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical Limits of Cell Migration: Control by ECM Space and Nuclear Deformation and Tuning by Proteolysis and Traction Force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef] [Green Version]
- Doyle, A.D.; Wang, F.W.; Matsumoto, K.; Yamada, K.M. One-Dimensional Topography Underlies Three-Dimensional Fibrillar Cell Migration. J. Cell Biol. 2009, 184, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, T.; Kim, E.; Riehl, B.D.; Esfahani, A.M.; Rosenbohm, J.; Yang, R.; Duan, B.; Lim, J.Y. The LINC Complex, Mechanotransduction, and Mesenchymal Stem Cell Function and Fate. J. Biol. Eng. 2019, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, T.J.; Lee, J.; Thodeti, C.K.; Lele, T. Actomyosin Tension Exerted on the Nucleus through Nesprin-1 Connections Influences Endothelial Cell Adhesion, Migration, and Cyclic Strain-Induced Reorientation. Biophys. J. 2010, 99, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Ketema, M.; Sonnenberg, A. Nesprin-3: A Versatile Connector between the Nucleus and the Cytoskeleton. Biochem. Soc. Trans. 2011, 39, 1719–1724. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarczyk, A.; Brouwer, T.B.; Pham, C.; Dekker, N.H.; van Noort, J. Probing Chromatin Structure with Magnetic Tweezers. Methods Mol. Biol. 2018, 1814, 297–323. [Google Scholar] [CrossRef]
- Kolimi, N.; Pabbathi, A.; Saikia, N.; Ding, F.; Sanabria, H.; Alper, J. Out-of-Equilibrium Biophysical Chemistry: The Case for Multidimensional, Integrated Single-Molecule Approaches. J. Phys. Chem. B 2021, 125, 10404–10418. [Google Scholar] [CrossRef]
- Kaczmarczyk, A.; Meng, H.; Ordu, O.; van Noort, J.; Dekker, N.H. Chromatin Fibers Stabilize Nucleosomes under Torsional Stress. Nat. Commun. 2020, 11, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa-Garrido, M.; Chapski, D.J.; Schmitt, A.D.; Kimball, T.H.; Karbassi, E.; Monte, E.; Balderas, E.; Pellegrini, M.; Shih, T.-T.; Soehalim, E.; et al. High-Resolution Mapping of Chromatin Conformation in Cardiac Myocytes Reveals Structural Remodeling of the Epigenome in Heart Failure. Circulation 2017, 136, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.M.; Miroshnikova, Y.A.; Biggs, L.C.; Whitefield, D.B.; Metge, F.; Boucas, J.; Vihinen, H.; Jokitalo, E.; Li, X.; García Arcos, J.M.; et al. Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage. Cell 2020, 181, 800–817. [Google Scholar] [CrossRef] [PubMed]
- Árnadóttir, J.; Chalfie, M. Eukaryotic Mechanosensitive Channels. Annu. Rev. Biophys. 2010, 39, 111–137. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.-L. Mechanisms of Hippo Pathway Regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Arthur, W.T.; Burridge, K. RhoA Inactivation by P190RhoGAP Regulates Cell Spreading and Migration by Promoting Membrane Protrusion and Polarity. Mol. Biol. Cell 2001, 12, 2711–2720. [Google Scholar] [CrossRef] [Green Version]
- Rafiq, N.B.M.; Nishimura, Y.; Plotnikov, S.V.; Thiagarajan, V.; Zhang, Z.; Shi, S.; Natarajan, M.; Viasnoff, V.; Kanchanawong, P.; Jones, G.E.; et al. A Mechano-Signalling Network Linking Microtubules, Myosin IIA Filaments and Integrin-Based Adhesions. Nat. Mater. 2019, 18, 638–649. [Google Scholar] [CrossRef] [Green Version]
- Trelstad, R.L.; Hayashi, K. Tendon Collagen Fibrillogenesis: Intracellular Subassemblies and Cell Surface Changes Associated with Fibril Growth. Dev. Biol. 1979, 71, 228–242. [Google Scholar] [CrossRef]
