Inhibition of Cdk5 Ameliorates Skeletal Bone Loss in Glucocorticoid-Treated Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Primary Murine Calvarial Osteoblasts
2.2. Small Interfering RNA (siRNA) Transfection
2.3. Murine Primary Calvarial Osteoblast Differentiation
2.4. PrestoBlue Cell Viability Assay
2.5. Alkaline Phosphatase (Alp) and Alizarin Red S (ARS) Staining
2.6. RNA Isolation, cDNA Synthesis, and Real-Time Polymerase Chain Reaction (RT-PCR)
2.7. Protein Isolation, Quantification, and Western Blotting
2.8. Animals
2.9. GIO Model
2.10. Fracture Healing Model
2.11. Biomechanical Testing of the Fractured Femurs
2.12. Microcomputed Tomography (µCT) Analysis
2.13. Histomorphometry
2.14. N-Terminal Propeptide of Type I Procollagen (PINP) and C-Terminal Telopeptides of Type I Collagen (CTX-I) ELISAs
2.15. Statistical Analysis
3. Results
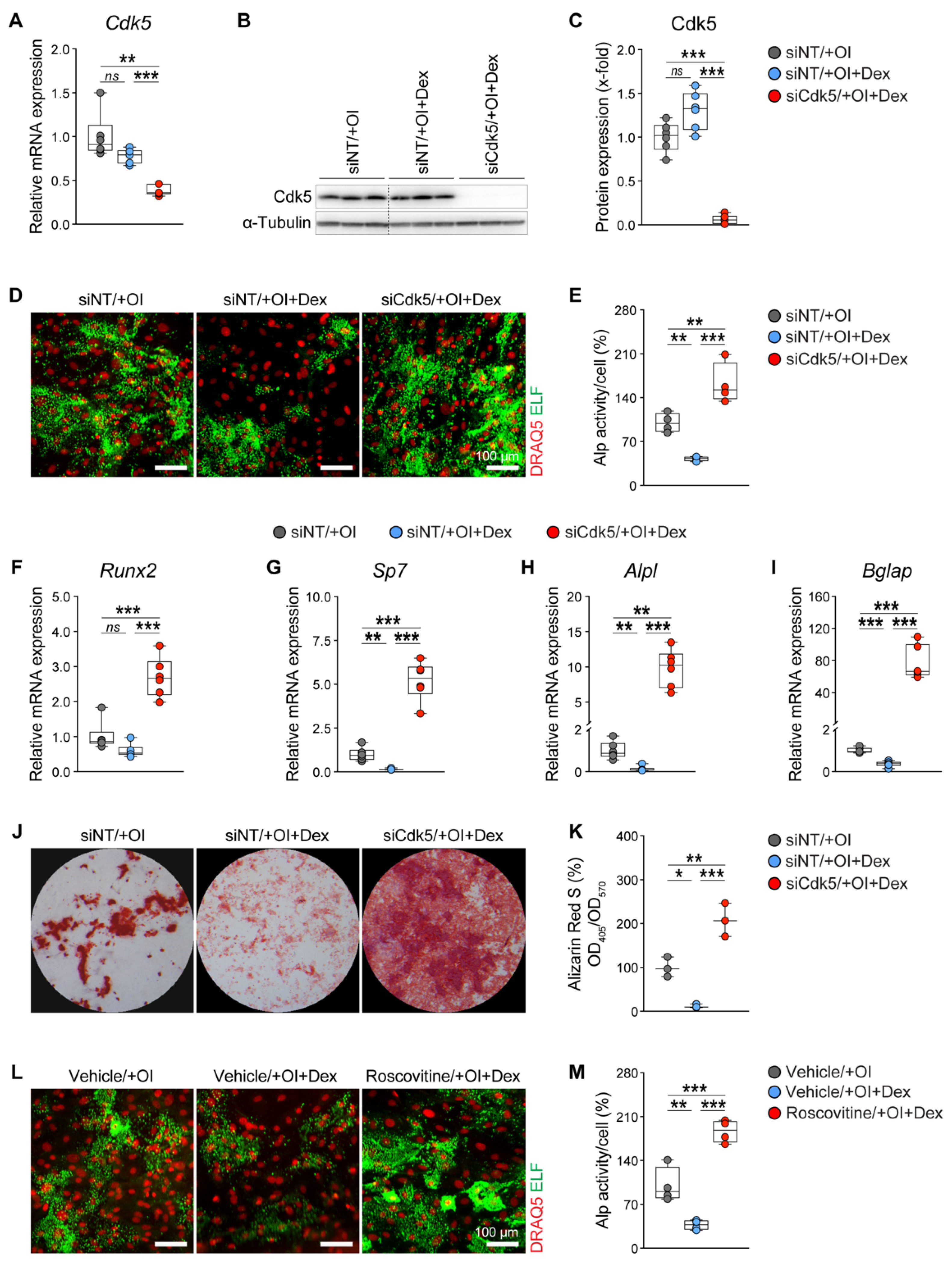
3.1. Cdk5 Deletion or Inhibition Antagonizes Suppressive Effects of GCs on Osteoblast Differentiation and Mineralization
3.2. Cdk5 Inhibition Antagonizes GC-Mediated Bone Loss by Reducing Osteoclastogenesis
3.3. Cdk5 Inhibition Does Not Reverse GC-Mediated Impaired Fracture Healing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartmann, K.; Koenen, M.; Schauer, S.; Wittig-Blaich, S.; Ahmad, M.; Baschant, U.; Tuckermann, J.P. Molecular Actions of Glucocorticoids in Cartilage and Bone During Health, Disease, and Steroid Therapy. Physiol. Rev. 2016, 96, 409–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agusti, A.; Fabbri, L.M.; Singh, D.; Vestbo, J.; Celli, B.; Franssen, F.M.E.; Rabe, K.F.; Papi, A. Inhaled corticosteroids in COPD: Friend or foe? Eur. Respir. J. 2018, 52, 1801219. [Google Scholar] [CrossRef]
- Ahmad, M.; Hachemi, Y.; Paxian, K.; Mengele, F.; Koenen, M.; Tuckermann, J. A Jack of All Trades: Impact of Glucocorticoids on Cellular Cross-Talk in Osteoimmunology. Front. Immunol. 2019, 10, 2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruscoli, S.; Febo, M.; Riccardi, C.; Migliorati, G. Glucocorticoid Therapy in Inflammatory Bowel Disease: Mechanisms and Clinical Practice. Front. Immunol. 2021, 12, 691480. [Google Scholar] [CrossRef]
- Buttgereit, F.; Burmester, G.R.; Lipworth, B.J. Optimised glucocorticoid therapy: The sharpening of an old spear. Lancet 2005, 365, 801–803. [Google Scholar] [CrossRef]
- Mazziotti, G.; Angeli, A.; Bilezikian, J.P.; Canalis, E.; Giustina, A. Glucocorticoid-induced osteoporosis: An update. Trends Endocrinol. Metab. TEM 2006, 17, 144–149. [Google Scholar] [CrossRef]
- Mazziotti, G.; Giustina, A.; Canalis, E.; Bilezikian, J.P. Treatment of glucocorticoid-induced osteoporsis. Ther. Adv. Musculoskelet. Dis. 2009, 1, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Koehnke, R.; Caldwell, J.R.; Brasington, R.; Burmeister, L.F.; Zimmerman, B.; Kohler, J.A.; Furst, D.E. Low dose long-term corticosteroid therapy in rheumatoid arthritis: An analysis of serious adverse events. Am. J. Med. 1994, 96, 115–123. [Google Scholar] [CrossRef]
