The Concept of Indeterminable NASH Inducted by Preoperative Diet and Metabolic Surgery: Analyses of Histopathological and Clinical Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment
2.3. Data Collection
2.3.1. Weight-Loss Effects
2.3.2. Metabolic Parameters
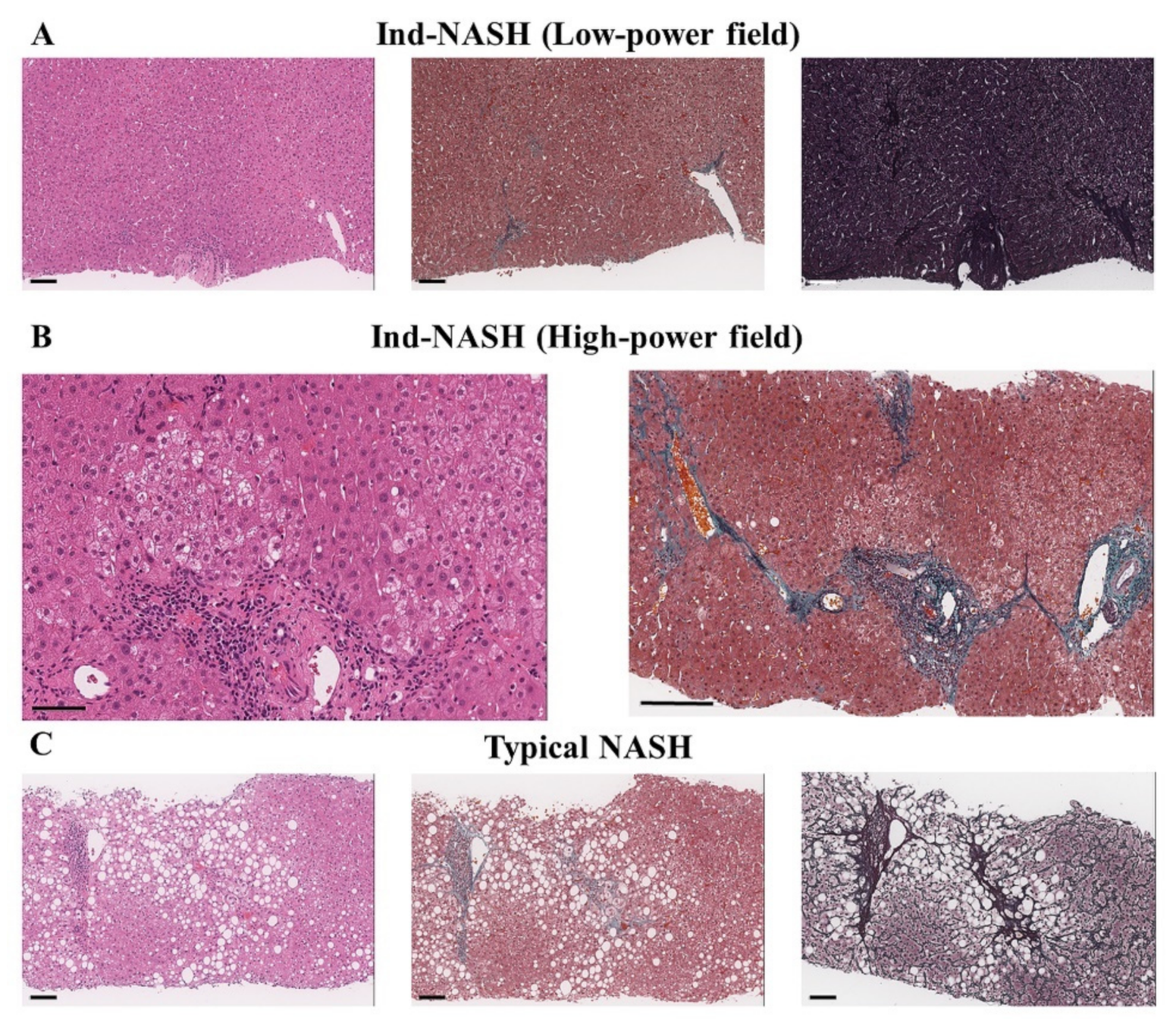
2.3.3. Histopathological Evaluation of Liver Biopsy
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Enrolled Patients
3.2. Weight-Loss and Metabolic Effects after LSG in Each Group
3.3. Histopathological Changes along with Protocol Liver Biopsy
3.3.1. Patient Flowchart along with Protocol Liver Biopsy
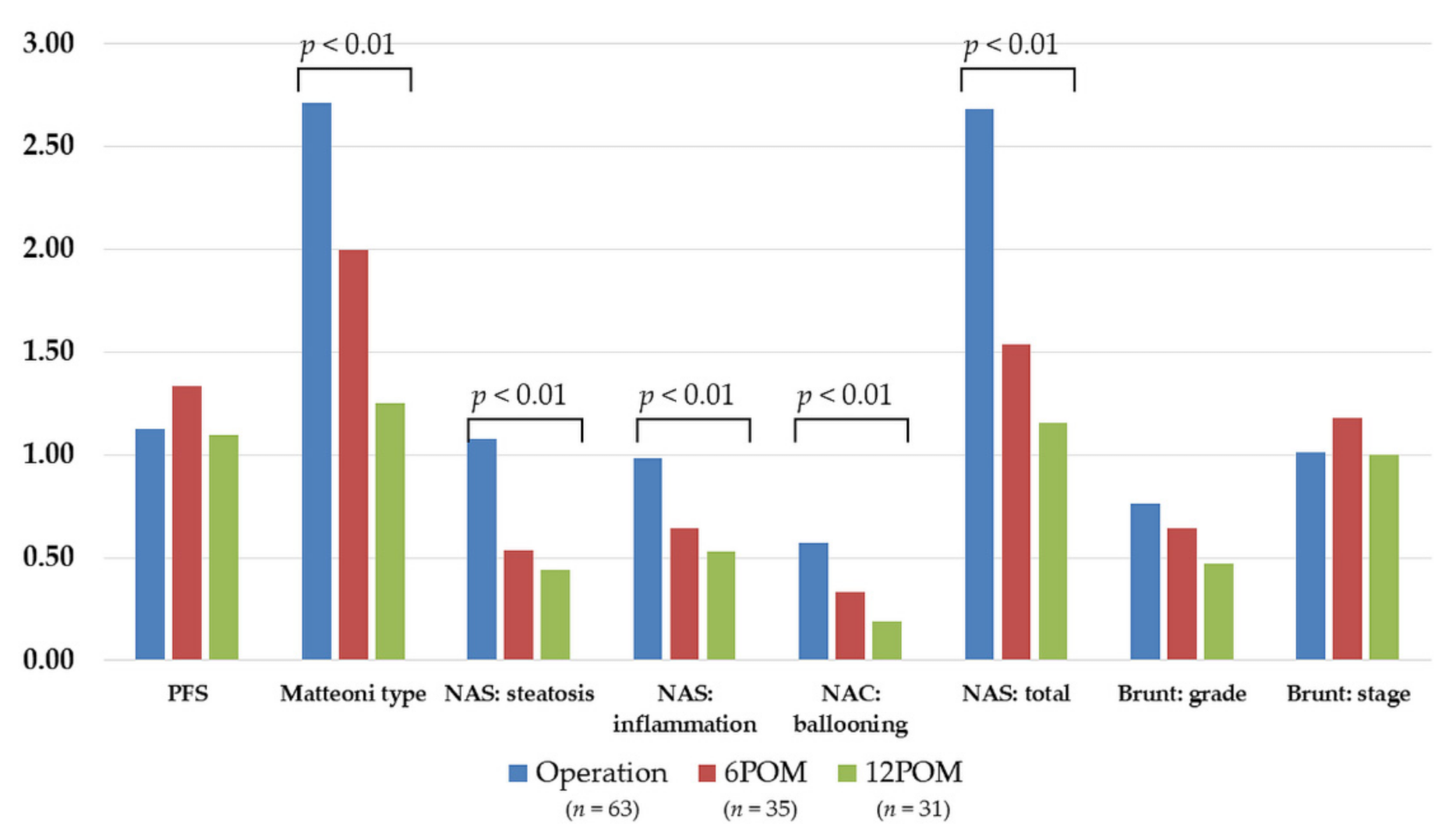
3.3.2. Histopathological Findings of the Protocol Liver Biopsy
3.4. Prognostic Factors for the Improvement of NASH and Clinical Features of Non-Improved Patients
3.4.1. Prognostic Factors for the Improvement of NASH
3.4.2. Relationships between L/S Ratio and the Distribution of Ind-NASH at Intraoperative Liver Biopsy
3.4.3. Clinical Features of Non-Improved Patients
3.4.4. Clinical Features of Non-Improve Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinowski, P.; Paluszkiewicz, R.; Ziarkiewicz-Wróblewska, B.; Wróblewski, T.; Remiszewski, P.; Grodzicki, M.; Krawczyk, M. Liver Function in Patients With Nonalcoholic Fatty Liver Disease Randomized to Roux-en-Y Gastric Bypass Versus Sleeve Gastrectomy: A Secondary Analysis of a Randomized Clinical Trial. Ann. Surg. 2017, 266, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Seki, Y.; Kakizaki, S.; Horiguchi, N.; Hashizume, H.; Tojima, H.; Yamazaki, Y.; Sato, K.; Kusano, M.; Yamada, M.; Kasama, K. Prevalence of nonalcoholic steatohepatitis in Japanese patients with morbid obesity undergoing bariatric surgery. J. Gastroenterol. 2016, 51, 281–289. [Google Scholar] [CrossRef]
- Nikai, H.; Ishida, K.; Umemura, A.; Baba, S.; Nitta, H.; Sugai, T.; Sasaki, A. Effects of Laparoscopic Sleeve Gastrectomy on Non-Alcoholic Steatohepatitis and Liver Fibrosis in Japanese Patients with Severe Obesity. Obes. Surg. 2020, 30, 2579–2587. [Google Scholar] [CrossRef]
