Integrative Identification of Genetic Loci Jointly Influencing Diabetes-Related Traits and Sleep Traits of Insomnia, Sleep Duration, and Chronotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Statistical Analysis
2.2.1. Conditional Quantile–Quantile (Q–Q) Plots
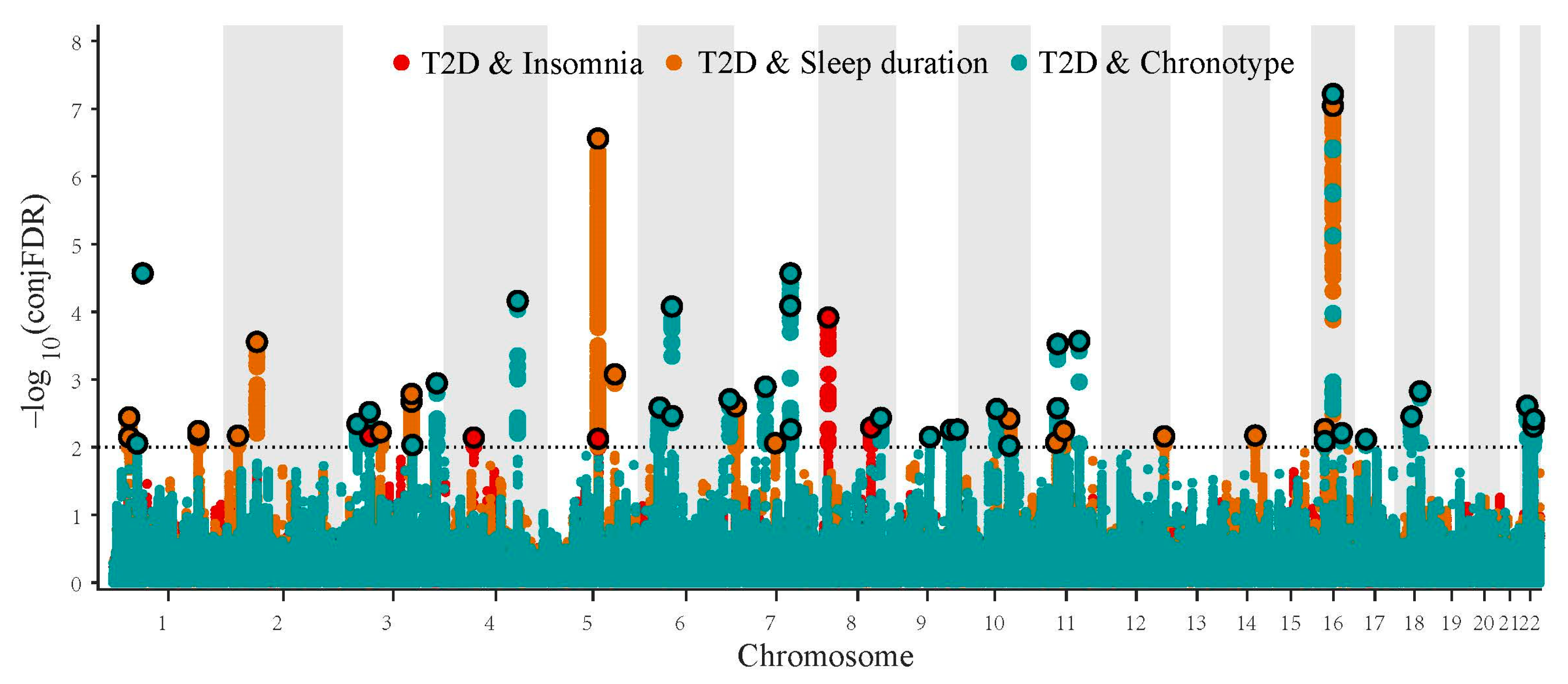
2.2.2. Identification for Pleiotropic Loci
2.2.3. Functional Annotation
2.2.4. Expression Analysis of Pleiotropic Genes
2.2.5. Mendelian Randomization Study
3. Results
3.1. Assessment of Pleiotropic Enrichment
3.2. Pleiotropic Gene Loci in Diabetes-Relevant Phenotype and Sleep Traits Identified with ConjFDR
3.3. Functional Annotation of Pleiotropic Gene
3.4. Differential Expression of Pleiotropic Genes
3.5. Mendelian Randomization Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic, M.; Keaton, J.M.; Lynch, J.A.; Miller, D.R.; Zhou, J.; Tcheandjieu, C.; Huffman, J.E.; Assimes, T.L.; Lorenz, K.; Zhu, X.; et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat. Genet. 2020, 52, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Almgren, P.; Lehtovirta, M.; Isomaa, B.; Sarelin, L.; Taskinen, M.R.; Lyssenko, V.; Tuomi, T.; Groop, L. Heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia Study. Diabetologia 2011, 54, 2811–2819. [Google Scholar] [CrossRef]
- Udler, M.S.; McCarthy, M.I.; Florez, J.C.; Mahajan, A. Genetic Risk Scores for Diabetes Diagnosis and Precision Medicine. Endocr. Rev. 2019, 40, 1500–1520. [Google Scholar] [CrossRef]
- Watanabe, K.; Stringer, S.; Frei, O.; Umićević Mirkov, M.; de Leeuw, C.; Polderman, T.J.C.; van der Sluis, S.; Andreassen, O.A.; Neale, B.M.; Posthuma, D. A global overview of pleiotropy and genetic architecture in complex traits. Nat. Genet. 2019, 51, 1339–1348. [Google Scholar] [CrossRef]
- Desikan, R.S.; Schork, A.J.; Wang, Y.; Thompson, W.K.; Dehghan, A.; Ridker, P.M.; Chasman, D.I.; McEvoy, L.K.; Holland, D.; Chen, C.H.; et al. Polygenic Overlap Between C-Reactive Protein, Plasma Lipids, and Alzheimer Disease. Circulation 2015, 131, 2061–2069. [Google Scholar] [CrossRef][Green Version]
- Andreassen, O.A.; Thompson, W.K.; Dale, A.M. Boosting the power of schizophrenia genetics by leveraging new statistical tools. Schizophr. Bull. 2014, 40, 13–17. [Google Scholar] [CrossRef]
- Schork, A.J.; Wang, Y.; Thompson, W.K.; Dale, A.M.; Andreassen, O.A. New statistical approaches exploit the polygenic architecture of schizophrenia--implications for the underlying neurobiology. Curr. Opin. Neurobiol. 2016, 36, 89–98. [Google Scholar] [CrossRef]