- Adami, G.; Saag, K.G. Glucocorticoid-induced osteoporosis update. Curr. Opin. Rheumatol. 2019, 31, 388–393. [Google Scholar] [CrossRef]
- Rauch, A.; Seitz, S.; Baschant, U.; Schilling, A.F.; Illing, A.; Stride, B.; Kirilov, M.; Mandic, V.; Takacz, A.; Schmidt-Ullrich, R.; et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010, 11, 517–531. [Google Scholar] [CrossRef] [Green Version]
- Hofbauer, L.C.; Zeitz, U.; Schoppet, M.; Skalicky, M.; Schuler, C.; Stolina, M.; Kostenuik, P.J.; Erben, R.G. Prevention of glucocorticoid-induced bone loss in mice by inhibition of RANKL. Arthritis Rheum. 2009, 60, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Heersche, J.N. Glucocorticoid-induced osteoporosis: Both in vivo and in vitro concentrations of glucocorticoids higher than physiological levels attenuate osteoblast differentiation. J. Bone Miner. Res. 1998, 13, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Mazziotti, G.; Giustina, A.; Bilezikian, J.P. Glucocorticoid-induced osteoporosis: Pathophysiology and therapy. Osteoporos. Int. 2007, 18, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, B.; White, W.; Tuckermann, J. Glucocorticoid-induced osteoporosis. In Glucocorticoid Signaling; Springer: Berlin/Heidelberg, Germany, 2015; pp. 179–215. [Google Scholar]
- Weinstein, R.S.; Jilka, R.L.; Parfitt, A.M.; Manolagas, S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Investig. 1998, 102, 274–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, R.S.; Hogan, E.A.; Borrelli, M.J.; Liachenko, S.; O’Brien, C.A.; Manolagas, S.C. The Pathophysiological Sequence of Glucocorticoid-Induced Osteonecrosis of the Femoral Head in Male Mice. Endocrinology 2017, 158, 3817–3831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manolagas, S.C. Steroids and osteoporosis: The quest for mechanisms. J. Clin. Investig. 2013, 123, 1919–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, E.L.; Williams, J.H.; Davie, M.W.; Marshall, M.J. Effects of dissociated glucocorticoids on OPG and RANKL in osteoblastic cells. Bone 2006, 38, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, M.; Xiong, J.; Fujiwara, Y.; Thostenson, J.D.; O’Brien, C.A. Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E587–E593. [Google Scholar] [CrossRef] [Green Version]
- Wildemann, B.; Ignatius, A.; Leung, F.; Taitsman, L.A.; Smith, R.M.; Pesántez, R.; Stoddart, M.J.; Richards, R.G.; Jupiter, J.B. Non-union bone fractures. Nat. Rev. Dis. Primers 2021, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.E.; Hachemi, Y.; Kemmler, J.; Koenen, M.; Tuckermann, J.; Ignatius, A. Induced global deletion of glucocorticoid receptor impairs fracture healing. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 2235–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachemi, Y.; Rapp, A.E.; Picke, A.K.; Weidinger, G.; Ignatius, A.; Tuckermann, J. Molecular mechanisms of glucocorticoids on skeleton and bone regeneration after fracture. J. Mol. Endocrinol. 2018, 61, R75–R90. [Google Scholar] [CrossRef] [PubMed]
