Sex-Specific Impact of Different Obesity/Metabolic Phenotypes on Long-Term Cardiovascular Outcomes in Acute Coronary Syndrome Patients
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Methods
2.2.1. Blood Pressure Measurements
2.2.2. Body Mass Index
2.2.3. Transthoracic Echocardiography (TTE)
2.2.4. Metabolic Status
2.2.5. Study Outcomes
2.3. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics According to Sex
3.2. Baseline Clinical Characteristics According to Obesity-Related Phenotypes
3.3. Baseline Clinical Characteristics According to Sex- and Obesity-Related Phenotypes
3.4. Cumulative Incidence of the Composite Outcome of Death, Fatal or Nonfatal Reinfarction with or without PCI and/or Stroke in ACS Patients, According to Sex and Obesity/Metabolic Phenotypes
3.5. Factors Associated with the Incidence of the Composite Outcome of Death, Fatal or Nonfatal Reinfarction with or without PCI and/or Stroke in ACS Men and Women
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steg, P.G.; James, S.K.; Atar, D.; Badano, L.P.; Lundqvist, C.B.; Borger, M.A.; Di Mario, C.; Dickstein, K.; Ducrocq, G.; Fernandez-Aviles, F.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2012, 33, 2569–2619. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cífková, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2013, 23, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Schiller, N.B.; Shah, P.M.; Crawford, M.; DeMaria, A.; Devereux, R.; Feigenbaum, H.; Gutgesell, H.; Reichek, N.; Sahn, D.; Schnittger, I.; et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J. Am. Soc. Echocardiogr. 1989, 2, 358–367. [Google Scholar] [CrossRef]
- Greenland, S. Modeling and variable selection in epidemiologic analysis. Am. J. Public Health 1989, 79, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Arnlöv, J.; Ingelsson, E.; Sundström, J.; Lind, L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation 2010, 121, 230–236. [Google Scholar]
- Aung, K.; Lorenzo, C.; Hinojosa, M.A.; Haffner, S.M. Risk of Developing Diabetes and Cardiovascular Disease in Metabolically Unhealthy Normal-Weight and Metabolically Healthy Obese Individuals. J. Clin. Endocrinol. Metab. 2014, 99, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Opio, J.; Croker, E.; Odongo, G.S.; Attia, J.; Wynne, K.; McEvoy, M. Metabolically healthy overweight/obesity are associated with increased risk of cardiovascular disease in adults, even in the absence of metabolic risk factors: A systematic review and meta-analysis of prospective cohort studies. Obes. Rev. 2020, 21, e13127. [Google Scholar] [CrossRef] [PubMed]
- Dooley, J.; Chang, A.M.; Salhi, R.A.; Hollander, J.E. Relationship between body mass index and prognosis of patients presenting with potential acute coronary syndromes. Acad. Emerg. Med. 2013, 20, 904–910. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kadakia, M.B.; Fox, C.S.; Scirica, B.M.; Murphy, S.A.; Bonaca, M.P.; Morrow, D.A. Central obesity and cardiovascular outcomes in patients with acute coronary syndrome: Observations from the MERLIN-TIMI 36 trial. Heart 2011, 97, 1782–1787. [Google Scholar] [CrossRef]