- Prager-Khoutorsky, M.; Lichtenstein, A.; Krishnan, R.; Rajendran, K.; Mayo, A.; Kam, Z.; Geiger, B.; Bershadsky, A.D. Fibroblast Polarization Is a Matrix-Rigidity-Dependent Process Controlled by Focal Adhesion Mechanosensing. Nat. Cell Biol. 2011, 13, 1457–1465. [Google Scholar] [CrossRef]
- Vogel, V.; Sheetz, M. Local Force and Geometry Sensing Regulate Cell Functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane Crosstalk between the Extracellular Matrix and the Cytoskeleton. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A. The Extracellular Matrix Viscoelasticity as a Regulator of Cell and Tissue Dynamics. Curr. Opin. Cell Biol. 2021, 72, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, Z.; Sha, D.; Ma, Y.; Kim, B.Y.S.; Jiang, W.; Yuan, Y.; Liu, C. Injectable, Viscoelastic Hydrogel Precisely Regulates Developmental Tissue Regeneration. Chem. Eng. J. 2021, in press. [Google Scholar] [CrossRef]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Ihsan, A.B.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical Hydrogels Composed of Polyampholytes Demonstrate High Toughness and Viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, H.; Wang, H.; Zhang, Z.; Yang, W.; Liu, W.; Li, Y.; Li, L. Biomaterial Stiffness Determines Stem Cell Fate. Life Sci. 2017, 178, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate Modulus Directs Neural Stem Cell Behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef] [Green Version]
- Thomas, K.; Engler, A.J.; Meyer, G.A. Extracellular Matrix Regulation in the Muscle Satellite Cell Niche. Connect. Tissue Res. 2015, 56, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, P.M.; Havenstrite, K.L.; Magnusson, K.E.G.; Sacco, A.; Leonardi, N.A.; Kraft, P.; Nguyen, N.K.; Thrun, S.; Lutolf, M.P.; Blau, H.M. Substrate Elasticity Regulates Skeletal Muscle Stem Cell Self-Renewal in Culture. Science 2010, 329, 1078–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.L.; Cooke, M.E.; Alliston, T. ECM Stiffness Primes the TGFβ Pathway to Promote Chondrocyte Differentiation. Mol. Biol. Cell 2012, 23, 3731–3742. [Google Scholar] [CrossRef]
- Calderwood, D.A. Talin Controls Integrin Activation. Biochem. Soc. Trans. 2004, 32, 434–437. [Google Scholar] [CrossRef]
- Stupack, D.G.; Cheresh, D.A. Get a Ligand, Get a Life: Integrins, Signaling and Cell Survival. J. Cell Sci. 2002, 115, 3729–3738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphries, J.D.; Chastney, M.R.; Askari, J.A.; Humphries, M.J. Signal Transduction via Integrin Adhesion Complexes. Curr. Opin. Cell Biol. 2019, 56, 14–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, G.; Malmsten, M.; Ermilov, E. Anionic Biopolyelectrolytes of the Syndecan/Perlecan Superfamily: Physicochemical Properties and Medical Significance. Adv. Colloid Interface Sci. 2014, 205, 275–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Provenzano, P.P.; Smith, C.L.; Levchenko, A. Matrix Nanotopography as a Regulator of Cell Function. J. Cell Biol. 2012, 197, 351–360. [Google Scholar] [CrossRef]
- Chaterji, S.; Lam, C.H.; Ho, D.S.; Proske, D.C.; Baker, A.B. Syndecan-1 Regulates Vascular Smooth Muscle Cell Phenotype. PLoS ONE 2014, 9, e89824. [Google Scholar] [CrossRef] [Green Version]