- Hammerschlag, A.R.; Stringer, S.; de Leeuw, C.A.; Sniekers, S.; Taskesen, E.; Watanabe, K.; Blanken, T.F.; Dekker, K.; Te Lindert, B.H.W.; Wassing, R.; et al. Genome-wide association analysis of insomnia complaints identifies risk genes and genetic overlap with psychiatric and metabolic traits. Nat. Genet. 2017, 49, 1584–1592. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Liao, D.; Pejovic, S.; Calhoun, S.; Karataraki, M.; Bixler, E.O. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care 2009, 32, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, E.M.; Dudley, K.A.; Sotres-Alvarez, D.; Zee, P.C.; Daviglus, M.L.; Shah, N.A.; Talavera, G.A.; Gallo, L.C.; Mattei, J.; Qi, Q.; et al. Joint associations of insomnia and sleep duration with prevalent diabetes: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). J. Diabetes 2016, 8, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, H.; Zhang, Y.; Liu, L.; Wang, J.; Wang, T. Investigating Causal Relations Between Sleep-Related Traits and Risk of Type 2 Diabetes Mellitus: A Mendelian Randomization Study. Front. Genet. 2020, 11, 607865. [Google Scholar] [CrossRef] [PubMed]
- Buxton, O.M.; Pavlova, M.; Reid, E.W.; Wang, W.; Simonson, D.C.; Adler, G.K. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010, 59, 2126–2133. [Google Scholar] [CrossRef]
- Leproult, R.; Deliens, G.; Gilson, M.; Peigneux, P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep 2015, 38, 707–715. [Google Scholar] [CrossRef]
- Dashti, H.S.; Redline, S.; Saxena, R. Polygenic risk score identifies associations between sleep duration and diseases determined from an electronic medical record biobank. Sleep 2019, 42, zsy247. [Google Scholar] [CrossRef]
- Byrne, E.M. The relationship between insomnia and complex diseases—Insights from genetic data. Genome Med. 2019, 11, 57. [Google Scholar] [CrossRef]
- Adan, A.; Archer, S.N.; Hidalgo, M.P.; Di Milia, L.; Natale, V.; Randler, C. Circadian typology: A comprehensive review. Chronobiol. Int. 2012, 29, 1153–1175. [Google Scholar] [CrossRef]
- Tonetti, L.; Adan, A.; Di Milia, L.; Randler, C.; Natale, V. Measures of circadian preference in childhood and adolescence: A review. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2015, 30, 576–582. [Google Scholar] [CrossRef]
- Baron, K.G.; Reid, K.J. Chapter 26—Relationship between Circadian Rhythms, Feeding, and Obesity. In Modulation of Sleep by Obesity, Diabetes, Age, and Diet; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 243–253. [Google Scholar]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N.; et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef]