- Umemura, A.; Sasaki, A.; Nitta, H.; Nikai, H.; Baba, S.; Takahara, T.; Hasegawa, Y.; Katagiri, H.; Kanno, S.; Ishigaki, Y. Prognostic factors and a new preliminary scoring system for remission of type 2 diabetes mellitus after laparoscopic sleeve gastrectomy. Surg. Today 2020, 50, 1056–1064. [Google Scholar] [CrossRef]
- Haruta, H.; Kasama, K.; Ohta, M.; Sasaki, A.; Yamamoto, H.; Miyazaki, Y.; Oshiro, T.; Naitoh, T.; Hosoya, Y.; Togawa, T.; et al. Long-Term Outcomes of Bariatric and Metabolic Surgery in Japan: Results of a Multi-Institutional Survey. Obes. Surg. 2016, 27, 754–762. [Google Scholar] [CrossRef]
- Nishijima, T.; Ohba, K.; Baba, S.; Kizawa, T.; Hosokawa, K.; Endo, F.; Yamashiro, Y.; Suwabe, A.; Sasaki, A.; Takahashi, K.; et al. Decrease of Plasma Soluble (Pro)renin Receptor by Bariatric Surgery in Patients with Obstructive Sleep Apnea and Morbid Obesity. Metab. Syndr. Relat. Disord. 2018, 16, 174–182. [Google Scholar] [CrossRef]
- Nikai, H.; Sasaki, A.; Umemura, A.; Takahashi, N.; Nitta, H.; Akasaka, R.; Kakisaka, K.; Kuroda, H.; Ishida, K.; Takikawa, Y. Predictive scoring system for advanced liver fibrosis in Japanese patients with severe obesity. Surg. Today 2021, 51, 1513–1520. [Google Scholar] [CrossRef]
- Bedossa, P.; FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014, 60, 565–575. [Google Scholar] [CrossRef]
- Sasaki, A.; Yokote, K.; Naitoh, T.; Fujikura, J.; Hayashi, K.; Hirota, Y.; Inagaki, N.; Ishigaki, Y.; Kasama, K.; Kikkawa, E.; et al. Metabolic surgery in treatment of obese Japanese patients with type 2 diabetes: A joint consensus statement from the Japanese Society for Treatment of Obesity, the Japan Diabetes Society, and the Japan Society for the Study of Obesity. Diabetol. Int. 2021, 13, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J.; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumida, Y.; Yoneda, M.; Hyogo, H.; Yamaguchi, K.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Imai, S.; Kanemasa, K.; et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J. Gastroenterol. 2011, 46, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef]