- Yim, E.K.F.; Darling, E.M.; Kulangara, K.; Guilak, F.; Leong, K.W. Nanotopography-Induced Changes in Focal Adhesions, Cytoskeletal Organization, and Mechanical Properties of Human Mesenchymal Stem Cells. Biomaterials 2010, 31, 1299–1306. [Google Scholar] [CrossRef] [Green Version]
- Teo, B.K.K.; Wong, S.T.; Lim, C.K.; Kung, T.Y.S.; Yap, C.H.; Ramagopal, Y.; Romer, L.H.; Yim, E.K.F. Nanotopography Modulates Mechanotransduction of Stem Cells and Induces Differentiation through Focal Adhesion Kinase. ACS Nano 2013, 7, 4785–4798. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in Development: A Growing Role for Contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Kolahi, K.S.; Donjacour, A.; Liu, X.; Lin, W.; Simbulan, R.K.; Bloise, E.; Maltepe, E.; Rinaudo, P. Effect of Substrate Stiffness on Early Mouse Embryo Development. PLoS ONE 2012, 7, e41717. [Google Scholar] [CrossRef]
- Abbas, Y.; Carnicer-Lombarte, A.; Gardner, L.; Thomas, J.; Brosens, J.J.; Moffett, A.; Sharkey, A.M.; Franze, K.; Burton, G.J.; Oyen, M.L. Tissue Stiffness at the Human Maternal–Fetal Interface. Hum. Reprod. 2019, 34, 1999–2008. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.J.; Costanzo, M.; Ruiz-Herrero, T.; Mönke, G.; Petrie, R.J.; Bergert, M.; Diz-Muñoz, A.; Mahadevan, L.; Hiiragi, T. Hydraulic Control of Mammalian Embryo Size and Cell Fate. Nature 2019, 571, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulou, E.; Galea, G.L.; Rolo, A.; Greene, N.D.E.; Copp, A.J. Neural Tube Closure: Cellular, Molecular and Biomechanical Mechanisms. Dev. Camb. Engl. 2017, 144, 552–566. [Google Scholar] [CrossRef] [Green Version]
- Greene, N.D.E.; Copp, A.J. Neural Tube Defects. Annu. Rev. Neurosci. 2014, 37, 221–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, H.; Robin, F.B.; Sherrard, K.M.; Munro, E.M. Sequential Contraction and Exchange of Apical Junctions Drives Zippering and Neural Tube Closure in a Simple Chordate. Dev. Cell 2015, 32, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, Y.; Suzuki, M.; Watanabe, T.; Yasue, N.; Tateo, I.; Adachi, T.; Ueno, N. Mechanical Roles of Apical Constriction, Cell Elongation, and Cell Migration during Neural Tube Formation in Xenopus. Biomech. Model. Mechanobiol. 2016, 15, 1733–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heisenberg, C.-P.; Bellaïche, Y. Forces in Tissue Morphogenesis and Patterning. Cell 2013, 153, 948–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, M.T.; Wallingford, J.B. Spatial and Temporal Analysis of PCP Protein Dynamics during Neural Tube Closure. eLife 2018, 7, e36456. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulou, E.; Hirst, C.S.; Galea, G.; Venturini, C.; Moulding, D.; Marshall, A.R.; Rolo, A.; De Castro, S.C.P.; Copp, A.J.; Greene, N.D.E. Spinal Neural Tube Closure Depends on Regulation of Surface Ectoderm Identity and Biomechanics by Grhl2. Nat. Commun. 2019, 10, 2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, S.T.; Dean, B.C.; Dean, D. A Computational Approach to Understand Phenotypic Structure and Constitutive Mechanics Relationships of Single Cells. Ann. Biomed. Eng. 2013, 41, 630–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunwald, G.B. Chapter 2—Cadherin Cell Adhesion Molecules in Normal and Abnormal Neural Development. In Handbook of Developmental Neurotoxicology; Slikker, W., Chang, L.W., Eds.; Academic Press: San Diego, CA, USA, 1998; pp. 43–60. ISBN 978-0-12-648860-9. [Google Scholar]