- Chen, J.; Spracklen, C.N.; Marenne, G.; Varshney, A.; Corbin, L.J.; Luan, J.a.; Willems, S.M.; Wu, Y.; Zhang, X.; Horikoshi, M.; et al. The trans-ancestral genomic architecture of glycemic traits. Nat. Genet. 2021, 53, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.R.; Watanabe, K.; Stringer, S.; Skene, N.; Bryois, J.; Hammerschlag, A.R.; de Leeuw, C.A.; Benjamins, J.S.; Muñoz-Manchado, A.B.; Nagel, M. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat. Genet. 2019, 51, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Smeland, O.B.; Frei, O.; Shadrin, A.; O’Connell, K.; Fan, C.-C.; Bahrami, S.; Holland, D.; Djurovic, S.; Thompson, W.K.; Dale, A.M. Discovery of shared genomic loci using the conditional false discovery rate approach. Hum. Genet. 2020, 139, 85–94. [Google Scholar] [CrossRef]
- Chelala, C.; Khan, A.; Lemoine, N.R. SNPnexus: A web database for functional annotation of newly discovered and public domain single nucleotide polymorphisms. Bioinformatics 2009, 25, 655–661. [Google Scholar] [CrossRef]
- Kircher, M.; Witten, D.M.; Jain, P.; O’roak, B.J.; Cooper, G.M.; Shendure, J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014, 46, 310–315. [Google Scholar] [CrossRef]
- Boyle, A.P.; Hong, E.L.; Hariharan, M.; Cheng, Y.; Schaub, M.A.; Kasowski, M.; Karczewski, K.J.; Park, J.; Hitz, B.C.; Weng, S. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012, 22, 1790–1797. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Võsa, U.; Claringbould, A.; Westra, H.-J.; Bonder, M.J.; Deelen, P.; Zeng, B.; Kirsten, H.; Saha, A.; Kreuzhuber, R.; Yazar, S.; et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 2021, 53, 1300–1310. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Prats-Puig, A.; Grau-Cabrera, P.; Riera-Pérez, E.; Cortés-Marina, R.; Fortea, E.; Soriano-Rodríguez, P.; de Zegher, F.; Ibánez, L.; Bassols, J.; López-Bermejo, A. Variations in the obesity genes FTO, TMEM18 and NRXN3 influence the vulnerability of children to weight gain induced by short sleep duration. Int. J. Obes. 2013, 37, 182–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stratigopoulos, G.; Padilla, S.L.; LeDuc, C.A.; Watson, E.; Hattersley, A.T.; McCarthy, M.I.; Zeltser, L.M.; Chung, W.K.; Leibel, R.L. Regulation of Fto/Ftm gene expression in mice and humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1185–R1196. [Google Scholar] [CrossRef]
