Loss of atm in Zebrafish as a Model of Ataxia–Telangiectasia Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance and the Generation of Mutants
2.2. Hematoxylin–Eosin Staining
2.3. Anti-Acid Staining
2.4. Zebrafish Behavioral Assays
2.5. Quantitative RT-PCR
| atm-F (PCR1): 5′-GAGTTGGCCCTCTGTCATGC-3′ |
| atm-R (PCR1): 5′-TATCAACGGGCACCAGGGAT-3′ |
| atm-F (PCR2): 5′-TGATTCTTTGCCCTTGGCCC-3′ |
| atm-R (PCR2): 5′-AACTGGTCCAAGTTCCCCCA-3′ |
| atm-F (PCR3): 5′-TCACTTCCAGACCTTCCCAGT-3′ |
| atm-R (PCR3): 5′-AGGAGAATCCACCGTTGAGCT-3′ |
| il-1β-F: 5′-TCCGCTCCACATCTCGTACT-3′ |
| il-1β-R: 5′-AACCGGGACATTTGACGGAC-3′ |
| il6-F: 5′-AGCAGGAATGGCTTTGAAGGG-3′ |
| il6-R: 5′-GTCAGGACGCTGTAGATTCGC-3′ |
| il8-F: 5′-GTAGATCCACGCTGTCGCTG-3′ |
| il8-R: 5′-TACAGTGTGGGCTTGGAGGG-3′ |
| tnfa-F: 5′-AGACCTTAGACTGGAGAGATGAC-3′ |
| tnfa-R: 5′-CAAAGACACCTGGCTGTAGAC-3′ |
| il10-F: 5′-GGAGACCATTCTGCCAACAGC-3′ |
| il10-R: 5′-TCTTGCATTTCACCATATCCCG-3′ |
| β-actin-F: 5′-GGGTATGGAATCTTGCGGTATC-3′ |
| β-actin-R: 5′-CTTCATGGTGGAAGGAGCAA-3′ |
| tgf1β-F: 5′-GCTGGAACTGTATCGCGGAG-3′ |
| tgf1β-R: 5′-ATCCGTGCTCTGCTGGTTTG-3′ |
| il4-F: 5′-AGCAGGAATGGCTTTGAAGGG-3′ |
| il4-R: 5′-TTCATTGTGCATTCCCCCGAG-3′ |
| il13-F: 5′-GCACTGTATTCGTCTCGGGT-3′ |
| il13-R: 5′-CGGACAGGCCAGAAACCTTTT-3′ |
| mmp9-F: 5′-GAGGGCCGCAATGATGGAAA-3′ |
| mmp9-R: 5′-CGATGCAAGGGGAAACCCTC-3′ |
| mmp13a-F: 5′-GCTGCGGAGTTCCAGACATC-3′ |
| mmp13a-R: 5′-AGTAACGTCAGCCCACACCT-3′ |
| plaua-F: 5′-CTCAGCCATGGTGCGTTGTA-3′ |
| plaua-R: 5′-TTTGTCTGTCGGTCCAAGCG-3′ |
| plaub-F: 5′-ACCGAGAACATGCTGTGTGC-3′ |
| plaub-R: 5′-GGCATAAACACCAGGCCGAA-3′ |
| ctsd-F: 5′-CGGGTATCTCAGCCAGGACA-3′ |
| ctsd-R: 5′-AGGAGGAACCCCATCGACAG-3′ |
| gpx1b-F: 5′-CAGATGAACGAGCTGCACGA-3′ |
| gpx1b-R: 5′-GGGCTCATAGCCATTGCCAG-3′ |
| sod1-F: 5′-ACCTGGGTAATGTGACCGCT-3′ |
| sod1-R: 5′-TCATTGCCACCCTTCCCCAA-3′ |
| sod2-F: 5′-AACCCCCTGTTAGGTGCTGT-3′ |
| sod2-R: 5′-GTTGCATGGTGCTTGCTGTG-3′ |
2.6. RNAscope® In Situ Hybridization Combined with Immunofluorescence
2.7. Whole-Mount In Situ Hybridization
2.8. Senescence-Associated β-Galactosidase Activity
2.9. Flow Cytometry Analysis
2.10. Immunohistochemistry
2.11. Quantification and Statistical Analysis Quantification and Statistical Analysis
3. Results
3.1. Establishment of an atm Fish Model