- Xu, J.-J.; Song, Y.; Jiang, P.; Jiang, L.; Zhao, X.-Y.; Gao, Z.; Li, J.-X.; Qiao, S.-B.; Gao, R.-L.; Yang, Y.-J.; et al. Effects of metabolic syndrome on onset age and long-term outcomes in patients with acute coronary syndrome. World J. Emerg. Med. 2021, 12, 36–41. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Peterson, C.M.; Thomas, D.M.; Heo, M.; Schuna, J.M. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes. Rev. 2015, 17, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Garawi, F.; Devries, K.; Thorogood, N.; Uauy, R. Global differences between women and men in the prevalence of obesity: Is there an association with gender inequality? Eur. J. Clin. Nutr. 2014, 68, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Chrysohoou, C.; Dilaveris, P.; Georgiopoulos, G.; Magkas, N.; Aggelopoulos, P.; Panagiotakos, D.B.; Tousoulis, D. Skeletal muscle mass in acute coronary syndrome prognosis: Gender-based analysis from Hellenic Heart Failure cohort. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 718–727. [Google Scholar] [CrossRef]
- Kouvari, M.; Chrysohoou, C.; Tsiamis, E.; Kosyfa, H.; Kalogirou, L.; Filippou, A.; Iosifidis, S.; Aggelopoulos, P.; Pitsavos, C.; Tousoulis, D. The “overweight paradox” in the prognosis of acute coronary syndrome for patients with heart failure—A truth for all? A 10-year follow-up study. Maturitas 2017, 102, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes. Facts 2017, 10, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, K.; Campbell, N.R.; Joffres, M.R.; McAlister, F.A.; Nichol, M.; Quach, S.; Johansen, H.L.; Tremblay, M.S. Blood pressure in Canadian adults. Health Rep. 2010, 21, 37–46. [Google Scholar]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar]
- The Emerging Risk Factors Collaboration Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet 2011, 377, 1085–1095. [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2006, 26, 968–976. [Google Scholar] [CrossRef]
- Poirier, P.; Eckel, R.H. Obesity and cardiovascular disease. Curr. Atheroscler. Rep. 2002, 4, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Montori, V.; Somers, V.K.; Korinek, J.; Thomas, R.; Allison, T.G.; Mookadam, F.; Lopez-Jimenez, F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: A systematic review of cohort studies. Lancet 2006, 368, 666–678. [Google Scholar] [CrossRef]
- Appleton, S.L.; Seaborn, C.J.; Visvanathan, R.; Hill, C.L.; Gill, T.K.; Taylor, A.W.; Adams, R.J.; North West Adelaide Health Study Team. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: A cohort study. Diabetes Care 2013, 36, 2388–2394. [Google Scholar] [CrossRef]
- Hamer, M.; Stamatakis, E. Metabolically Healthy Obesity and Risk of All-Cause and Cardiovascular Disease Mortality. J. Clin. Endocrinol. Metab. 2012, 97, 2482–2488. [Google Scholar] [CrossRef]
- Voulgari, C.; Tentolouris, N.; Dilaveris, P.; Tousoulis, D.; Katsilambros, N.; Stefanadis, C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. J. Am. Coll. Cardiol. 2011, 58, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.; Lattuada, G.; Piemonti, L.; Garancini, M.P.; Ragogna, F.; Villa, M.; Mannino, S.; Crosignani, P.; Bosi, E.; Luzi, L.; et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: The Cremona Study. Diabetes Care 2011, 34, 210–215. [Google Scholar] [CrossRef]
- Choi, K.M.; Cho, H.J.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Baik, S.H.; Choi, D.S.; Kim, N.H. Higher mortality in metabolically obese normal-weight people than in metabolically healthy obese subjects in elderly Koreans. Clin. Endocrinol. 2013, 79, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Mørkedal, B.; Vatten, L.J.; Romundstad, P.R.; Laugsand, L.E.; Janszky, I. Risk of Myocardial Infarction and Heart Failure Among Metabolically Healthy But Obese Individuals. J. Am. Coll. Cardiol. 2014, 63, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Roberson, L.L.; Aneni, E.C.; Maziak, W.; Agatston, A.S.; Feldman, T.; Rouseff, M.; Tran, T.; Blaha, M.J.; Santos, R.D.; Sposito, A.C.; et al. Beyond BMI: The “Metabolically healthy obese” phenotype & its association with clinical/subclinical cardiovascular disease and all-cause mortality—A systematic review. BMC Public Health 2014, 14, 14. [Google Scholar] [CrossRef]