- Gerken, T.; Girard, C.A.; Tung, Y.C.; Webby, C.J.; Saudek, V.; Hewitson, K.S.; Yeo, G.S.; McDonough, M.A.; Cunliffe, S.; McNeill, L.A.; et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007, 318, 1469–1472. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Morrison, C. The brain, appetite, and obesity. Annu. Rev. Psychol. 2008, 59, 55–92. [Google Scholar] [CrossRef]
- Héron, L.; Virsolvy, A.; Apiou, F.; Le Cam, A.; Bataille, D. Isolation, characterization, and chromosomal localization of the human ENSA gene that encodes alpha-endosulfine, a regulator of beta-cell K(ATP) channels. Diabetes 1999, 48, 1873–1876. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kubota, M.; Kosaki, R.; Kosaki, K.; Ishiguro, A. A severe form of autosomal recessive spinocerebellar ataxia associated with novel PMPCA variants. Brain Dev. 2021, 43, 464–469. [Google Scholar] [CrossRef]
- Choquet, K.; Zurita-Rendón, O.; La Piana, R.; Yang, S.; Dicaire, M.J.; Boycott, K.M.; Majewski, J.; Shoubridge, E.A.; Brais, B.; Tétreault, M. Autosomal recessive cerebellar ataxia caused by a homozygous mutation in PMPCA. Brain A J. Neurol. 2016, 139, e19. [Google Scholar] [CrossRef]
- Jobling, R.K.; Assoum, M.; Gakh, O.; Blaser, S.; Raiman, J.A.; Mignot, C.; Roze, E.; Dürr, A.; Brice, A.; Lévy, N.; et al. PMPCA mutations cause abnormal mitochondrial protein processing in patients with non-progressive cerebellar ataxia. Brain A J. Neurol. 2015, 138, 1505–1517. [Google Scholar] [CrossRef]
- Corbi, G.; Conti, V.; Russomanno, G.; Longobardi, G.; Furgi, G.; Filippelli, A.; Ferrara, N. Adrenergic signaling and oxidative stress: A role for sirtuins? Front. Physiol. 2013, 4, 324. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Wefers, J.; Meier, J.J. Treatment of type 2 diabetes: Challenges, hopes, and anticipated successes. Lancet. Diabetes Endocrinol. 2021, 9, 525–544. [Google Scholar] [CrossRef]
- Lotti, S.; Pagliai, G.; Colombini, B.; Sofi, F.; Dinu, M. Chronotype Differences in Energy Intake, Cardiometabolic Risk Parameters, Cancer and Depression: A Systematic Review with Meta-analysis of Observational Studies. Adv. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.M.; Hadjiconstantinou, M.; Brady, E.M.; Bodicoat, D.H.; Henson, J.J.; Hall, A.P.; Davies, M.J. Chronotype and well-being in adults with established type 2 diabetes: A cross-sectional study. Diabet. Med. A J. Br. Diabet. Assoc. 2021, e14690. [Google Scholar] [CrossRef]
- Brown, M.R.; Sen, S.K.; Mazzone, A.; Her, T.K.; Xiong, Y.; Lee, J.-H.; Javeed, N.; Colwell, C.S.; Rakshit, K.; LeBrasseur, N.K.; et al. Time-restricted feeding prevents deleterious metabolic effects of circadian disruption through epigenetic control of β; cell function. Sci. Adv. 2021, 7, eabg6856. [Google Scholar] [CrossRef] [PubMed]