- Hachemi, Y.; Rapp, A.E.; Lee, S.; Dorn, A.K.; Krüger, B.T.; Kaiser, K.; Ignatius, A.; Tuckermann, J. Intact Glucocorticoid Receptor Dimerization Is Deleterious in Trauma-Induced Impaired Fracture Healing. Front. Immunol. 2020, 11, 628287. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.V.; Gamradt, S.C.; Asnis, P.; Vickery, B.H.; Avnur, Z.; Hill, E.; Bostrom, M. Systemic corticosteroids inhibit bone healing in a rabbit ulnar osteotomy model. Acta Orthop. Scand. 2000, 71, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Lyritis, G.; Papadopoulou, Z.; Nikiforidis, P.; Batrinos, M.; Varonos, D. Effect of cortisone and an anabolic steroid upon plasma hydroxyproline during fracture healing in rabbits. Acta Orthop. Scand. 1975, 46, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Newman, R.J.; Francis, M.J.; Duthie, R.B. Nuclear magnetic resonance studies of experimentally induced delayed fracture union. Clin. Orthop. Relat. Res. 1987, 216, 253–261. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Akhter, M.P.; Gao, X.; Wang, X.Y.; Wang, X.B.; Zhao, G.; Wei, X.; Wu, H.J.; Chen, H.; Wang, D.; et al. Glucocorticoid-induced delayed fracture healing and impaired bone biomechanical properties in mice. Clin. Interv. Aging 2018, 13, 1465–1474. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, O.H.; Aspenberg, P. Glucocorticoids inhibit shaft fracture healing but not metaphyseal bone regeneration under stable mechanical conditions. Bone Jt. Res. 2015, 4, 170–175. [Google Scholar] [CrossRef]
- Allen, C.S.; Yeung, J.H.; Vandermeer, B.; Homik, J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst. Rev. 2016, 10, Cd001347. [Google Scholar] [CrossRef] [PubMed]
- Bultink, I.E.; Baden, M.; Lems, W.F. Glucocorticoid-induced osteoporosis: An update on current pharmacotherapy and future directions. Expert Opin. Pharm. 2013, 14, 185–197. [Google Scholar] [CrossRef]
- Saag, K.G.; Shane, E.; Boonen, S.; Marin, F.; Donley, D.W.; Taylor, K.A.; Dalsky, G.P.; Marcus, R. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N. Engl. J. Med. 2007, 357, 2028–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saag, K.G.; Wagman, R.B.; Geusens, P.; Adachi, J.D.; Messina, O.D.; Emkey, R.; Chapurlat, R.; Wang, A.; Pannacciulli, N.; Lems, W.F. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: A multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. 2018, 6, 445–454. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Kuntz, D.; Verdickt, W.; Wouters, M.; Guillevin, L.; Menkès, C.J.; Nielsen, K. Prophylactic use of alfacalcidol in corticosteroid-induced osteoporosis. Osteoporos. Int. 1999, 9, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; LaValley, M.P.; Simms, R.W.; Felson, D.T. The role of vitamin D in corticosteroid-induced osteoporosis: A meta-analytic approach. Arthritis Rheum. 1999, 42, 1740–1751. [Google Scholar] [CrossRef]
- Homik, J.; Suarez-Almazor, M.E.; Shea, B.; Cranney, A.; Wells, G.; Tugwell, P. Calcium and vitamin D for corticosteroid-induced osteoporosis. Cochrane Database Syst. Rev. 2000, 1998, Cd000952. [Google Scholar] [CrossRef]