- Holderbaum, M.; Casagrande, D.S.; Sussenbach, S.; Buss, C. Effects of very low calorie diets on liver size and weight loss in the preoperative period of bariatric surgery: A systematic review. Surg. Obes. Relat. Dis. 2018, 14, 237–244. [Google Scholar] [CrossRef]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef]
- Umemura, A.; Sasaki, A.; Nitta, H.; Baba, S.; Ando, T.; Kajiwara, T.; Ishigaki, Y. Pancreas volume reduction and metabolic effects in Japanese patients with severe obesity following laparoscopic sleeve gastrectomy. Endocr. J. 2017, 64, 487–498. [Google Scholar] [CrossRef] [Green Version]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gils Contreras, A.; Bonada Sanjaume, A.; Montero Jaime, M.; Rabassa Soler, A.; Sabench Pereferrer, F.; Molina López, A.; Becerra Tomás, N.; Del Castillo Déjardin, D.; Salas-Salvadó, J. Effects of Two Preoperatory Weight Loss Diets on Hepatic Volume, Metabolic Parameters, and Surgical Complications in Morbid Obese Bariatric Surgery Candidates: A Randomized Clinical Trial. Obes. Surg. 2018, 28, 3756–3768. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Lok, A.S. Use of Liver Imaging and Biopsy in Clinical Practice. N. Engl. J. Med. 2017, 377, 756–768. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Eslam, M.; Sarin, S.K.; Wong, V.W.; Fan, J.G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Lassailly, G.; Caiazzo, R.; Buob, D.; Pigeyre, M.; Verkindt, H.; Labreuche, J.; Raverdy, V.; Leteurtre, E.; Dharancy, S.; Louvet, A.; et al. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology 2015, 149, 379–388, quiz e315–e376. [Google Scholar] [CrossRef] [Green Version]
- Lassailly, G.; Caiazzo, R.; Ntandja-Wandji, L.C.; Gnemmi, V.; Baud, G.; Verkindt, H.; Ningarhari, M.; Louvet, A.; Leteurtre, E.; Raverdy, V.; et al. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology 2020, 159, 1290–1301.e5. [Google Scholar] [CrossRef]