- Reis-Canaan, J.C.; Canaan, M.M.; Costa, P.D.; Rodrigues-Juliatte, T.P.; Pereira, M.C.A.; Castelo, P.M.; Pardi, V.; M Murata, R.; Pereira, L.J. Association between Chronotype and Nutritional, Clinical and Sociobehavioral Characteristics of Adults Assisted by a Public Health Care System in Brazil. Nutrients 2021, 13, 2260. [Google Scholar] [CrossRef] [PubMed]

| Locus No. | SNP | Gene | Chr:Pos | A1/A2 | Z Score Type 2 Diabetes | Z Score Sleep Traits | ConjFDR | p-Value Type 2 Diabetes | p-Value Sleep Traits |
|---|---|---|---|---|---|---|---|---|---|
| Type 2 diabetes and insomnia | |||||||||
| 1 | rs2820290 | NAV1 | 1:201783682 | A/G | −3.90 | −4.62 | 0.01 | 9.69 × 10−5 | 3.88 × 10−6 |
| 1 | rs2820290 | IPO9-AS1 | 1:201783682 | A/G | −3.90 | −4.62 | 0.01 | 9.69 × 10−5 | 3.88 × 10−6 |
| 2 | rs4688760 | RBM6 | 3:49980596 | C/T | −5.67 | −4.42 | 0.01 | 1.44 × 10−8 | 9.82 × 10−6 |
| 3 | rs67073213 | Upstream: SPATA18; Downstream: RP11-588F10.1 | 4:53286872 | A/G | 3.87 | −4.48 | 0.01 | 1.10 × 10−4 | 7.57 × 10−6 |
| 4 | rs26434 | PAM | 5:102363402 | C/T | 5.90 | −4.40 | 0.01 | 3.58 × 10−9 | 1.10× 10−5 |
| 5 | rs4526367 | MSRA | 8:10213462 | G/A | −4.96 | 5.49 | 0.00 | 7.10 × 10−7 | 3.99 × 10−8 |
| 6 | rs4735334 | NDUFAF6 | 8:95955292 | G/A | 3.98 | 4.63 | 0.01 | 6.91 × 10−5 | 3.70 × 10−6 |
| 6 | rs4735334 | TP53INP1 | 8:95955292 | G/A | 3.98 | 4.63 | 0.01 | 6.91 × 10−5 | 3.70 × 10−6 |
| Type 2 diabetes and sleep duration | |||||||||
| 7 | rs4949329 | PUM1 | 1:31440361 | T/C | −4.04 | 4.78 | 0.00 | 5.29 × 10−5 | 1.80 × 10−6 |
| 8 | rs61780511 | Upstream: PUM1; Downstream: SEPW1P | 1:31546006 | G/A | 3.83 | −4.12 | 0.01 | 1.27 × 10−4 | 3.81 × 10−5 |
| 9 | rs12137232 | LMOD1 | 1:201885446 | T/G | −3.90 | 4.36 | 0.01 | 9.69 × 10−5 | 1.30 × 10−5 |
| 10 | rs6711622 | DNMT3A | 2:25531350 | A/G | −3.85 | 4.14 | 0.01 | 1.19 × 10−4 | 3.51 × 10−5 |
| 11 | rs1641155 | LINC01122 | 2:58965211 | G/T | 4.69 | 4.90 | 0.00 | 2.75 × 10−6 | 9.36 × 10−7 |
| 12 | rs12485697 | Upstream: RP11-231I13.2; Downstream: COX6CP6 | 3:70543116 | T/C | 3.89 | −4.16 | 0.01 | 1.01 × 10−4 | 3.15 × 10−5 |
| 13 | rs9844666 | PCCB | 3:135974216 | A/G | 4.19 | 4.60 | 0.00 | 2.77 × 10−5 | 4.27 × 10−6 |
| 14 | rs1291921 | PCCB | 3:136036226 | A/G | −4.35 | −4.50 | 0.00 | 1.36 × 10−5 | 6.85 × 10−6 |
| 15 | rs11242483 | PAM | 5:102323766 | T/C | 6.07 | 6.28 | 0.00 | 1.31 × 10−9 | 3.39 × 10−10 |
| 16 | rs329124 | JADE2 | 5:133865452 | G/A | 5.14 | −4.65 | 0.00 | 2.80 × 10−7 | 3.30 × 10−6 |