- Raterman, H.G.; Bultink, I.E.M.; Lems, W.F. Current Treatments and New Developments in the Management of Glucocorticoid-induced Osteoporosis. Drugs 2019, 79, 1065–1087. [Google Scholar] [CrossRef]
- Lane, N.E.; Sanchez, S.; Modin, G.W.; Genant, H.K.; Pierini, E.; Arnaud, C.D. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J. Clin. Investig. 1998, 102, 1627–1633. [Google Scholar] [CrossRef]
- Amiche, M.A.; Lévesque, L.E.; Gomes, T.; Adachi, J.D.; Cadarette, S.M. Effectiveness of Oral Bisphosphonates in Reducing Fracture Risk Among Oral Glucocorticoid Users: Three Matched Cohort Analyses. J. Bone Miner. Res. 2018, 33, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.R.; Smolen, L.J.; Klein, T.M.; Klein, R.W. The cost effectiveness of teriparatide as a first-line treatment for glucocorticoid-induced and postmenopausal osteoporosis patients in Sweden. BMC Musculoskelet. Disord. 2012, 13, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, E.; Nanes, M. Advances in treatment of glucocorticoid-induced osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 411–417. [Google Scholar] [CrossRef]
- Kawano, T.; Miyakoshi, N.; Kasukawa, Y.; Hongo, M.; Tsuchie, H.; Sato, C.; Fujii, M.; Suzuki, M.; Akagawa, M.; Ono, Y.; et al. Effects of combined therapy of alendronate and low-intensity pulsed ultrasound on metaphyseal bone repair after osteotomy in the proximal tibia of glucocorticoid-induced osteopenia rats. Osteoporos Sarcopenia 2017, 3, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Doyon, A.R.; Ferries, I.K.; Li, J. Glucocorticoid attenuates the anabolic effects of parathyroid hormone on fracture repair. Calcif. Tissue Int. 2010, 87, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.A.; Drake, M.T. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin. Proc. 2009, 84, 632–637, quiz 638. [Google Scholar] [CrossRef] [Green Version]
- Vahle, J.L.; Long, G.G.; Sandusky, G.; Westmore, M.; Ma, Y.L.; Sato, M. Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol. Pathol. 2004, 32, 426–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, P.D. Safety of parathyroid hormone for the treatment of osteoporosis. Curr. Osteoporos. Rep. 2008, 6, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Pautke, C.; Van den Wyngaert, T.; Niepel, D.; Schiødt, M. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018, 69, 177–187. [Google Scholar] [CrossRef]
- Hildebrand, G.K.; Kasi, A. Denosumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Dhavan, R.; Tsai, L.H. A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2001, 2, 749–759. [Google Scholar] [CrossRef]
- Chang, K.H.; Vincent, F.; Shah, K. Deregulated Cdk5 triggers aberrant activation of cell cycle kinases and phosphatases inducing neuronal death. J. Cell Sci. 2012, 125, 5124–5137. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.D.; Julien, J.P. Cyclin-dependent kinase 5 in amyotrophic lateral sclerosis. Neurosignals 2003, 12, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Vila, I.; Rife, M.; Lizcano, J.M.; Alberch, J.; Gines, S. Dopaminergic and glutamatergic signaling crosstalk in Huntington’s disease neurodegeneration: The role of p25/cyclin-dependent kinase 5. J. Neurosci. 2008, 28, 10090–10101. [Google Scholar] [CrossRef] [Green Version]