- Kramer, C.K.; Zinman, B.; Retnakaran, R. Are Metabolically Healthy Overweight and Obesity Benign Conditions?: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2013, 159, 758. [Google Scholar] [CrossRef] [PubMed]
- Eckel, N.; Meidtner, K.; Kalle-Uhlmann, T.; Stefan, N.; Schulze, M.B. Metabolically healthy obesity and cardiovascular events: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2015, 23, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Song, Y.; Chen, Y.; Hui, R.; Zhang, W. Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 2013, 168, 4761–4768. [Google Scholar] [CrossRef]
- Zheng, R.; Zhou, D.; Zhu, Y. The long-term prognosis of cardiovascular disease and all-cause mortality for metabolically healthy obesity: A systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Mongraw-Chaffin, M.; Foster, M.C.; Anderson, C.A.; Burke, G.L.; Haq, N.; Kalyani, R.R.; Ouyang, P.; Sibley, C.T.; Tracy, R.; Woodward, M.; et al. Metabolically Healthy Obesity, Transition to Metabolic Syndrome, and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 71, 1857–1865. [Google Scholar] [CrossRef]
- Fingeret, M.; Marques-Vidal, P.; Vollenweider, P. Incidence of type 2 diabetes, hypertension, and dyslipidemia in metabolically healthy obese and non-obese. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1036–1044. [Google Scholar] [CrossRef]
- Russo, G.T.; Giandalia, A.; Romeo, E.L.; Alibrandi, A.; Horvath, K.V.; Asztalos, B.F.; Cucinotta, D. Markers of Systemic Inflammation and Apo-AI Containing HDL Subpopulations in Women with and without Diabetes. Int. J. Endocrinol. 2014, 2014, 607924. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Trotta, M.C.; Pieretti, G.; Gatta, G.; Ferraro, G.; Nicoletti, G.F.; Onofrio, N.D.; Balestrieri, M.L.; Amico, M.D.; Abbatecola, A.; et al. MicroRNAs modulation and clinical outcomes at 1 year of follow-up in obese patients with pre-diabetes treated with metformin vs. placebo. Geol. Rundsch. 2021, 58, 1381–1393. [Google Scholar] [CrossRef]
- Sasso, F.C.; Rinaldi, L.; Lascar, N.; Marrone, A.; Pafundi, P.C.; Adinolfi, L.E.; Marfella, R. Role of Tight Glycemic Control during Acute Coronary Syndrome on CV Outcome in Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 3106056. [Google Scholar] [CrossRef]
- Marfella, R.; Sasso, F.C.; Cacciapuoti, F.; Portoghese, M.; Rizzo, M.R.; Siniscalchi, M.; Carbonara, O.; Ferraraccio, F.; Torella, M.; Petrella, A.; et al. Tight Glycemic Control May Increase Regenerative Potential of Myocardium during Acute Infarction. J. Clin. Endocrinol. Metab. 2012, 97, 933–942. [Google Scholar] [CrossRef]
- Kalantzi, K.; Korantzopoulos, P.; Tzimas, P.; Katsouras, C.S.; Goudevenos, J.A.; Milionis, H.J. The relative value of metabolic syndrome and cardiovascular risk score estimates in premature acute coronary syndromes. Am. Heart J. 2008, 155, 534–540. [Google Scholar] [CrossRef]
- Mirza, A.J.; Taha, A.Y.; Khdhir, B.R. Risk factors for acute coronary syndrome in patients below the age of 40 years. Egypt. Heart J. 2018, 70, 233–235. [Google Scholar] [CrossRef]