| 17 | rs62442924 | MAD1L1 | 7:1989976 | T/C | −4.15 | 5.16 | 0.00 | 3.33 × 10−5 | 2.47 × 10−7 |
| 18 | rs7790729 | AUTS2 | 7:69598649 | T/C | 3.76 | 4.07 | 0.01 | 1.69 × 10−4 | 4.77 × 10−5 |
| 19 | rs3121426 | Upstream: 5-Mar; Downstream: MARK2P9 | 10:94153435 | T/G | −6.69 | −4.29 | 0.00 | 2.19 × 10−11 | 1.82 × 10−5 |
| 20 | rs11037564 | HSD17B12 | 11:43708725 | C/T | −3.77 | 5.01 | 0.01 | 1.65 × 10−4 | 5.41 × 10−7 |
| 21 | rs174533 | MYRF | 11:61549025 | A/G | −3.90 | 4.44 | 0.01 | 9.69 × 10−5 | 8.82 × 10−6 |
| 21 | rs174533 | TMEM258 | 11:61549025 | A/G | −3.90 | 4.44 | 0.01 | 9.69 × 10−5 | 8.82 × 10−6 |
| 22 | rs12820906 | PITPNM2 | 12:123493123 | G/A | −5.41 | 4.11 | 0.01 | 6.39 × 10−8 | 3.90 × 10−5 |
| 23 | rs12433645 | NRXN3 | 14:80028314 | T/C | −4.30 | −4.12 | 0.01 | 1.75 × 10−5 | 3.75 × 10−5 |
| 24 | rs4780887 | PDILT | 16:20393562 | C/A | −3.92 | 4.23 | 0.01 | 8.78 × 10−5 | 2.39 × 10−5 |
| 25 | rs8047587 | FTO | 16:53798622 | T/G | 16.19 | −6.66 | 0.00 | 6.29 × 10−59 | 2.66 × 10−11 |
| Type 2 diabetes and chronotype | |||||||||
| 26 | rs148262742 | Upstream: CDKN2C; Downstream: MIR4421 | 1:51472241 | C/T | −5.34 | −3.95 | 0.01 | 9.40 × 10−8 | 7.93 × 10−5 |
| 27 | rs12140153 | INADL | 1:62579891 | T/G | −5.18 | −6.10 | 0.00 | 2.21 × 10−7 | 1.03 × 10−9 |
| 28 | rs903518 | UBE2E2 | 3:23336968 | G/A | −3.93 | −4.22 | 0.00 | 8.32 × 10−5 | 2.42 × 10−5 |
| 29 | rs78580841 | CCDC12 | 3:46986452 | T/C | 4.05 | 4.78 | 0.00 | 5.03 × 10−5 | 1.72 × 10−6 |
| 30 | rs1679147 | MRAS | 3:138097537 | A/G | 4.45 | −3.93 | 0.01 | 8.72 × 10−6 | 8.49 × 10−5 |
| 31 | rs17774982 | ST6GAL1 | 3:186684460 | C/T | −4.88 | −4.51 | 0.00 | 1.07 × 10−6 | 6.39 × 10−6 |
| 32 | rs1296328 | RP11-775H9.2 | 4:137083193 | A/C | 4.98 | 6.11 | 0.00 | 6.37 × 10−7 | 9.73 × 10−10 |
| 33 | rs1265945 | EHMT2 | 6:31861815 | G/A | −4.10 | 4.71 | 0.00 | 4.15 × 10−5 | 2.51 × 10−6 |
| 34 | rs734597 | Upstream: RPS17P5; Downstream: RP4-753D5.3 | 6:50836279 | A/G | 6.06 | 5.12 | 0.00 | 1.35 × 10−9 | 2.99 × 10−7 |
| 35 | rs4434471 | Upstream: FTH1P5; Downstream: RP3-437C15.2 | 6:51146875 | G/A | 4.02 | 4.37 | 0.00 | 5.94 × 10−5 | 1.24 × 10−5 |
| 36 | rs66930764 | Upstream: RP5-826L7.1; Downstream: RP1-230L10.1 | 6:164103243 | A/G | −5.02 | −4.38 | 0.00 | 5.24 × 10−7 | 1.21 × 10−5 |
| 37 | rs11555134 | GRB10 | 7:50659193 | T/C | 4.30 | −5.30 | 0.00 | 1.75 × 10−5 | 1.17 × 10−7 |
| 38 | rs77655131 | ORAI2 | 7:102086552 | T/C | 4.94 | −5.54 | 0.00 | 7.70 × 10−7 | 3.03 × 10−8 |
| 39 | rs11496066 | FBXL13 | 7:102486254 | C/T | −5.18 | 5.90 | 0.00 | 2.21 × 10−7 | 3.62 × 10−9 |