- Lopes, J.P.; Agostinho, P. Cdk5: Multitasking between physiological and pathological conditions. Prog. Neurobiol. 2011, 94, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Krüger, B.T.; Kroll, T.; Vettorazzi, S.; Dorn, A.-K.; Mengele, F.; Lee, S.; Nandi, S.; Yilmaz, D.; Stolz, M.; et al. Inhibition of Cdk5 increases osteoblast differentiation, bone mass and improves fracture healing. Bone Res. 2021. [Google Scholar] [CrossRef]
- Ahmad, M.; Kroll, T.; Jakob, J.; Rauch, A.; Ploubidou, A.; Tuckermann, J. Cell-based RNAi screening and high-content analysis in primary calvarian osteoblasts applied to identification of osteoblast differentiation regulators. Sci. Rep. 2018, 8, 14045. [Google Scholar] [CrossRef] [Green Version]
- Jonason, J.H.; O’Keefe, R.J. Isolation and culture of neonatal mouse calvarial osteoblasts. Methods Mol. Biol. 2014, 1130, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, S.; Baschant, U.; Rauch, A.; Rauner, M. Instructions for producing a mouse model of glucocorticoid-induced osteoporosis. BoneKEy Rep. 2014, 3, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röntgen, V.; Blakytny, R.; Matthys, R.; Landauer, M.; Wehner, T.; Göckelmann, M.; Jermendy, P.; Amling, M.; Schinke, T.; Claes, L.; et al. Fracture healing in mice under controlled rigid and flexible conditions using an adjustable external fixator. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2010, 28, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Mason, Z.D.; Chien, K.B.; Pfeiffer, A.J.; Barnes, G.L.; Einhorn, T.A.; Gerstenfeld, L.C. Micro-computed tomography assessment of fracture healing: Relationships among callus structure, composition, and mechanical function. Bone 2009, 44, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Najafova, Z.; Liu, P.; Wegwitz, F.; Ahmad, M.; Tamon, L.; Kosinsky, R.L.; Xie, W.; Johnsen, S.A.; Tuckermann, J. RNF40 exerts stage-dependent functions in differentiating osteoblasts and is essential for bone cell crosstalk. Cell Death Differ. 2020. [Google Scholar] [CrossRef]
- Liu, P.; Lee, S.; Knoll, J.; Rauch, A.; Ostermay, S.; Luther, J.; Malkusch, N.; Lerner, U.H.; Zaiss, M.M.; Neven, M.; et al. Loss of menin in osteoblast lineage affects osteocyte-osteoclast crosstalk causing osteoporosis. Cell Death Differ. 2017, 24, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Amling, M.; Priemel, M.; Holzmann, T.; Chapin, K.; Rueger, J.M.; Baron, R.; Demay, M.B. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: Formal histomorphometric and biomechanical analyses. Endocrinology 1999, 140, 4982–4987. [Google Scholar] [CrossRef]
- Li, J.; Zhang, N.; Huang, X.; Xu, J.; Fernandes, J.C.; Dai, K.; Zhang, X. Dexamethasone shifts bone marrow stromal cells from osteoblasts to adipocytes by C/EBPalpha promoter methylation. Cell Death Dis. 2013, 4, e832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luppen, C.A.; Smith, E.; Spevak, L.; Boskey, A.L.; Frenkel, B. Bone morphogenetic protein-2 restores mineralization in glucocorticoid-inhibited MC3T3-E1 osteoblast cultures. J. Bone Miner. Res. 2003, 18, 1186–1197. [Google Scholar] [CrossRef]