- Rabkin, S.W.; Mathewson, F.A.; Hsu, P.-H. Relation of body weight to development of ischemic heart disease in a cohort of young north American men after a 26 year observation period: The manitoba study. Am. J. Cardiol. 1977, 39, 452–458. [Google Scholar] [CrossRef]
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Gurm, H.S.; Whitlow, P.L.; Kip, K.E. The impact of body mass index on short- and long-term outcomes inpatients undergoing coronary revascularization. Insights from the bypass angioplasty revascularization investigation (BARI). J. Am. Coll. Cardiol. 2002, 39, 834–840. [Google Scholar] [CrossRef]
- Yeh, T.-L.; Chen, H.-H.; Tsai, S.-Y.; Lin, C.-Y.; Liu, S.-J.; Chien, K.-L. The Relationship between Metabolically Healthy Obesity and the Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1228. [Google Scholar] [CrossRef] [PubMed]
- El Moheb, M.; Jia, Z.; Qin, H.; El Hechi, M.W.; Nordestgaard, A.T.; Lee, J.M.; Han, K.; Kaafarani, H.M. The Obesity Paradox in Elderly Patients Undergoing Emergency Surgery: A Nationwide Analysis. J. Surg. Res. 2021, 265, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, M.; Franchi, C.; Nobili, A.; Mannucci, P.M.; Ardoino, I.; REPOSI Investigators. Defining Aging Phenotypes and Related Outcomes: Clues to Recognize Frailty in Hospitalized Older Patients. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2017, 72, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Paolisso, G.; Marfella, R. Impact of Sex Differences in Incident and Recurrent Coronary Events and All-Cause Mortality. J. Am. Coll. Cardiol. 2021, 77, 829–830. [Google Scholar] [CrossRef]
- Giandalia, A.; Alibrandi, A.; Giorgianni, L.; Piano, F.L.; Consolo, F.; Elia, G.L.; Asztalos, B.; Cucinotta, D.; Squadrito, G.; Russo, G.T. Resistin levels and inflammatory and endothelial dysfunction markers in obese postmenopausal women with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2021, 13, 98. [Google Scholar] [CrossRef]
- Giandalia, A.; Giuffrida, A.; Gembillo, G.; Cucinotta, D.; Squadrito, G.; Santoro, D.; Russo, G. Gender Differences in Diabetic Kidney Disease: Focus on Hormonal, Genetic and Clinical Factors. Int. J. Mol. Sci. 2021, 22, 5808. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Handisurya, A. Metabolic diseases and associated complications: Sex and gender matter! Eur. J. Clin. Investig. 2009, 39, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Huebschmann, A.G.; Huxley, R.; Kohrt, W.M.; Zeitler, P.; Regensteiner, J.G.; Reusch, J.E.B. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia 2019, 62, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Gatta, G.; Pieretti, G.; Viola, L.; Sacra, C.; Di Grezia, G.; Musto, L.; Minelli, S.; La Forgia, D.; Capodieci, M.; et al. Pre-Menopausal Breast Fat Density Might Predict MACE During 10 Years of Follow-up. JACC Cardiovasc. Imaging 2020, 14, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Hioki, H.; Miura, T.; Motoki, H.; Kobayashi, H.; Kobayashi, M.; Nakajima, H.; Sekimura, N.; Mawatari, E.; Akanuma, H.; Sato, T.; et al. Lean body mass index prognostic value for cardiovascular events in patients with coronary artery disease. Heart Asia 2015, 7, 12–18. [Google Scholar] [CrossRef]
- Peters, S.; Huxley, R.R.; Woodward, M. Diabetes as risk factor for incident coronary heart disease in women compared with men: A systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014, 57, 1542–1551. [Google Scholar] [CrossRef]