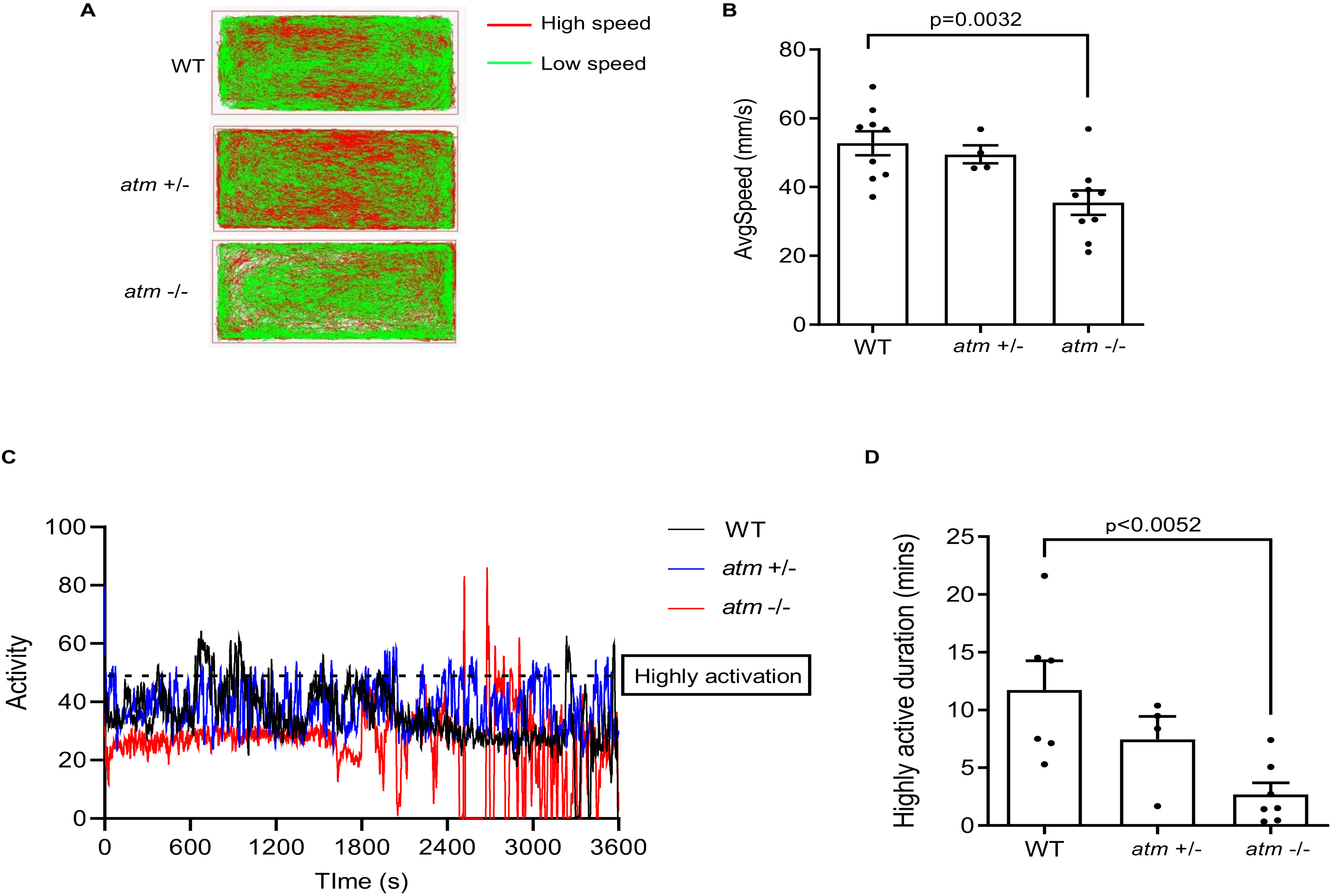
3.2. ATM−/− Fish Show Motor Disability and Coordination Disorder
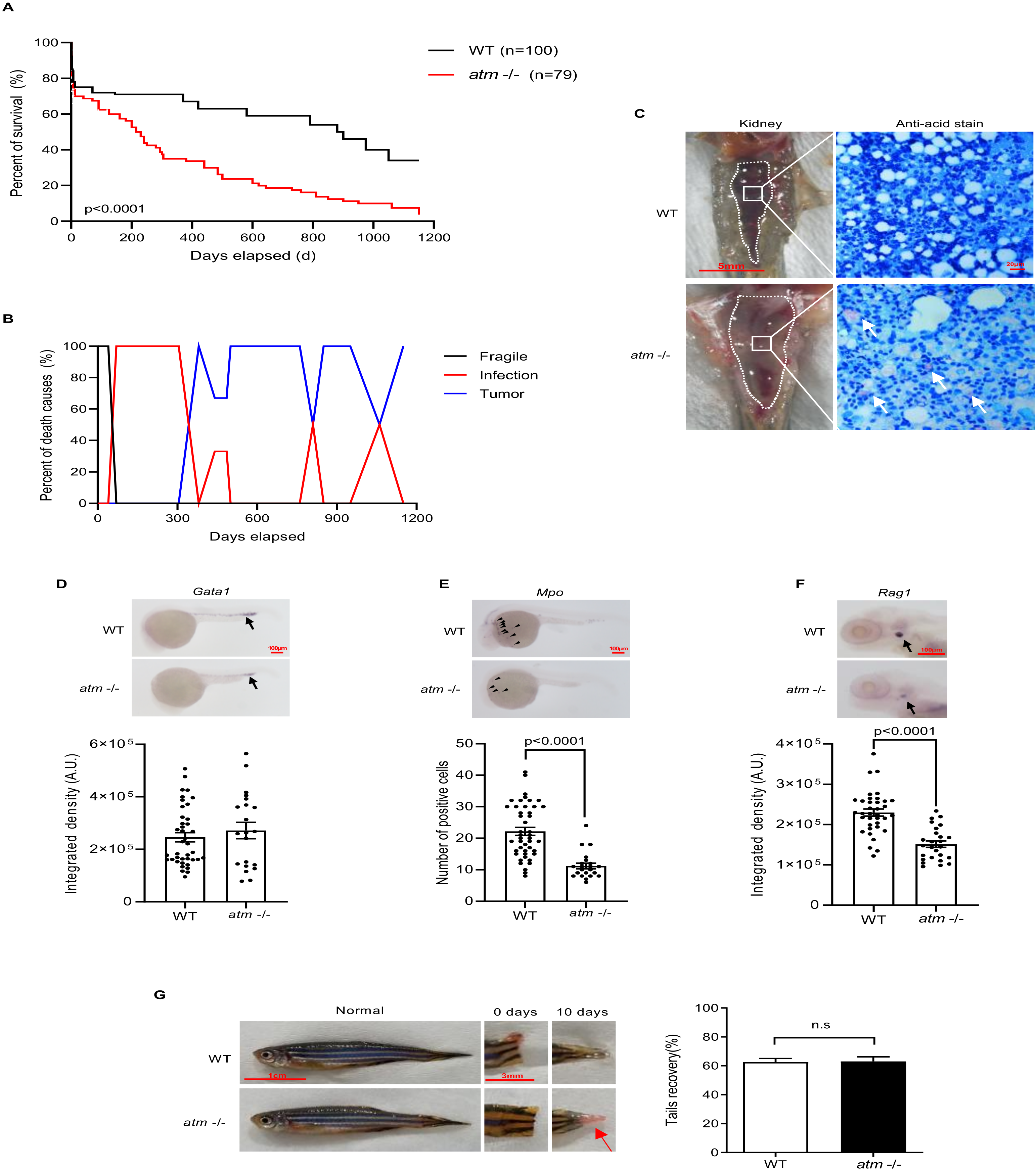
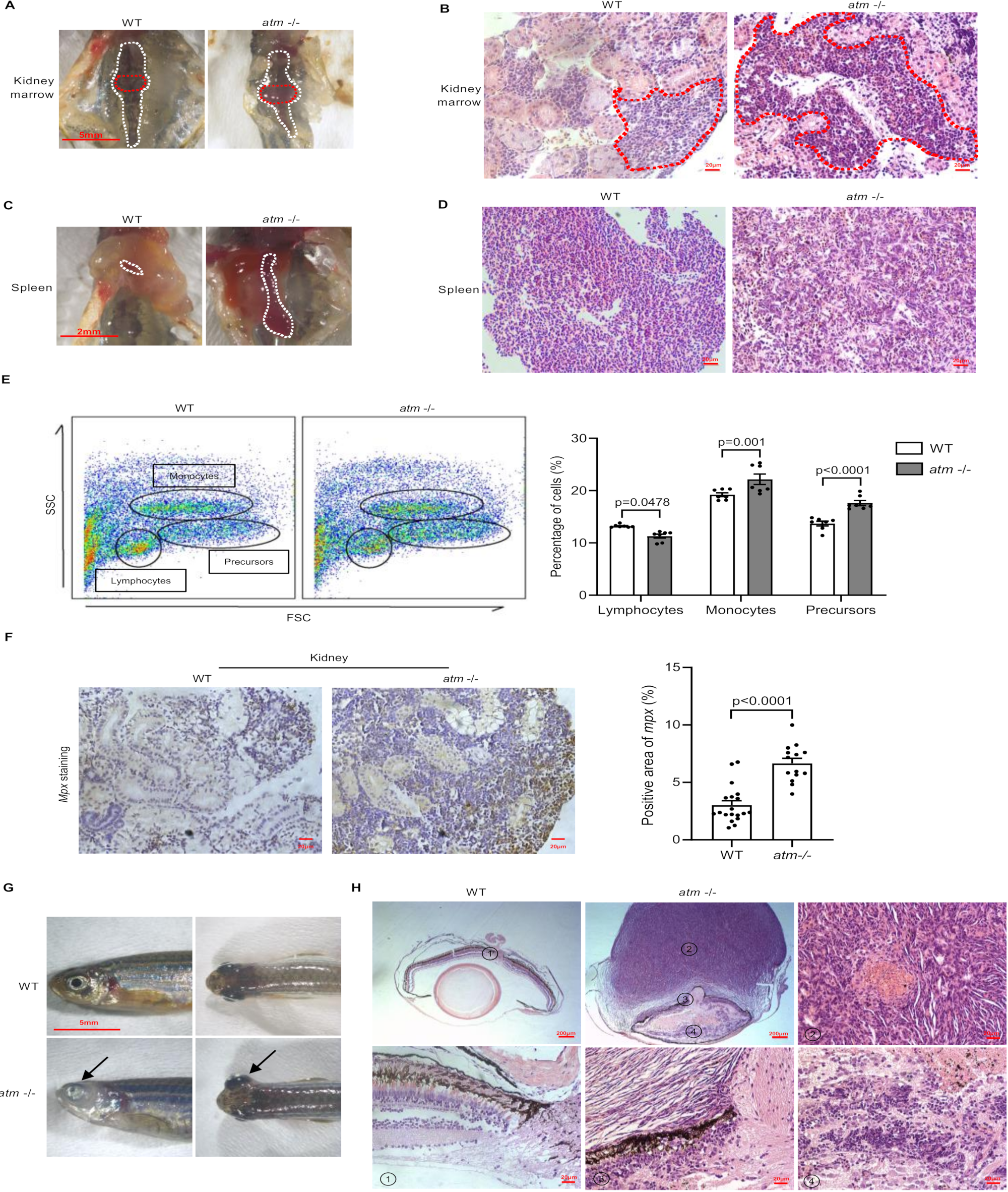
3.3. Lack of atm Causes Immune Deficiency during Zebrafish Development
3.4. Lack of atm Does Not Impair Fish Tail Regeneration
3.5. Atm Loss Promotes Tumorigenesis in Zebrafish
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothblum-Oviatt, C.; Wright, J.; Lefton-Greif, M.A.; McGrath-Morrow, S.A.; Crawford, T.O.; Lederman, H.M. Ataxia telangiectasia: A review. Orphanet J. Rare Dis. 2016, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Concannon, P.; Gatti, R.A. Diversity of ATM gene mutations detected in patients with ataxia-telangiectasia. Hum. Mutat. 1997, 10, 100–107. [Google Scholar] [CrossRef]
- Lee, J.-H.; Paull, T.T. Cellular functions of the protein kinase ATM and their relevance to human disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 796–814. [Google Scholar] [CrossRef] [PubMed]
- Barlow, C.; Hirotsune, S.; Paylor, R.; Liyanage, M.; Eckhaus, M.; Collins, F.; Shiloh, Y.; Crawley, J.N.; Ried, T.; Tagle, D.; et al. Atm-Deficient Mice: A Paradigm of Ataxia Telangiectasia. Cell 1996, 86, 159–171. [Google Scholar] [CrossRef]
- Xu, Y.; Ashley, T.; Brainerd, E.E.; Bronson, R.T.; Meyn, M.S.; Baltimore, D. Targeted disruption of ATM leads to growth retardation. chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996, 10, 2411–2422. [Google Scholar] [CrossRef]
- Quek, H.; Luff, J.; Cheung, K.; Kozlov, S.; Gatei, M.; Lee, C.S.; Bellingham, M.C.; Noakes, P.G.; Lim, Y.C.; Barnett, N.L.; et al. A rat model of ataxia-telangiectasia: Evidence for a neurodegenerative phenotype. Hum. Mol. Genet. 2017, 26, 109–123. [Google Scholar] [CrossRef]
- Petersen, A.J.; Rimkus, S.A.; Wassarman, D.A. ATM kinase inhibition in glial cells activates the innate immune response and causes neurodegeneration in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, E656. [Google Scholar] [CrossRef]
- Amirifar, P.; Ranjouri, M.R.; Yazdani, R.; Abolhassani, H.; Aghamohammadi, A. Ataxia-telangiectasia: A review of clinical features and molecular pathology. Pediatr. Allergy Immunol. 2019, 30, 277–288. [Google Scholar] [CrossRef]