- Martin, T.J.; Ng, K.W. Mechanisms by which cells of the osteoblast lineage control osteoclast formation and activity. J. Cell. Biochem. 1994, 56, 357–366. [Google Scholar] [CrossRef]
- Thomas, G.P.; Baker, S.U.; Eisman, J.A.; Gardiner, E.M. Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. J. Endocrinol. 2001, 170, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Compston, J. Glucocorticoid-induced osteoporosis. Horm. Res. 2003, 60 (Suppl. 3), 77–79. [Google Scholar] [CrossRef]
- Tsai, L.H.; Takahashi, T.; Caviness, V.S., Jr.; Harlow, E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development 1993, 119, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zhang, T.; Michowski, W.; Rebecca, V.W.; Xiao, M.; Ferretti, R.; Suski, J.M.; Bronson, R.T.; Paulo, J.A.; Frederick, D.; et al. Targeting the cyclin-dependent kinase 5 in metastatic melanoma. Proc. Natl. Acad. Sci. USA 2020, 117, 8001–8012. [Google Scholar] [CrossRef] [PubMed]
- Dorand, R.D.; Nthale, J.; Myers, J.T.; Barkauskas, D.S.; Avril, S.; Chirieleison, S.M.; Pareek, T.K.; Abbott, D.W.; Stearns, D.S.; Letterio, J.J.; et al. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science 2016, 353, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Askew, D.; Pareek, T.K.; Eid, S.; Ganguly, S.; Tyler, M.; Huang, A.Y.; Letterio, J.J.; Cooke, K.R. Cyclin-dependent kinase 5 activity is required for allogeneic T-cell responses after hematopoietic cell transplantation in mice. Blood 2017, 129, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Ersek, A.; Santo, A.I.; Vattakuzhi, Y.; George, S.; Clark, A.R.; Horwood, N.J. Strain dependent differences in glucocorticoid-induced bone loss between C57BL/6J and CD-1 mice. Sci. Rep. 2016, 6, 36513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brent, M.B.; Thomsen, J.S.; Brüel, A. Short-term glucocorticoid excess blunts abaloparatide-induced increase in femoral bone mass and strength in mice. Sci. Rep. 2021, 11, 12258. [Google Scholar] [CrossRef] [PubMed]
- Thiele, S.; Ziegler, N.; Tsourdi, E.; De Bosscher, K.; Tuckermann, J.P.; Hofbauer, L.C.; Rauner, M. Selective glucocorticoid receptor modulation maintains bone mineral density in mice. J. Bone Miner. Res. 2012, 27, 2242–2250. [Google Scholar] [CrossRef]
- Yao, W.; Cheng, Z.; Pham, A.; Busse, C.; Zimmermann, E.A.; Ritchie, R.O.; Lane, N.E. Glucocorticoid-induced bone loss in mice can be reversed by the actions of parathyroid hormone and risedronate on different pathways for bone formation and mineralization. Arthritis Rheum. 2008, 58, 3485–3497. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, S.; Baschant, U.; Thiele, S.; Tuckermann, J.; Hofbauer, L.C.; Rauner, M. Glucocorticoids suppress Wnt16 expression in osteoblasts in vitro and in vivo. Sci. Rep. 2018, 8, 8711. [Google Scholar] [CrossRef]