- Sumida, Y.; Nakajima, A.; Itoh, Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 475–485. [Google Scholar] [CrossRef]
- Okanoue, T.; Ebise, H.; Kai, T.; Mizuno, M.; Shima, T.; Ichihara, J.; Aoki, M. A simple scoring system using type IV collagen 7S and aspartate aminotransferase for diagnosing nonalcoholic steatohepatitis and related fibrosis. J. Gastroenterol. 2017, 53, 129–139. [Google Scholar] [CrossRef]
- Dietrich, C.G.; Rau, M.; Geier, A. Screening for nonalcoholic fatty liver disease-when, who and how? World J. Gastroenterol. 2021, 27, 5803–5821. [Google Scholar] [CrossRef] [PubMed]
- Green, R.M.; Flamm, S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology 2002, 123, 1367–1384. [Google Scholar] [CrossRef] [Green Version]
- Sinaiko, A.R.; Steinberger, J.; Moran, A.; Prineas, R.J.; Vessby, B.; Basu, S.; Tracy, R.; Jacobs, D.R., Jr. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation 2005, 111, 1985–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohn, A.; Catino, M.; Capanna, R.; Giannini, C.; Marcovecchio, M.; Chiarelli, F. Increased oxidative stress in prepubertal severely obese children: Effect of a dietary restriction-weight loss program. J. Clin. Endocrinol. Metab. 2005, 90, 2653–2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabré, N.; Luciano-Mateo, F.; Fernández-Arroyo, S.; Baiges-Gayà, G.; Hernández-Aguilera, A.; Fibla, M.; Fernández-Julià, R.; París, M.; Sabench, F.; Castillo, D.D.; et al. Laparoscopic sleeve gastrectomy reverses non-alcoholic fatty liver disease modulating oxidative stress and inflammation. Metabolism 2019, 99, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.H.; Adam, S.; Liu, Y.; Azmi, S.; Dhage, S.; Syed, A.A.; Ammori, B.J.; Donn, R.; Heald, A.; Gibson, M.J.; et al. Effect of bariatric surgery on plasma levels of oxidised phospholipids, biomarkers of oxidised LDL and lipoprotein(a). J. Clin. Lipidol. 2020, 15, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Jensen, D.; Hart, J.; Mohanty, S.R. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 2013, 33, 1398–1405. [Google Scholar] [CrossRef]