- Wang, Y.; O’Neil, A.; Jiao, Y.; Wang, L.; Huang, J.; Lan, Y.; Zhu, Y.; Yu, C. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: A systematic review and meta-analysis of 5,162,654 participants. BMC Med. 2019, 17, 136. [Google Scholar] [CrossRef]
- Roque, D.; Ferreira, J.; Monteiro, S.; Costa, M.; Gil, V. Understanding a woman’s heart: Lessons from 14,177 women with acute coronary syndrome. Rev. Port. Cardiol. 2020, 39, 57–72. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, J.; Liu, J.; Yang, N.; Smith, S.C., Jr.; Huo, Y.; Fonarow, G.C.; Ge, J.; Taubert, K.A.; Morgan, L.; et al. Sex Differences in In-Hospital Management and Outcomes of Patients With Acute Coronary Syndrome. Circulation 2019, 139, 1776–1785. [Google Scholar] [CrossRef]
- Poirier, P.; Martin, J.; Marceau, P.; Biron, S.; Marceau, S. Impact of bariatric surgery on cardiac structure, function and clinical manifestations in morbid obesity. Expert Rev. Cardiovasc. Ther. 2004, 2, 193–201. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.; Larson, M.; Kannel, W.B.; Vasan, R.S. Obesity and the Risk of Heart Failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef]

| TOTAL (674 pts) | MALE (504 pts) | FEMALE (170 pts) | p Value | MHNW (368 pts) | MUNW (178 pts) | MHO (40 pts) | MUO (88 pts) | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Demographic Characteristics | |||||||||
| Age, (year) | 64.0 ± 11.6 | 62.4 ± 11.4 | 68.8 ± 11.1 | <0.001 ** | 64.1 ± 12.1 | 64.3 ± 11.7 | 63.2 ± 10.6 | 63.5 ± 10.2 | 0.924 # |
| Cardiovascular risk factors | |||||||||
| Smokers, (%) | 38.6 | 46.0 | 16.5 | <0.001 * | 40.2 | 42.1 | 35.0 | 26.1 | 0.062 * |
| Hypertension, (%) | 65.7 | 62.5 | 75.3 | 0.002 * | 58.4 | 71.3 | 62.5 | 86.4 | <0.001 * |
| Diabetes mellitus, (%) | 28.0 | 25.6 | 35.3 | 0.020 * | 19.0 | 44.9 | 12.5 | 38.6 | <0.001 * |
| Clinical parameters | |||||||||
| Systolic BP, (mmHg) | 142.6 ± 23.3 | 141.3 ± 23.7 | 146.4 ± 21.4 | 0.022 ** | 139.0 ± 22.9 | 149.7 ± 23.6 | 133.6 ± 21.8 | 146.3 ± 20.6 | <0.001 # |
| Diastolic BP, (mmHg) | 79.6 ± 13.1 | 79.5 ± 13.0 | 80.0 ± 13.6 | 0.676 | 78.2 ± 13.0 | 79.6 ± 12.4 | 81.2 ± 13.4 | 84.8 ± 13.6 | 0.001 # |
| Heart rate, (beats/min) | 71.9 ± 13.2 | 70.5 ± 12.8 | 75.8 ± 13.7 | <0.001 ** | 71.3 ± 12.5 | 72.4 ± 13.8 | 69.8 ± 17.2 | 74.3 ± 12.9 | 0.320 # |
| Ejection fraction, (%) | 53.8 ± 11.1 | 53.3 ± 10.8 | 55.3 ± 12.0 | 0.142 | 54.3 ± 11.0 | 52.3 ± 11.9 | 56.5 ± 12.4 | 54.3 ± 8.2 | 0.316 # |
| E/A | 0.3 ± 0.4 | 0.4 ± 0.4 | 0.3 ± 0.5 | 0.493 | 0.2 ± 0.4 | 0.4 ± 0.4 | 0.1 ± 0.3 | 0.4 ± 0.5 | 0.09 # |
| Extent of coronary artery disease at baseline | |||||||||
| No significant coronary artery disease, (%) | 9.8 | 10.1 | 9.5 | 0.547 * | 12.8 | 2.8 | 20.8 | 6.8 | 0.005 * |
| 1-vessel disease, (%) | 36.9 | 37.7 | 34.7 | 0.485 * | 37.0 | 39.9 | 35.0 | 31.8 | |
| 2-vessel disease, (%) | 24.5 | 25.0 | 22.4 | 0.480 * | 24.2 | 27.0 | 20.0 | 22.7 | |
| 3-vessel disease, (%) | 28.8 | 30.2 | 24.7 | 0.175 * | 26.1 | 30.3 | 25.0 | 38.6 | |
| Coronary revascularization at baseline | |||||||||
| Single drug-eluting stent, (%) | 43.6 | 45.2 | 38.8 | 0.145 * | 41.0 | 48.9 | 45.0 | 43.2 | 0.386 * |
| Multiple drug-eluting stents with overlapping, (%) | 12.0 | 12.3 | 11.2 | 0.697 * | 12.2 | 12.4 | 7.5 | 12.5 | 0.843 * |
| Coronary artery bypass graft surgery, (%) | 10.5 | 11.1 | 8.8 | 0.402 * | 9.8 | 11.2 | 10.0 | 12.5 | 0.568 * |
| Biochemical markers | |||||||||