- Matsushita, T.; Chan, Y.Y.; Kawanami, A.; Balmes, G.; Landreth, G.E.; Murakami, S. Extracellular signal-regulated kinase 1 (ERK1) and ERK2 play essential roles in osteoblast differentiation and in supporting osteoclastogenesis. Mol. Cell Biol. 2009, 29, 5843–5857. [Google Scholar] [CrossRef] [Green Version]
- Kassel, O.; Sancono, A.; Kratzschmar, J.; Kreft, B.; Stassen, M.; Cato, A.C. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001, 20, 7108–7116. [Google Scholar] [CrossRef] [Green Version]
- Postnov, A.; De Schutter, T.; Sijbers, J.; Karperien, M.; De Clerck, N. Glucocorticoid-induced osteoporosis in growing mice is not prevented by simultaneous intermittent PTH treatment. Calcif. Tissue Int. 2009, 85, 530–537. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. The RANKL/RANK/OPG pathway. Curr. Osteoporos. Rep. 2007, 5, 98–104. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udagawa, N.; Takahashi, N.; Yasuda, H.; Mizuno, A.; Itoh, K.; Ueno, Y.; Shinki, T.; Gillespie, M.T.; Martin, T.J.; Higashio, K.; et al. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 2000, 141, 3478–3484. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Chakraborty, M.; Chatterjee, D.; Horne, W.C.; Lomri, A.; Ravesloot, J.H. Biology of the Osteoclast. Handb. Exp. Pharmacol. 1993, 107, 111–147. [Google Scholar]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Do, P.A.; Lee, C.H. The Role of CDK5 in Tumours and Tumour Microenvironments. Cancers 2020, 13, 101. [Google Scholar] [CrossRef]
- Cicenas, J.; Kalyan, K.; Sorokinas, A.; Stankunas, E.; Levy, J.; Meskinyte, I.; Stankevicius, V.; Kaupinis, A.; Valius, M. Roscovitine in cancer and other diseases. Ann. Transl. Med. 2015, 3, 135. [Google Scholar] [CrossRef]
- Kino, T.; Ichijo, T.; Amin, N.D.; Kesavapany, S.; Wang, Y.; Kim, N.; Rao, S.; Player, A.; Zheng, Y.L.; Garabedian, M.J.; et al. Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: Clinical implications for the nervous system response to glucocorticoids and stress. Mol. Endocrinol. 2007, 21, 1552–1568. [Google Scholar] [CrossRef]
- Pfänder, P.; Fidan, M.; Burret, U.; Lipinski, L.; Vettorazzi, S. Cdk5 Deletion Enhances the Anti-inflammatory Potential of GC-Mediated GR Activation During Inflammation. Front. Immunol. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Kwee, B.J.; Mooney, D.J.; Duda, G.N. Boon and Bane of Inflammation in Bone Tissue Regeneration and Its Link with Angiogenesis. Tissue Eng. Part B Rev. 2015, 21, 354–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebl, J.; Zhang, S.; Moser, M.; Agalarov, Y.; Demir, C.S.; Hager, B.; Bibb, J.A.; Adams, R.H.; Kiefer, F.; Miura, N.; et al. Cdk5 controls lymphatic vessel development and function by phosphorylation of Foxc2. Nat. Commun. 2015, 6, 7274. [Google Scholar] [CrossRef] [Green Version]
- Merk, H.; Zhang, S.; Lehr, T.; Muller, C.; Ulrich, M.; Bibb, J.A.; Adams, R.H.; Bracher, F.; Zahler, S.; Vollmar, A.M.; et al. Inhibition of endothelial Cdk5 reduces tumor growth by promoting non-productive angiogenesis. Oncotarget 2016, 7, 6088–6104. [Google Scholar] [CrossRef]