| 40 | rs62482405 | PSMC2 | 7:102987583 | G/T | 4.36 | −4.09 | 0.01 | 1.29 × 10−5 | 4.23 × 10−5 |
| 41 | rs3808478 | TRPS1 | 8:116678277 | C/T | −4.00 | 4.86 | 0.00 | 6.43 × 10−5 | 1.15 × 10−6 |
| 42 | rs6559752 | C9orf64 | 9:86570075 | T/C | −3.80 | 4.17 | 0.01 | 1.44 × 10−4 | 3.09 × 10−5 |
| 43 | rs6478623 | DENND1A | 9:126315123 | G/T | 3.88 | −4.68 | 0.01 | 1.06 × 10−4 | 2.81 × 10−6 |
| 44 | rs11145756 | SEC16A | 9:139364585 | G/A | −4.63 | 4.09 | 0.01 | 3.64 × 10−6 | 4.24 × 10−5 |
| 45 | rs10998304 | TET1 | 10:70342775 | C/T | 4.08 | −4.36 | 0.00 | 4.41 × 10−5 | 1.28 × 10−5 |
| 46 | rs143539037 | CPEB3 | 10:93827055 | T/C | 5.92 | −3.92 | 0.01 | 3.26 × 10−9 | 8.80 × 10−5 |
| 47 | rs11039307 | Upstream: FAM180B; Downstream: C1QTNF4 | 11:47611152 | T/C | 5.22 | 4.83 | 0.00 | 1.77 × 10−7 | 1.37 × 10−6 |
| 48 | rs11039358 | FNBP4 | 11:47746962 | G/A | 4.52 | 4.30 | 0.00 | 6.10 × 10−6 | 1.72 × 10−5 |
| 49 | rs4237555 | Upstream: MTNR1B; Downstream: RPL26P31 | 11:92725803 | C/T | −7.86 | −4.85 | 0.00 | 3.84 × 10−15 | 1.22 × 10−6 |
| 50 | rs4606726 | PDILT | 16:20383700 | G/A | −3.83 | −3.97 | 0.01 | 1.27 × 10−4 | 7.10 × 10−5 |
| 51 | rs8047587 | FTO | 16:53798622 | T/G | 16.19 | 7.42 | 0.00 | 6.29 × 10−59 | 1.19 × 10−13 |
| 52 | rs217184 | TXNL4B | 16:72105965 | C/T | −3.98 | 4.06 | 0.01 | 6.91 × 10−5 | 4.99 × 10−5 |
| 52 | rs217184 | HPR | 16:72105965 | C/T | −3.98 | 4.06 | 0.01 | 6.91 × 10−5 | 4.99 × 10−5 |
| 53 | rs3816511 | PEMT | 17:17409401 | G/A | 3.77 | 4.79 | 0.01 | 1.61 × 10−4 | 1.63 × 10−6 |
| 54 | rs1371319 | Upstream: RP11-687D19.1; Downstream: RN7SKP182 | 18:36277087 | C/T | 4.50 | 4.22 | 0.00 | 6.94 × 10−6 | 2.45 × 10−5 |
| 55 | rs17596995 | TCF4 | 18:53166594 | A/G | −4.25 | −4.94 | 0.00 | 2.12 × 10−5 | 7.66 × 10−7 |
| 56 | rs5762622 | TTC28 | 22:28835458 | A/G | 4.28 | −4.32 | 0.00 | 1.87 × 10−5 | 1.57 × 10−5 |
| 57 | rs5757906 | TNRC6B | 22:40687757 | C/T | −3.91 | −4.27 | 0.00 | 9.24 × 10−5 | 1.91 × 10−5 |
| 58 | rs28741121 | XRCC6 | 22:42025823 | A/G | −4.07 | −4.19 | 0.00 | 4.72 × 10−5 | 2.74 × 10−5 |
| Genes | Type 2 Diabetes | Insomnia | Chronotype | Sleep Duration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NIV | OR | 95% CI | p | NIV | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| ENSA | 2 | 1.10 | 1.10 (0.98–1.23) | 0.1119 | 2 | 1.14 | 1.14 (1.05–1.24) | 0.0013 | 0.96 | 0.96 (0.88–1.06) | 0.4454 | 1.01 | 1.01 (0.97–1.05) | 0.6732 |
| CPEB3 | 1 | 1.43 | 1.43 (1.30–1.56) | 0.0000 | ||||||||||
| MYBPC3 | 6 | 0.95 | 0.95 (0.92–0.99) | 0.0173 | 5 | 1.02 | 1.02 (0.98–1.06) | 0.2920 | 1.00 | 1.00 (0.98–1.01) | 0.5591 | 0.99 | 0.99 (0.98–1.00) | 0.0880 |