| Total cholesterol, (mg/dL) | 181.7 ± 44.1 | 180.3 ± 44.5 | 185.6 ± 42.7 | 0.174 | 179.5 ± 43.0 | 185.7 ± 43.5 | 184.6 ± 49.9 | 181.3 ± 47.0 | 0.461 # |
| HDL cholesterol, (mg/dL) | 44.9 ± 13.6 | 43.7 ± 12.9 | 48.6 ± 14.9 | <0.001 ** | 49.0 ± 13.8 | 36.4 ± 8.9 | 52.0 ± 11.8 | 42.1 ± 12.5 | <0.001 # |
| LDL cholesterol, (mg/dL) | 107.0 ± 39.6 | 106.8 ± 40.2 | 107.6 ± 37.7 | 0.821 | 108.1 ± 38.8 | 107.3 ± 40.7 | 107.4 ± 45.8 | 101.3 ± 37.6 | 0.565 # |
| Triglyceride (mg/dL) | 146.5 ± 90.3 | 150.1 ± 94.3 | 136.3 ± 76.8 | 0.088 | 111.7 ± 47.2 | 209.3 ± 104.9 | 107.0 ± 35.6 | 181.9 ± 123.7 | <0.001 # |
| Fasting glucose, (mg/dL) | 126.4 ± 59.7 | 123.9 ± 56.5 | 133.9 ± 67.7 | 0.058 | 114.7 ± 59.1 | 151.8 ± 58.6 | 104.4 ± 45.0 | 134.2 ± 52.2 | <0.001 # |
| Creatinine, (mg/dL) | 1.0 ± 0.8 | 1.0 ± 0.7 | 0.9 ± 0.8 | 0.299 | 1.0 ± 0.9 | 1.0 ± 0.8 | 0.9 ± 0.3 | 0.9 ± 0.4 | 0.746 # |
| C-reactive protein, (mg/dL) | 10.5 ± 21.0 | 10.4 ± 20.3 | 10.8 ± 23.6 | 0.879 | 8.5 ± 14.6 | 11.4 ± 25.2 | 12.4 ± 12.7 | 15.4 ± 30.4 | 0.280 # |
| Medication following coronary revascularization | |||||||||
| Antiplatelet therapy, (%) | 99.5 | 99.1 | 98.6 | 0.540 * | 99.4 | 99.6 | 99.2 | 100.0 | 0.954 * |
| Statins, (%) | 99.3 | 97.2 | 96.2 | 0.612 * | 99.7 | 99.4 | 98.9 | 99.5 | 0.944 * |
| Diuretics, (%) | 28.3 | 27.5 | 26.4 | 0.654 * | 27.4 | 27.7 | 28.6 | 29.7 | 0.554 * |
| ACE inhibitor, (%) | 97.4 | 97.2 | 96.2 | 0.167 * | 97.6 | 96.6 | 98.1 | 97.3 | 0.898 * |
| Beta-blocker, (%) | 95.5 | 95.3 | 94.4 | 0.343 * | 95.2 | 95.1 | 96.5 | 95.3 | 0.789 * |
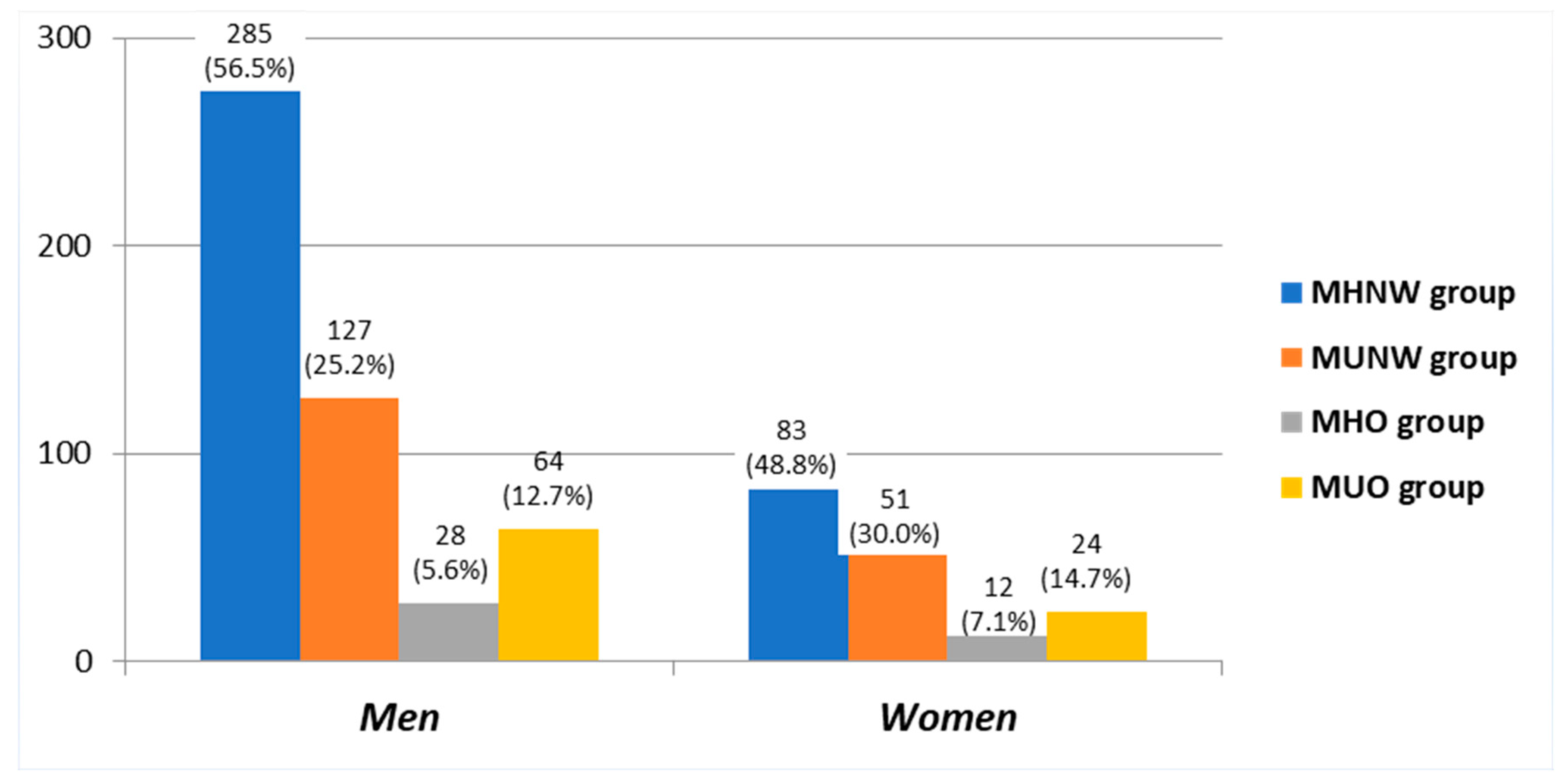
| (A) | MHNW (285 pts) | MUNW (127 pts) | MHO (28 pts) | MUO (64 pts) | p Value |
| Demographic characteristics | |||||
| Age, (year) | 62.9 ± 11.9 | 61.9 ± 11.1 | 60.9 ± 10.7 | 62.5 ± 9.8 | 0.765 # |
| Cardiovascular risk factors | |||||
| Smokers, (%) | 46.7 | 52.1 | 46.4 | 31.3 | 0.058 * |
| Hypertension, (%) | 55.4 | 68.5 | 53.6 | 85.9 | <0.001 * |
| Diabetes mellitus, (%) | 17.5 | 40.9 | 7.1 | 39.1 | <0.001 * |
| Clinical parameters | |||||
| Systolic BP, (mmHg) | 136.8 ± 22.9 | 150.6 ± 23.9 | 131.1 ± 24.0 | 146.5 ± 19.1 | <0.001 # |
| Diastolic BP, (mmHg) | 77.7 ± 12.9 | 81.3 ± 12.0 | 79.1 ± 12.9 | 84.2 ± 13.7 | 0.003 # |
| Heart rate, (beats/min) | 69.9 ± 12.0 | 71.1 ± 12.9 | 69.2 ± 18.6 | 72.9 ± 13.1 | 0.511 # |
| Ejection fraction, (%) | 53.7 ± 10.9 | 52.5 ± 11.7 | 54.6 ± 11.5 | 53.6 ± 7.3 | 0.829 # |
| E/A | 0.3 ± 0.4 | 0.4 ± 0.4 | 0.1 ± 0.3 | 0.4 ± 0.5 | 0.113 # |
| Extent of coronary artery disease at baseline | |||||
| No significant coronary artery disease, (%) | 9.5 | 1.6 | 14.3 | 4.7 | 0.007 * |
| 1-vessel disease, (%) | 38.6 | 37.1 | 46.4 | 31.3 | |
| 2-vessel disease, (%) | 24.2 | 27.6 | 21.4 | 25.1 | |
| 3-vessel disease, (%) | 27.7 | 33.9 | 17.9 | 39.1 | |
| Single drug-eluting stent, (%) | 43.5 | 46.5 | 50.1 | 48.4 | 0.817 * |
| Multiple drug-eluting stents with overlapping, (%) | 13.7 | 10.2 | 7.9 | 10.9 | 0.759 * |
| Coronary artery bypass graft surgery, (%) | 10.2 | 13.4 | 7.1 | 12.5 | 0.787 * |
| Biochemical markers | |||||
| Total cholesterol, (mg/dL) | 178.9 ± 43.1 | 183.9 ± 44.7 | 178.5 ± 49.1 | 180.6 ± 48.8 | 0.753 # |
| HDL cholesterol, (mg/dL) | 47.6 ± 13.3 | 35.3 ± 8.6 | 48.9 ± 11.0 | 41.2 ± 10.0 | <0.001 # |
| LDL cholesterol, (mg/dL) | 108.9 ± 39.4 | 105.8 ± 41.6 | 107.7 ± 46.6 | 99.2 ± 38.5 | 0.379 # |
| Triglyceride (mg/dL) | 113.4 ± 49.2 | 217.1 ± 104.4 | 108.2 ± 39.3 | 198.1 ± 139.2 | <0.001 # |