- Weber, G.F.; Bjerke, M.A.; DeSimone, D.W. A Mechanoresponsive Cadherin-Keratin Complex Directs Polarized Protrusive Behavior and Collective Cell Migration. Dev. Cell 2012, 22, 104–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowotschin, S.; Hadjantonakis, A.-K.; Campbell, K. The Endoderm: A Divergent Cell Lineage with Many Commonalities. Dev. Camb. Engl. 2019, 146, dev150920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maître, J.-L.; Berthoumieux, H.; Krens, S.F.G.; Salbreux, G.; Jülicher, F.; Paluch, E.; Heisenberg, C.-P. Adhesion Functions in Cell Sorting by Mechanically Coupling the Cortices of Adhering Cells. Science 2012, 338, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.; Singh, S.S.; Velankar, S.; Kumta, P.N.; Banerjee, I. Inducing Endoderm Differentiation by Modulating Mechanical Properties of Soft Substrates. J. Tissue Eng. Regen. Med. 2015, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varner, V.D.; Taber, L.A. Not Just Inductive: A Crucial Mechanical Role for the Endoderm during Heart Tube Assembly. Development 2012, 139, 1680–1690. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Jackson, T.R.; Davidson, L.A. On the Role of Mechanics in Driving Mesenchymal-to-Epithelial Transitions. Semin. Cell Dev. Biol. 2017, 67, 113–122. [Google Scholar] [CrossRef]
- Kashkooli, L.; Rozema, D.; Espejo-Ramirez, L.; Lasko, P.; Fagotto, F. Ectoderm to Mesoderm Transition by Down-Regulation of Actomyosin Contractility. PLoS Biol. 2021, 19, e3001060. [Google Scholar] [CrossRef]
- Xue, X.; Sun, Y.; Resto-Irizarry, A.; Yuan, Y.; Aw Yong, K.M.; Zheng, Y.; Weng, S.; Shao, Y.; Chai, Y.; Studer, L.; et al. Mechanics-Guided Embryonic Patterning of Neuroectoderm Tissue from Human Pluripotent Stem Cells. Nat. Mater. 2018, 17, 633–641. [Google Scholar] [CrossRef]
- Fortunato, A.; Boddy, A.; Mallo, D.; Aktipis, A.; Maley, C.C.; Pepper, J.W. Natural Selection in Cancer Biology: From Molecular Snowflakes to Trait Hallmarks. Cold Spring Harb. Perspect. Med. 2017, 7, a029652. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.J. Non-Random Selection of Cancer-Causing Mutations in Tissue-Specific Stem Cells Cause Cancer. J. Clin. Oncol. Res. 2020, 8, 1063. [Google Scholar]
- Weissman, I.L. Stem Cells Are Units of Natural Selection for Tissue Formation, for Germline Development, and in Cancer Development. Proc. Natl. Acad. Sci. USA. 2015, 112, 8922–8928. [Google Scholar] [CrossRef] [Green Version]
- Phan, T.G.; Croucher, P.I. The Dormant Cancer Cell Life Cycle. Nat. Rev. Cancer 2020, 20, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Hupfer, A.; Brichkina, A.; Koeniger, A.; Keber, C.; Denkert, C.; Pfefferle, P.; Helmprobst, F.; Pagenstecher, A.; Visekruna, A.; Lauth, M. Matrix Stiffness Drives Stromal Autophagy and Promotes Formation of a Protumorigenic Niche. Proc. Natl. Acad. Sci. USA 2021, 118, e2105367118. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; McEwen, G.D.; Harihar, S.; Baker, S.M.; DeWald, D.B.; Zhou, A. BRMS1 Expression Alters the Ultrastructural, Biomechanical and Biochemical Properties of MDA-MB-435 Human Breast Carcinoma Cells: An AFM and Raman Microspectroscopy Study. Cancer Lett. 2010, 293, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Lekka, M.; Pogoda, K.; Gostek, J.; Klymenko, O.; Prauzner-Bechcicki, S.; Wiltowska-Zuber, J.; Jaczewska, J.; Lekki, J.; Stachura, Z. Cancer Cell Recognition--Mechanical Phenotype. Micron 2012, 43, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.; Lekka, M.; Stylianopoulos, T. AFM Assessing of Nanomechanical Fingerprints for Cancer Early Diagnosis and Classification: From Single Cell to Tissue Level. Nanoscale 2018, 10, 20930–20945. [Google Scholar] [CrossRef] [PubMed]