- Driessen, G.J.; Ijspeert, H.; Weemaes, C.M.R.; Haraldsson, Á.; Trip, M.; Warris, A.; van der Flier, M.; Wulffraat, N.; Verhagen, M.M.M.; Taylor, M.A.; et al. Antibody deficiency in patients with ataxia telangiectasia is caused by disturbed B- and T-cell homeostasis and reduced immune repertoire diversity. J. Allergy Clin. Immunol. 2013, 131, 1367–1375. [Google Scholar] [CrossRef]
- Waldmann, T.A.; Broder, S.; Goldman, C.K.; Frost, K.; Korsmeyer, S.J.; Medici, M.A. Disorders of B cells and helper T cells in the pathogenesis of the immunoglobulin deficiency of patients with ataxia telangiectasia. J. Clin. Investig. 1983, 71, 282–295. [Google Scholar] [CrossRef]
- Kraus, M.; Lev, A.; Simon, A.J.; Levran, I.; Nissenkorn, A.; Levi, Y.B.; Berkun, Y.; Efrati, O.; Amariglio, N.; Rechavi, G.; et al. Disturbed B and T cell homeostasis and neogenesis in patients with ataxia telangiectasia. J. Clin. Immunol. 2014, 34, 561–572. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Crawford, T.O.; Winkelstein, J.A.; Carson, K.A.; Lederman, H.M. Immunodeficiency and infections in ataxia-telangiectasia. J. Pediatr. 2004, 144, 505–511. [Google Scholar] [CrossRef]
- Ghiasy, S.; Parvaneh, L.; Azizi, G.; Sadri, G.; Zaki dizaji, M.; Abolhassani, H.; Aghamohammadi, A. The clinical significance of complete class switching defect in Ataxia telangiectasia patients. Expert Rev. Clin. Immunol. 2017, 13, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Guo, C.; Boboila, C.; Oksenych, V.; Cheng, H.-L.; Zhang, Y.; Wesemann, D.R.; Yuen, G.; Patel, H.; Goff, P.H.; et al. ATM damage response and XLF repair factor are functionally redundant in joining DNA breaks. Nature 2011, 469, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Borghesani, P.R.; Alt, F.W.; Bottaro, A.; Davidson, L.; Aksoy, S.; Rathbun, G.A.; Roberts, T.M.; Swat, W.; Segal, R.A.; Gu, Y. Abnormal development of Purkinje cells and lymphocytes in Atm mutant mice. Proc. Natl. Acad. Sci. USA 2000, 97, 3336. [Google Scholar] [CrossRef]