| Fasting glucose, (mg/dL) | 115.1 ± 60.9 | 145.8 ± 50.8 | 100.5 ± 31.3 | 130.1 ± 40.7 | <0.001 # |
| Creatinine, (mg/dL) | 1.0 ± 0.9 | 1.0 ± 0.7 | 0.9 ± 0.3 | 1.0 ± 0.4 | 0.905 # |
| C-reactive protein, (mg/dL) | 9.4 ± 15.5 | 9.7 ± 21.9 | 12.4 ± 12.7 | 16.1 ± 35.5 | 0.440 # |
| Medication following coronary revascularization | |||||
| Antiplatelet therapy, (%) | 98.4 | 97.6 | 97.1 | 99.9 | 0.564 * |
| Statins, (%) | 99.3 | 99.1 | 97.3 | 98.8 | 0.173 * |
| Diuretics, (%) | 28.4 | 27.9 | 29.4 | 29.1 | 0.223 * |
| ACE inhibitor, (%) | 96.5 | 97.6 | 98.6 | 98.2 | 0.431 * |
| Beta-blocker, (%) | 94.6 | 96.1 | 97.1 | 96.1 | 0.551 * |
| (B) | MHNW (83 pts) | MUNW (51 pts) | MHO (12 pts) | MUO (24 pts) | p Value |
| Demographic characteristics | |||||
| Age, (year) | 68.5 ± 11.6 | 70.4 ± 11.1 | 68.1 ± 8.1 | 11.1 ± 10.9 | 0.549 # |
| Cardiovascular risk factors | |||||
| Smokers, (%) | 18.1 | 17.6 | 8.3 | 16.0 | 0.792 * |
| Hypertension, (%) | 68.7 | 78.4 | 83.3 | 84.1 | 0.210 * |
| Diabetes mellitus, (%) | 24.1 | 54.9 | 25.1 | 36.1 | 0.003 * |
| Clinical parameters | |||||
| Systolic BP, (mmHg) | 146.7 ± 21.2 | 147.5 ± 22.8 | 140.2 ± 14.3 | 146.1 ± 23.7 | 0.839 # |
| Diastolic BP, (mmHg) | 80.1 ± 13.4 | 75.5 ± 12.4 | 86.4 ± 13.7 | 86.1 ± 13.7 | 0.007 # |
| Heart rate, (beats/min) | 75.9 ± 12.9 | 75.9 ± 15.5 | 71.6 ± 14.1 | 77.1 ± 12.4 | 0.841 # |
| Ejection fraction, (%) | 56.9 ± 11.4 | 51.9 ± 12.5 | 63.1 ± 15.3 | 55.8 ± 10.5 | 0.191 # |
| E/A | 0.3 ± 0.5 | 0.4 ± 0.5 | 0.3 ± 0.4 | 0.7 ± 0.4 | 0.391 # |
| Extent of coronary artery disease at baseline | |||||
| No significant coronary artery disease, (%) | 24.1 | 5.9 | 33.3 | 12.1 | 0.367 * |
| 1-vessel disease, (%) | 31.1 | 47.1 | 8.3 | 32.0 | |
| 2-vessel disease, (%) | 24.1 | 25.5 | 16.7 | 20.1 | |
| 3-vessel disease, (%) | 20.5 | 21.6 | 41.7 | 36.1 | |
| Single drug-eluting stent, (%) | 32.5 | 54.9 | 33.3 | 32.0 | 0.045 * |
| Multiple drug-eluting stents with overlapping, (%) | 7.2 | 17.6 | 0.0 | 16.1 | 0.129 * |
| Coronary artery bypass graft surgery, (%) | 8.4 | 5.9 | 0.0 | 12.1 | 0.603 * |
| Biochemical markers | |||||
| Total cholesterol, (mg/dL) | 181.6 ± 42.6 | 190.1 ± 40.7 | 199.1 ± 50.8 | 183.4 ± 42.8 | 0.476 # |
| HDL cholesterol, (mg/dL) | 54.2 ± 14.0 | 39.3 ± 9.2 | 60.3 ± 10.1 | 45.1 ± 17.7 | <0.001 # |
| LDL cholesterol, (mg/dL) | 105.4 ± 37.1 | 111.2 ± 38.5 | 106.6 ± 45.9 | 107.5 ± 35.3 | 0.869 # |
| Triglyceride (mg/dL) | 106.1 ± 39.4 | 192.1 ± 104.8 | 104.1 ± 26.5 | 138.6 ± 52.8 | <0.001 # |
| Fasting glucose, (mg/dL) | 113.4 ± 53.0 | 166.9 ± 73.1 | 113.6 ± 68.1 | 145.3 ± 73.8 | <0.001 # |
| Creatinine, (mg/dL) | 0.9 ± 0.8 | 1.1 ± 1.0 | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.513 # |
| C-reactive protein, (mg/dL) | 5.1 ± 12.6 | 16.1 ± 33.3 | 11.6 ± 22.1 | 13.9 ± 15.8 | 0.194 # |
| Medication following coronary revascularization | |||||
| Antiplatelet therapy, (%) | 99.3 | 96.4 | 95.9 | 98.4 | 0.453 * |
| Statins, (%) | 98.2 | 98.4 | 97.3 | 99.3 | 0.226 * |
| Diuretics, (%) | 30.1 | 28.6 | 28.5 | 29.6 | 0.331 * |
| ACE inhibitor, (%) | 97.4 | 96.4 | 97.8 | 99.4 | 0.546 * |
| Beta-blocker, (%) | 95.9 | 97.1 | 98.2 | 97.3 | 0.662 * |
| MHNW | MUNW | MHO | MUO | |
|---|---|---|---|---|
| Number of person-years | 1951.05 | 780.95 | 207.30 | 438.87 |
| Rate of CVD events (100 person-years) | 8.4 | 14.6 | 12.5 | 20.4 |
| Univariate risk ratio a (95% CI) | 1.0 | 1.86 (1.06–3.24) c | 1.55 (0.56–4.25) c | 2.79 (1.48–5.27) c |
| Multivariate risk ratio b (95% CI) | 1.0 | 2.01 (1.19–3.38) c | 1.24 (0.77–1.99) c | 1.38 (1.13–1.67) c |