- Osmulski, P.A.; Cunsolo, A.; Chen, M.; Qian, Y.; Lin, C.-L.; Hung, C.-N.; Mahalingam, D.; Kirma, N.B.; Chen, C.-L.; Taverna, J.A.; et al. Contacts with Macrophages Promote an Aggressive Nanomechanical Phenotype of Circulating Tumor Cells in Prostate Cancer. Cancer Res. 2021, 81, 4110–4123. [Google Scholar] [CrossRef]
- Smolyakov, G.; Thiebot, B.; Campillo, C.; Labdi, S.; Severac, C.; Pelta, J.; Dague, É. Elasticity, Adhesion, and Tether Extrusion on Breast Cancer Cells Provide a Signature of Their Invasive Potential. ACS Appl. Mater. Interfaces 2016, 8, 27426–27431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, J.R.; Pabijan, J.; Garcia, R.; Lekka, M. The Softening of Human Bladder Cancer Cells Happens at an Early Stage of the Malignancy Process. Beilstein, J. Nanotechnol. 2014, 5, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Chivukula, V.K.; Krog, B.L.; Nauseef, J.T.; Henry, M.D.; Vigmostad, S.C. Alterations in Cancer Cell Mechanical Properties after Fluid Shear Stress Exposure: A Micropipette Aspiration Study. Cell Health Cytoskelet. 2015, 7, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogoda, K.; Pięta, E.; Roman, M.; Piergies, N.; Liberda, D.; Wróbel, T.P.; Janmey, P.A.; Paluszkiewicz, C.; Kwiatek, W.M. In Search of the Correlation between Nanomechanical and Biomolecular Properties of Prostate Cancer Cells with Different Metastatic Potential. Arch. Biochem. Biophys. 2021, 697, 108718. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kuramochi, H.; Takahashi, A.; Imai, K.; Katsuta, N.; Nakayama, T.; Fujiki, H.; Suganuma, M. Higher Cell Stiffness Indicating Lower Metastatic Potential in B16 Melanoma Cell Variants and in (−)-Epigallocatechin Gallate-Treated Cells. J. Cancer Res. Clin. Oncol. 2012, 138, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Abidine, Y.; Constantinescu, A.; Laurent, V.M.; Sundar Rajan, V.; Michel, R.; Laplaud, V.; Duperray, A.; Verdier, C. Mechanosensitivity of Cancer Cells in Contact with Soft Substrates Using AFM. Biophys. J. 2018, 114, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-T.; Osmulski, P.; Wang, Y.; Huang, Y.-W.; Liu, L.; Ruan, J.; Jin, V.X.; Kirma, N.B.; Gaczynska, M.E.; Huang, T.H.-M. EpCAM-Regulated Transcription Exerts Influences on Nanomechanical Properties of Endometrial Cancer Cells That Promote Epithelial-to-Mesenchymal Transition. Cancer Res. 2016, 76, 6171–6182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, F.; Lin, T.-C.; Wang, L.; Chen, S.; Chen, X.; Yiu, P.M.; Tsui, O.K.C.; Chu, J.; Kiang, C.-H.; Park, H. Mechanical Responses of Breast Cancer Cells to Substrates of Varying Stiffness Revealed by Single-Cell Measurements. J. Phys. Chem. Lett. 2020, 11, 7643–7649. [Google Scholar] [CrossRef]
- Yousafzai, M.S.; Coceano, G.; Bonin, S.; Niemela, J.; Scoles, G.; Cojoc, D. Investigating the Effect of Cell Substrate on Cancer Cell Stiffness by Optical Tweezers. J. Biomech. 2017, 60, 266–269. [Google Scholar] [CrossRef]