- Suarez, F.; Mahlaoui, N.; Canioni, D.; Andriamanga, C.; Dubois d’Enghien, C.; Brousse, N.; Jais, J.-P.; Fischer, A.; Hermine, O.; Stoppa-Lyonnet, D. Incidence. Presentation, and Prognosis of Malignancies in Ataxia-Telangiectasia: A Report from the French National Registry of Primary Immune Deficiencies. J. Clin. Oncol. 2014, 33, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Reiman, A.; Srinivasan, V.; Barone, G.; Last, J.I.; Wootton, L.L.; Davies, E.G.; Verhagen, M.M.; Willemsen, M.A.; Weemaes, C.M.; Byrd, P.J.; et al. Lymphoid tumours and breast cancer in ataxia telangiectasia; substantial protective effect of residual ATM kinase activity against childhood tumours. Br. J. Cancer 2011, 105, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Metcalfe, J.A.; Thick, J.; Mak, Y.F. Leukemia and lymphoma in ataxia telangiectasia. Blood 1996, 87, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Elson, A.; Wang, Y.; Daugherty, C.J.; Morton, C.C.; Zhou, F.; Campos-Torres, J.; Leder, P. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc. Natl. Acad. Sci. USA 1996, 93, 13084. [Google Scholar] [CrossRef]
- Grunwald, D.J.; Eisen, J.S. Headwaters of the zebrafish—Emergence of a new model vertebrate. Nat. Rev. Genet. 2002, 3, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Amatruda, J.F.; Shepard, J.L.; Stern, H.M.; Zon, L.I. Zebrafish as a cancer model system. Cancer Cell 2002, 1, 229–231. [Google Scholar] [CrossRef]
- Zon, L.I. Zebrafish: A New Model for Human Disease. Genome Res. 1999, 9, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Imamura, S.; Kishi, S. Molecular cloning and functional characterization of zebrafish ATM. Int. J. Biochem. Cell Biol. 2005, 37, 1105–1116. [Google Scholar] [CrossRef]
- Knapp, D.; Tanaka, E.M. Regeneration and reprogramming. Curr. Opin. Genet. Dev. 2012, 22, 485–493. [Google Scholar] [CrossRef]
- Kyritsis, N.; Kizil, C.; Zocher, S.; Kroehne, V.; Kaslin, J.; Freudenreich, D.; Iltzsche, A.; Brand, M. Acute Inflammation Initiates the Regenerative Response in the Adult Zebrafish Brain. Science 2012, 338, 1353–1356. [Google Scholar] [CrossRef]
- Bohaud, C.; Johansen, M.D.; Jorgensen, C.; Ipseiz, N.; Kremer, L.; Djouad, F. The Role of Macrophages During Zebrafish Injury and Tissue Regeneration Under Infectious and Non-Infectious Conditions. Front. Immunol. 2021, 12, 2879. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Adhikary, S.; Hui, S.P. Decoding the proregenerative competence of regulatory T cells through complex tissue regeneration in zebrafish. Clin. Exp. Immunol. 2021, 206, 346–353. [Google Scholar] [CrossRef]