| (A) | MHNW | MUNW | MHO | MUO |
| Number of person-years | 1471.39 | 574.39 | 132.35 | 299.16 |
| Rate of CVD events (100 person-years) | 1.9 | 3.3 | 3.7 | 8.3 |
| Univariate risk ratio (95% CI) | 1.0 | 1.17 (0.66–2.08) c | 1.45 (0.52–3.88) c | 2.4 (1.01–1.33) c |
| Multivariate risk ratio a (95% CI) | 1.0 | 1.11 (0.71–1.98) c | 1.39 (0.61–3.11) c | 2.2 (1.11–1.54) c |
| (B) | MHNW | MUNW | MHO | MUO |
| Number of person-years | 479.66 | 206.56 | 74.94 | 139.90 |
| Rate of CVD events (100 person-years) | 0.6 | 3.4 | 0.0 | 2.1 |
| Univariate risk ratio (95% CI) | 1.0 | 3.6 (1.09–12.03) c | 0.9 (0.88–0.96) c | 2.1 (0.51–8.21) c |
| Multivariate risk ratio b (95% CI) | 1.0 | 3.2 (1.23–9.98) | 0.0 | 1.8 (0.49–7.44) c |
| TOTAL Exp(B) | p Value * | Male Exp(B) | p Value * | Female Exp(B) | p Value * | |
|---|---|---|---|---|---|---|
| Male sex | 0.524 | 0.425 | - | - | ||
| Age | 1.054 | 0.119 | 1.102 | 0.019 | 0.566 | 0.997 |
| BMI | 1.216 | 0.022 | 1.251 | 0.038 | 1.169 | 0.667 |
| Systolic Blood Pressure | 0.973 | 0.122 | 0.958 | 0.141 | 0.777 | 0.563 |
| Diastolic Blood Pressure | 1.049 | 0.113 | 1.044 | 0.179 | 1.322 | 0.543 |
| Heart rate | 0.975 | 0.207 | 0.954 | 0.061 | 0.877 | 0.876 |
| Ejection fraction | 1.022 | 0.465 | 1.061 | 0.116 | 1.121 | 0.121 |
| E/A | 2.266 | 0.145 | 1.397 | 0.609 | 1.875 | 0.512 |
| Total Cholesterol | 1.015 | 0.369 | 1.020 | 0.269 | 0.987 | 0.867 |
| HDL Cholesterol | 0.973 | 0.332 | 0.905 | 0.023 | 0.676 | 0.771 |
| LDL Cholesterol | 0.983 | 0.313 | 0.982 | 0.330 | 0.232 | 0.911 |
| Triglycerides | 1.002 | 0.088 | 1.002 | 0.128 | 0.991 | 0.879 |
| Fasting glucose | 0.998 | 0.720 | 0.987 | 0.076 | 1.02 | 0.783 |
| Creatinine | 0.368 | 0.295 | 0.345 | 0.285 | 0.451 | 0.913 |
| C reactive protein | 1.000 | 0.978 | 1.007 | 0.614 | 0.999 | 0.985 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imbalzano, E.; Russo, G.T.; Giandalia, A.; Sciacqua, A.; Orlando, L.; Russo, V.; Perticone, M.; Cicero, A.F.G.; Versace, A.G.; Di Micco, P.; et al. Sex-Specific Impact of Different Obesity/Metabolic Phenotypes on Long-Term Cardiovascular Outcomes in Acute Coronary Syndrome Patients. Biomedicines 2022, 10, 424. https://doi.org/10.3390/biomedicines10020424
Imbalzano E, Russo GT, Giandalia A, Sciacqua A, Orlando L, Russo V, Perticone M, Cicero AFG, Versace AG, Di Micco P, et al. Sex-Specific Impact of Different Obesity/Metabolic Phenotypes on Long-Term Cardiovascular Outcomes in Acute Coronary Syndrome Patients. Biomedicines. 2022; 10(2):424. https://doi.org/10.3390/biomedicines10020424
Chicago/Turabian StyleImbalzano, Egidio, Giuseppina T. Russo, Annalisa Giandalia, Angela Sciacqua, Luana Orlando, Vincenzo Russo, Maria Perticone, Arrigo F. G. Cicero, Antonio Giovanni Versace, Pierpaolo Di Micco, and et al. 2022. "Sex-Specific Impact of Different Obesity/Metabolic Phenotypes on Long-Term Cardiovascular Outcomes in Acute Coronary Syndrome Patients" Biomedicines 10, no. 2: 424. https://doi.org/10.3390/biomedicines10020424
APA StyleImbalzano, E., Russo, G. T., Giandalia, A., Sciacqua, A., Orlando, L., Russo, V., Perticone, M., Cicero, A. F. G., Versace, A. G., Di Micco, P., Ciconte, V. A., Dattilo, G., Squadrito, G., & Vatrano, M. (2022). Sex-Specific Impact of Different Obesity/Metabolic Phenotypes on Long-Term Cardiovascular Outcomes in Acute Coronary Syndrome Patients. Biomedicines, 10(2), 424. https://doi.org/10.3390/biomedicines10020424