- Liebl, J.; Weitensteiner, S.B.; Vereb, G.; Takacs, L.; Furst, R.; Vollmar, A.M.; Zahler, S. Cyclin-dependent kinase 5 regulates endothelial cell migration and angiogenesis. J. Biol. Chem. 2010, 285, 35932–35943. [Google Scholar] [CrossRef] [Green Version]
- Liebl, J.; Krystof, V.; Vereb, G.; Takacs, L.; Strnad, M.; Pechan, P.; Havlicek, L.; Zatloukal, M.; Furst, R.; Vollmar, A.M.; et al. Anti-angiogenic effects of purine inhibitors of cyclin dependent kinases. Angiogenesis 2011, 14, 281–291. [Google Scholar] [CrossRef]
- Peng, Y.; Lv, S.; Li, Y.; Zhu, J.; Chen, S.; Zhen, G.; Cao, X.; Wu, S.; Crane, J.L. Glucocorticoids Disrupt Skeletal Angiogenesis Through Transrepression of NF-κB-Mediated Preosteoclast Pdgfb Transcription in Young Mice. J. Bone Miner. Res. 2020, 35, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Tuckermann, J.; Adams, R.H. The endothelium-bone axis in development, homeostasis and bone and joint disease. Nat. Rev. Rheumatol. 2021, 17, 608–620. [Google Scholar] [CrossRef] [PubMed]




| Gene Symbol | Gene Name | Gene ID | Reverse Primer (5′–3′) |
|---|---|---|---|
| Non-targeting | - | - | UAAGGCUAUGAAGAGAUAC |
| AUGUAUUGGCCUGUAUUAG | |||
| AUGAACGUGAAUUGCUCAA UGGUUUACAUGUCGACUAA | |||
| Cdk5 | Cyclin-dependent kinase 5 | 12568 | GGAGAUCUGUCUACUCAAA |
| UAUAAGCCCUACCCAAUGU GCAACGUGCUACAUAGGGA CAACAUCCUUGGUGAACGU |
| Gene Symbol | Gene ID | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|
| Cdk5 | 12568 | TGGACCCTGAGATTGTGAAGT | GACAGAATCCCAGGCCTTTC |
| Runx2 | 12393 | TGTTCTCTGATCGCCTCAGTG | CCTGGGATCTGTAATCTGACTCT |
| Sp7 | 170574 | CCCACCCTTCCCTCACTCAT | CCTTGTACCACGAGCCATAGG |
| Alpl | 11647 | GCTGATCATTCCCACGTTTT | CTGGGCCTGGTAGTTGTTGT |
| Bglap | 12096 | TCTGACAAAGCCTTCATGTCCA | CGGTCTTCAAGCCATACTGGTC |
| Rankl | 21943 | TCACCATTCGGATGAGTCTG | ACTTGTGGCTCTGATGTTCC |
| Opg | 18383 | CCTGAGGCCCAGCCATTT | CTTGGCCCAGCCTCGAT |
| Actb | 11461 | CCTTGCCCTGACCACTCTTA | ACACTGGGCTGCAATACACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, B.T.; Steppe, L.; Vettorazzi, S.; Haffner-Luntzer, M.; Lee, S.; Dorn, A.-K.; Ignatius, A.; Tuckermann, J.; Ahmad, M. Inhibition of Cdk5 Ameliorates Skeletal Bone Loss in Glucocorticoid-Treated Mice. Biomedicines 2022, 10, 404. https://doi.org/10.3390/biomedicines10020404
Krüger BT, Steppe L, Vettorazzi S, Haffner-Luntzer M, Lee S, Dorn A-K, Ignatius A, Tuckermann J, Ahmad M. Inhibition of Cdk5 Ameliorates Skeletal Bone Loss in Glucocorticoid-Treated Mice. Biomedicines. 2022; 10(2):404. https://doi.org/10.3390/biomedicines10020404
Chicago/Turabian StyleKrüger, Benjamin Thilo, Lena Steppe, Sabine Vettorazzi, Melanie Haffner-Luntzer, Sooyeon Lee, Ann-Kristin Dorn, Anita Ignatius, Jan Tuckermann, and Mubashir Ahmad. 2022. "Inhibition of Cdk5 Ameliorates Skeletal Bone Loss in Glucocorticoid-Treated Mice" Biomedicines 10, no. 2: 404. https://doi.org/10.3390/biomedicines10020404
APA StyleKrüger, B. T., Steppe, L., Vettorazzi, S., Haffner-Luntzer, M., Lee, S., Dorn, A.-K., Ignatius, A., Tuckermann, J., & Ahmad, M. (2022). Inhibition of Cdk5 Ameliorates Skeletal Bone Loss in Glucocorticoid-Treated Mice. Biomedicines, 10(2), 404. https://doi.org/10.3390/biomedicines10020404