- Iribarne, M. Inflammation induces zebrafish regeneration. Neural Regen. Res. 2021, 16, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Lin, W.-C.; Wang, C.-H.; Ho, Y.-J.; Chiang, I.P.; Peng, C.-T.; Wu, K.-H. Child with Ataxia Telangiectasia Developing Acute Myeloid Leukemia. J. Clin. Oncol. 2010, 28, e213–e214. [Google Scholar] [CrossRef] [PubMed]
- Brioli, A.; Parisi, S.; Iacobucci, I.; Cavo, M.; Papayannidis, C.; Zannetti, B.A.; Martinelli, G.; Baccarani, M. Patient with ataxia telangiectasia who developed acute myeloid leukemia. Leuk. Lymphoma 2011, 52, 1818–1820. [Google Scholar] [CrossRef]
- Dutzmann, C.M.; Spix, C.; Popp, I.; Kaiser, M.; Erdmann, F.; Erlacher, M.; Dörk, T.; Schindler, D.; Kalb, R.; Kratz, C.P. Cancer in Children with Fanconi Anemia and Ataxia-Telangiectasia—A Nationwide Register-Based Cohort Study in Germany. J. Clin. Oncol. 2021, 40, 32–39. [Google Scholar] [CrossRef]
- Masri, A.T.; Bakri, F.G.; Al-Hadidy, A.M.; Musharbash, A.F.; Al-Hussaini, M. Ataxia-Telangiectasia Complicated by Craniopharyngioma—A New Observation. Pediatr. Neurol. 2006, 35, 287–288. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Xiao, A.; Wang, Z.; Hu, Y.; Wu, Y.; Luo, Z.; Yang, Z.; Zu, Y.; Li, W.; Huang, P.; Tong, X.; et al. Chromosomal deletions and inversions mediated by TALENs and CRISPR/Cas in zebrafish. Nucleic Acids Res. 2013, 41, e141. [Google Scholar] [CrossRef]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.T.; Ma, X.J.; Luo, Y. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef]
- Holowiecki, A.; O’Shields, B.; Jenny, M.J. Spatiotemporal expression and transcriptional regulation of heme oxygenase and biliverdin reductase genes in zebrafish (Danio rerio) suggest novel roles during early developmental periods of heightened oxidative stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 191, 138–151. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Claes, K.B.M.; Ferreira, M.G.; Henriques, C.M.; van Eeden, F.; Varga, M.; Vierstraete, J.; Mione, M.C. The Zebrafish as an Emerging Model to Study DNA Damage in Aging, Cancer and Other Diseases. Front. Cell Dev. Biol. 2019, 6, 178. [Google Scholar] [CrossRef]