- Cherla, D.V.; Rodriguez, N.A.; Vangoitsenhoven, R.; Singh, T.; Mehta, N.; McCullough, A.J.; Brethauer, S.A.; Schauer, P.R.; Aminian, A. Impact of sleeve gastrectomy and Roux-en-Y gastric bypass on biopsy-proven non-alcoholic fatty liver disease. Surg. Endosc. 2019, 34, 2266–2272. [Google Scholar] [CrossRef]
- Pedersen, J.S.; Rygg, M.O.; Serizawa, R.R.; Kristiansen, V.B.; Albrechtsen, N.J.W.; Gluud, L.L.; Madsbad, S.; Bendtsen, F. Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Non-Alcoholic Fatty Liver Disease: A 12-Month Follow-Up Study with Paired Liver Biopsies. J. Clin. Med. 2021, 10, 3783. [Google Scholar] [CrossRef]
- De Brito E Silva, M.B.; Tustumi, F.; de Miranda Neto, A.A.; Dantas, A.C.B.; Santo, M.A.; Cecconello, I. Gastric Bypass Compared with Sleeve Gastrectomy for Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Obes. Surg. 2021, 31, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Imajo, K.; Sharpton, S.; Jung, J.; Kawamura, N.; Yoneda, M.; Valasek, M.A.; Behling, C.; Sirlin, C.B.; Nakajima, A.; et al. Magnetic resonance elastography plus Fibrosis-4 versus FibroScan-aspartate aminotransferase in detection of candidates for pharmacological treatment of NASH-related fibrosis. Hepatology 2021, 75, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Fujiwara, Y.; Abe, T.; Nagasawa, T.; Oguri, T.; Noguchi, S.; Kamiyama, N.; Takikawa, Y. Two-dimensional shear wave elastography and ultrasound-guided attenuation parameter for progressive non-alcoholic steatohepatitis. PLoS ONE 2021, 16, e0249493. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, L.; Aggarwal, S.; Shalimar Yadav, R.; Dattagupta, S.; Garg, H.; Agarwal, S. Bariatric Surgery in Nonalcoholic Fatty Liver Disease (NAFLD): Impact Assessment Using Paired Liver Biopsy and Fibroscan. Obes. Surg. 2020, 31, 617–626. [Google Scholar] [CrossRef]