- Rianna, C.; Kumar, P.; Radmacher, M. The Role of the Microenvironment in the Biophysics of Cancer. Semin. Cell Dev. Biol. 2018, 73, 107–114. [Google Scholar] [CrossRef]
- Filipe, E.C.; Chitty, J.L.; Cox, T.R. Charting the Unexplored Extracellular Matrix in Cancer. Int. J. Exp. Pathol. 2018, 99, 58–76. [Google Scholar] [CrossRef] [Green Version]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular Matrix (ECM) Stiffness and Degradation as Cancer Drivers. J. Cell. Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Amos, S.E.; Choi, Y.S. The Cancer Microenvironment: Mechanical Challenges of the Metastatic Cascade. Front. Bioeng. Biotechnol. 2021, 9, 625859. [Google Scholar] [CrossRef]
- Pratt, S.J.P.; Lee, R.M.; Martin, S.S. The Mechanical Microenvironment in Breast Cancer. Cancers 2020, 12, 1452. [Google Scholar] [CrossRef]
- Cox, T.R. The Matrix in Cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Tschumperlin, D.J.; Lagares, D. Mechano-Therapeutics: Targeting Mechanical Signaling in Fibrosis and Tumor Stroma. Pharmacol. Ther. 2020, 212, 107575. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Cox, T.R.; Erler, J.T. The Rationale for Targeting the LOX Family in Cancer. Nat. Rev. Cancer 2012, 12, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eble, J.A.; Niland, S. The Extracellular Matrix in Tumor Progression and Metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef]
- Mezzenga, R.; Mitsi, M. The Molecular Dance of Fibronectin: Conformational Flexibility Leads to Functional Versatility. Biomacromolecules 2019, 20, 55–72. [Google Scholar] [CrossRef]
- Rick, J.W.; Chandra, A.; Dalle Ore, C.; Nguyen, A.T.; Yagnik, G.; Aghi, M.K. Fibronectin in Malignancy: Cancer-Specific Alterations, Protumoral Effects, and Therapeutic Implications. Semin. Oncol. 2019, 46, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Beck, K. Laminin 332 Processing Impacts Cellular Behavior. Cell Adhes. Migr. 2013, 7, 122–134. [Google Scholar] [CrossRef] [Green Version]
- Eckert, R.L.; Fisher, M.L.; Grun, D.; Adhikary, G.; Xu, W.; Kerr, C. Transglutaminase Is a Tumor Cell and Cancer Stem Cell Survival Factor: TRANSGLUTAMINASE 2 IN CANCER. Mol. Carcinog. 2015, 54, 947–958. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Ma, M.; Du, Z.; Liu, Z.; Gong, X. Quantitative Evaluation of Tissue Stiffness around Lesion by Sound Touch Elastography in the Diagnosis of Benign and Malignant Breast Lesions. PLoS ONE 2019, 14, e0219943. [Google Scholar] [CrossRef] [Green Version]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix Metalloproteinases (MMPs), the Main Extracellular Matrix (ECM) Enzymes in Collagen Degradation, as a Target for Anticancer Drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesanakurti, D.; Chetty, C.; Dinh, D.H.; Gujrati, M.; Rao, J.S. Role of MMP-2 in the Regulation of IL-6/Stat3 Survival Signaling via Interaction with A5β1 Integrin in Glioma. Oncogene 2013, 32, 327–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent Advances in Hydrogels for Cartilage Tissue Engineering. Eur. Cell. Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin-Based Injectable Biomaterials for Bone Tissue Engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; Del Bene, M.; Rampini, A.; Mattei, L.; Casali, C.; Vetrano, I.G.; Gennari, A.G.; Sdao, S.; Saini, M.; Sconfienza, L.M.; et al. Intraoperative Strain Elastosonography in Brain Tumor Surgery. Oper. Neurosurg. 2019, 17, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Li, R.; Lu, G.; Jiang, L.; Kang, H.; Kirk Shung, K.; Humayun, M.S.; Zhou, Q. Ultrasonic Elastography to Assess Biomechanical Properties of the Optic Nerve Head and Peripapillary Sclera of the Eye. Ultrasonics 2021, 110, 106263. [Google Scholar] [CrossRef]