- Lavin, M.F.; Shiloh, Y. The genetic defect in ataxia-telangiectasia. Annu. Rev. Immunol. 1997, 15, 177–202. [Google Scholar] [CrossRef]
- Aguado, J.; Chaggar, H.K.; Gómez-Inclán, C.; Shaker, M.R.; Leeson, H.C.; Mackay-Sim, A.; Wolvetang, E.J. Inhibition of the cGAS-STING pathway ameliorates the premature senescence hallmarks of Ataxia-Telangiectasia brain organoids. Aging Cell 2021, 20, e13468. [Google Scholar] [CrossRef]
- Ammann, A.J.; Hong, R. Autoimmune phenomena in ataxia telangiectasia. J. Pediatr. 1971, 78, 821–826. [Google Scholar] [CrossRef]
- McGrath-Morrow, S.A.; Collaco, J.M.; Detrick, B.; Lederman, H.M. Serum Interleukin-6 Levels and Pulmonary Function in Ataxia-Telangiectasia. J. Pediatr. 2016, 171, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Chiam, L.Y.T.; Verhagen, M.M.M.; Haraldsson, A.; Wulffraat, N.; Driessen, G.J.; Netea, M.G.; Weemaes, C.M.R.; Seyger, M.M.B.; van Deuren, M. Cutaneous Granulomas in Ataxia Telangiectasia and Other Primary Immunodeficiencies: Reflection of Inappropriate Immune Regulation? Dermatology 2011, 223, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Shoimer, I.; Wright, N.; Haber, R.M. Noninfectious Granulomas: A Sign of an Underlying Immunodeficiency? J. Cutan. Med. Surg. 2016, 20, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, M.; Feng, Y.; Jones, R.A.; Martin, P. Inflammation drives wound hyperpigmentation in zebrafish by recruiting pigment cells to sites of tissue damage. Dis. Models Mech. 2013, 6, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Da Silva-Álvarez, S.; Guerra-Varela, J.; Sobrido-Cameán, D.; Quelle, A.; Barreiro-Iglesias, A.; Sánchez, L.; Collado, M. Cell senescence contributes to tissue regeneration in zebrafish. Aging Cell 2020, 19, e13052. [Google Scholar] [CrossRef]
- Yun, M.H.; Davaapil, H.; Brockes, J.P. Recurrent turnover of senescent cells during regeneration of a complex structure. eLife 2015, 4, e05505. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.M.; Vijg, J.; Van Steeg, H.; Dolle, M.E.; et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef]
- Pulido, T.; Velarde, M.C.; Alimirah, F. The senescence-associated secretory phenotype: Fueling a wound that never heals. Mech. Ageing Dev. 2021, 199, 111561. [Google Scholar] [CrossRef]