| MYRF | 1 | 0.94 | 0.94 (0.90–0.98) | 0.0049 | 1 | 1.03 | 1.03 (1.00–1.07) | 0.0581 | 1.00 | 1.00 (0.98–1.01) | 0.8016 | 1.01 | 1.01 (1.00–1.03) | 0.1302 |
| KLHL29 | 2 | 0.99 | 0.99 (0.91–1.07) | 0.7417 | 2 | 0.89 | 0.89 (0.83–0.95) | 0.0008 | 1.00 | 1.00 (0.97–1.03) | 0.9629 | 1.03 | 1.03 (0.99–1.06) | 0.1057 |
| DNMT3A | 1 | 1.07 | 1.07 (0.98–1.15) | 0.1161 | ||||||||||
| XRCC6 | 2 | 1.06 | 1.06 (0.91–1.23) | 0.4503 | 2 | 1.03 | 1.03 (1.00–1.07) | 0.0469 | 0.97 | 0.97 (0.96–0.99) | 0.0002 | 1.01 | 1.01 (0.99–1.03) | 0.1748 |
| PCCB | 3 | 1.03 | 1.03 (0.96–1.1) | 0.4567 | 3 | 1.00 | 1.00 (0.97–1.02) | 0.8452 | 1.01 | 1.01 (0.99–1.02) | 0.3804 | 1.00 | 1.00 (0.96–1.03) | 0.8049 |
| MAD1L1 | 4 | 1.00 | 1.00 (0.97–1.04) | 0.8834 | 4 | 1.02 | 1.02 (0.99–1.04) | 0.1612 | 1.00 | 1(0.98–1.02) | 0.7269 | 0.99 | 0.99 (0.97–1.00) | 0.0167 |
| PMPCA | 1 | 0.74 | 0.74 (0.62–0.87) | 0.0003 | 1 | 0.93 | 0.93 (0.82–1.07) | 0.3157 | 1.15 | 1.15 (1.08–1.22) | 0.0000 | 0.99 | 0.99 (0.93–1.06) | 0.7955 |
| INPP5E | 4 | 1.10 | 1.10 (1.07–1.13) | 0.0000 | 4 | 1.00 | 1.00 (0.98–1.02) | 0.9185 | 0.98 | 0.98 (0.97–0.99) | 0.0001 | 1.00 | 1.00 (0.99–1.02) | 0.8376 |
| SEC16A | 2 | 1.08 | 1.08 (1.05–1.12) | 0.0000 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhou, Z.; Li, X.; Yan, Z.; Ding, K.; Xiao, H.; Wu, Y.; Wu, T.; Chen, D. Integrative Identification of Genetic Loci Jointly Influencing Diabetes-Related Traits and Sleep Traits of Insomnia, Sleep Duration, and Chronotypes. Biomedicines 2022, 10, 368. https://doi.org/10.3390/biomedicines10020368
Ma Y, Zhou Z, Li X, Yan Z, Ding K, Xiao H, Wu Y, Wu T, Chen D. Integrative Identification of Genetic Loci Jointly Influencing Diabetes-Related Traits and Sleep Traits of Insomnia, Sleep Duration, and Chronotypes. Biomedicines. 2022; 10(2):368. https://doi.org/10.3390/biomedicines10020368
Chicago/Turabian StyleMa, Yujia, Zechen Zhou, Xiaoyi Li, Zeyu Yan, Kexin Ding, Han Xiao, Yiqun Wu, Tao Wu, and Dafang Chen. 2022. "Integrative Identification of Genetic Loci Jointly Influencing Diabetes-Related Traits and Sleep Traits of Insomnia, Sleep Duration, and Chronotypes" Biomedicines 10, no. 2: 368. https://doi.org/10.3390/biomedicines10020368
APA StyleMa, Y., Zhou, Z., Li, X., Yan, Z., Ding, K., Xiao, H., Wu, Y., Wu, T., & Chen, D. (2022). Integrative Identification of Genetic Loci Jointly Influencing Diabetes-Related Traits and Sleep Traits of Insomnia, Sleep Duration, and Chronotypes. Biomedicines, 10(2), 368. https://doi.org/10.3390/biomedicines10020368