- Pagé, G.; Tardieu, M.; Besret, L.; Blot, L.; Lopes, J.; Sinkus, R.; Van Beers, B.E.; Garteiser, P. Assessing Tumor Mechanics by MR Elastography at Different Strain Levels. J. Magn. Reson. Imaging 2019, 50, 1982–1989. [Google Scholar] [CrossRef]
- Huml, M.; Silye, R.; Zauner, G.; Hutterer, S.; Schilcher, K. Brain Tumor Classification Using AFM in Combination with Data Mining Techniques. BioMed Res. Int. 2013, 2013, 176519. [Google Scholar] [CrossRef] [Green Version]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.L.; Rich, J.N. Cancer Stem Cells in Glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef] [Green Version]
- Pobbati, A.V.; Hong, W. A Combat with the YAP/TAZ-TEAD Oncoproteins for Cancer Therapy. Theranostics 2020, 10, 3622–3635. [Google Scholar] [CrossRef] [PubMed]
- Chiquet-Ehrismann, R.; Orend, G.; Chiquet, M.; Tucker, R.P.; Midwood, K.S. Tenascins in Stem Cell Niches. Matrix Biol. 2014, 37, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, J.; Brösicke, N.; Theocharidis, U.; Faissner, A. The Extracellular Matrix Niche Microenvironment of Neural and Cancer Stem Cells in the Brain. Int. J. Biochem. Cell Biol. 2016, 81, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Senthebane, D.A.; Jonker, T.; Rowe, A.; Thomford, N.E.; Munro, D.; Dandara, C.; Wonkam, A.; Govender, D.; Calder, B.; Soares, N.C.; et al. The Role of Tumor Microenvironment in Chemoresistance: 3D Extracellular Matrices as Accomplices. Int. J. Mol. Sci. 2018, 19, 2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweizer, J.; Bowden, P.E.; Coulombe, P.A.; Langbein, L.; Lane, E.B.; Magin, T.M.; Maltais, L.; Omary, M.B.; Parry, D.A.D.; Rogers, M.A.; et al. New Consensus Nomenclature for Mammalian Keratins. J. Cell Biol. 2006, 174, 169–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shmelev, M.E.; Titov, S.I.; Belousov, A.S.; Farniev, V.M.; Zhmenia, V.M.; Lanskikh, D.V.; Penkova, A.O.; Kumeiko, V.V. Cell and Tissue Nanomechanics: From Early Development to Carcinogenesis. Biomedicines 2022, 10, 345. https://doi.org/10.3390/biomedicines10020345
Shmelev ME, Titov SI, Belousov AS, Farniev VM, Zhmenia VM, Lanskikh DV, Penkova AO, Kumeiko VV. Cell and Tissue Nanomechanics: From Early Development to Carcinogenesis. Biomedicines. 2022; 10(2):345. https://doi.org/10.3390/biomedicines10020345
Chicago/Turabian StyleShmelev, Mikhail E., Sergei I. Titov, Andrei S. Belousov, Vladislav M. Farniev, Valeriia M. Zhmenia, Daria V. Lanskikh, Alina O. Penkova, and Vadim V. Kumeiko. 2022. "Cell and Tissue Nanomechanics: From Early Development to Carcinogenesis" Biomedicines 10, no. 2: 345. https://doi.org/10.3390/biomedicines10020345
APA StyleShmelev, M. E., Titov, S. I., Belousov, A. S., Farniev, V. M., Zhmenia, V. M., Lanskikh, D. V., Penkova, A. O., & Kumeiko, V. V. (2022). Cell and Tissue Nanomechanics: From Early Development to Carcinogenesis. Biomedicines, 10(2), 345. https://doi.org/10.3390/biomedicines10020345