- Berghmans, S.; Murphey, R.D.; Wienholds, E.; Neuberg, D.; Kutok, J.L.; Fletcher, C.D.M.; Morris, J.P.; Liu, T.X.; Schulte-Merker, S.; Kanki, J.P.; et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 407. [Google Scholar] [CrossRef] [PubMed]
- Botthof, J.G.; Bielczyk-Maczyńska, E.; Ferreira, L.; Cvejic, A. Loss of the homologous recombination gene rad51 leads to Fanconi anemia-like symptoms in zebrafish. Proc. Natl. Acad. Sci. USA 2017, 114, E4452. [Google Scholar] [CrossRef] [PubMed]
- Shive, H.R.; West, R.R.; Embree, L.J.; Golden, C.D.; Hickstein, D.D. BRCA2 and TP53 collaborate in tumorigenesis in zebrafish. PLoS ONE 2014, 9, e87177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, C.; Guo, T.; Qin, Y. Meiotic Recombination Defects and Premature Ovarian Insufficiency. Front. Cell Dev. Biol. 2021, 9, 349. [Google Scholar] [CrossRef]
- Shiloh, Y.; Ziv, Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013, 14, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Shive, H.R.; West, R.R.; Embree, L.J.; Azuma, M.; Sood, R.; Liu, P.; Hickstein, D.D. brca2 in zebrafish ovarian development. spermatogenesis, and tumorigenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 19350. [Google Scholar] [CrossRef]
- Rodriguez-Mari, A.; Canestro, C.; Bremiller, R.A.; Nguyen-Johnson, A.; Asakawa, K.; Kawakami, K.; Postlethwait, J.H. Sex reversal in zebrafish fancl mutants is caused by Tp53-mediated germ cell apoptosis. PLoS Genet. 2010, 6, e1001034. [Google Scholar] [CrossRef]
- Hoche, F.; Seidel, K.; Theis, M.; Vlaho, S.; Schubert, R.; Zielen, S.; Kieslich, M. Neurodegeneration in Ataxia Telangiectasia: What Is New? What Is Evident? Neuropediatrics 2012, 43, 119–129. [Google Scholar] [CrossRef]
- Pearson, T.S. More Than Ataxia: Hyperkinetic Movement Disorders in Childhood Autosomal Recessive Ataxia Syndromes. Tremor Other Hyperkinetic Mov. 2016, 6, 368. [Google Scholar] [CrossRef]
- Kamsler, A.; Daily, D.; Hochman, A.; Stern, N.; Shiloh, Y.; Rotman, G.; Barzilai, A. Increased Oxidative Stress in Ataxia Telangiectasia Evidenced by Alterations in Redox State of Brains from Atm-deficient Mice. Cancer Res. 2001, 61, 1849. [Google Scholar]
- Sahama, I.; Sinclair, K.; Pannek, K.; Lavin, M.; Rose, S. Radiological Imaging in Ataxia Telangiectasia: A Review. Cerebellum 2014, 13, 521–530. [Google Scholar] [CrossRef]
- Wehner, D.; Weidinger, G. Signaling networks organizing regenerative growth of the zebrafish fin. Trends Genet. 2015, 31, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Ditch, S.; Paull, T.T. The ATM protein kinase and cellular redox signaling: Beyond the DNA damage response. Trends Biochem. Sci. 2012, 37, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.J.; Dodson, M.L. Impaired Glutathione Biosynthesis in Cultured Human Ataxia-Telangiectasia Cells. Cancer Res. 1987, 47, 4576. [Google Scholar] [PubMed]
- Chakravarti, D.; Lee, R.; Multani, A.S.; Santoni, A.; Keith, Z.; Hsu, W.-H.; Chang, K.; Reyes, L.; Rashid, A.; Wu, C.-J.; et al. Telomere dysfunction instigates inflammation in inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2021, 118, e2024853118. [Google Scholar] [CrossRef]
- Carneiro, M.C.; de Castro, I.P.; Ferreira, M.G. Telomeres in aging and disease: Lessons from zebrafish. Dis. Models Mech. 2016, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]

| Pigment Recovery | ||
| Genotypes | Yes | No |
| WT | 12 | 0 |
| atm−/− | 2 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Wang, P.; Chen, J.; Ying, Y.; Chen, Y.; Gilson, E.; Lu, Y.; Ye, J. Loss of atm in Zebrafish as a Model of Ataxia–Telangiectasia Syndrome. Biomedicines 2022, 10, 392. https://doi.org/10.3390/biomedicines10020392
Chen K, Wang P, Chen J, Ying Y, Chen Y, Gilson E, Lu Y, Ye J. Loss of atm in Zebrafish as a Model of Ataxia–Telangiectasia Syndrome. Biomedicines. 2022; 10(2):392. https://doi.org/10.3390/biomedicines10020392
Chicago/Turabian StyleChen, Kehua, Peng Wang, Jingrun Chen, Yiling Ying, Yi Chen, Eric Gilson, Yiming Lu, and Jing Ye. 2022. "Loss of atm in Zebrafish as a Model of Ataxia–Telangiectasia Syndrome" Biomedicines 10, no. 2: 392. https://doi.org/10.3390/biomedicines10020392
APA StyleChen, K., Wang, P., Chen, J., Ying, Y., Chen, Y., Gilson, E., Lu, Y., & Ye, J. (2022). Loss of atm in Zebrafish as a Model of Ataxia–Telangiectasia Syndrome. Biomedicines, 10(2), 392. https://doi.org/10.3390/biomedicines10020392