| All Patients (n = 63) | NASH (n = 35) | NAFL (n = 18) | Ind-NASH (n = 10) | p-Value (Control: NASH) | |
|---|---|---|---|---|---|
| Age, years | 43.3 ± 12.3 | 41.8 ± 13.9 | 44.4 ± 8.2 | 46.4 ± 13.0 | NAFL: 0.5814 Ind-NASH: 0.6729 |
| Male, n (%) | 32 (50.8) | 15 (42.9) | 9 (50.0) | 8 (80.0) | NAFL: 0.8598 Ind-NASH: 0.0807 |
| Body weight, kg | 119.1 ± 20.5 | 114.7 ± 18.8 | 119.0 ± 18.9 | 134.9 ± 22.9 | NAFL: 0.6352 * Ind-NASH: 0.0245 |
| BMI, kg/m2 | 43.6 ± 6.3 | 42.6 ± 5.2 | 43.1 ± 5.6 | 48.1 ± 9.1 | NAFL: 0.9670 Ind-NASH: 0.1237 |
| Comorbidities, n HT, n (%) T2D, n (%) HL, n (%) HU, n (%) OSA, n (%) | 5.7 ± 2.2 53 (84.1) 40 (63.5) 45 (71.4) 30 (47.6) 60 (95.2) | 5.7 ± 2.1 28 (80.0) 28 (80.0) 25 (71.4) 12 (34.3) 33 (94.3) | 5.4 ± 2.4 15 (83.3) 6 (33.3) 13 (72.2) 10 (55.6) 17 (94.4) | 6.2 ± 2.3 10 (100.0) 6 (60.0) 7 (70.0) 8 (80.0) 10 (100.0) | |
| FBG, mg/dL | 123.9 ± 42.1 | 135.8 ± 41.9 | 112.1 ± 41.2 | 101.2 ± 30.5 | * NAFL: 0.0063 * Ind-NASH: 0.0185 |
| IRI, μU/mL | 23.1 ± 18.8 | 25.6 ± 18.2 | 19.0 ± 9.4 | 21.1 ± 32.2 | NAFL: 0.4055 * Ind-NASH: 0.0369 |
| HbA1c, % | 7.2 ± 1.7 | 7.7 ± 1.5 | 6.6 ± 1.9 | 6.6 ± 1.5 | * NAFL: 0.0031 Ind-NASH: 0.0619 |
| HOMA-IR, no unit | 7.4 ± 7.6 | 8.7 ± 7.4 | 4.9 ± 2.1 | 7.1 ± 13.5 | * NAFL: 0.0496 * Ind-NASH: 0.0188 |
| HOMA-β, no unit | 172.7 ± 121.5 | 162.5 ± 119.4 | 197.7 ± 123.1 | 162.5 ± 135.6 | NAFL: 0.3235 Ind-NASH: 0.9762 |
| C-peptide, ng/mL | 2.9 ± 1.3 | 3.3 ± 1.4 | 2.7 ± 0.9 | 2.2 ± 1.0 | NAFL: 0.3366 Ind-NASH: 0.0952 |
| TC, mg/dL | 187.6 ± 36.5 | 185.4 ± 36.9 | 191.4 ± 40.4 | 188.2 ± 30.1 | NAFL: 0.8699 Ind-NASH: 0.9980 |
| TG, mg/dL | 138.9 ± 81.2 | 147.3 ± 94.3 | 128.2 ± 68.2 | 128.8 ± 49.1 | NAFL: 0.6288 Ind-NASH: 0.9921 |
| LDL-C, mg/dL | 120.0 ± 30.9 | 114.9 ± 30.0 | 127.2 ± 34.8 | 124.7 ± 25.1 | NAFL: 0.4969 Ind-NASH: 0.5634 |
| HDL-C, mg/dL | 43.5 ± 10.3 | 45.2 ± 11.9 | 42.9 ± 7.6 | 38.7 ± 6.7 | NAFL: 0.8597 Ind-NASH: 0.2546 |
| AST, IU/L | 47.7 ± 40.8 | 63.2 ± 47.7 | 26.8 ± 16.3 | 31.0 ± 16.7 | * NAFL: 0.0001 * Ind-NASH: 0.0202 |
| ALT, IU/L | 67.9 ± 62.3 | 90.4 ± 70.7 | 39.9 ± 37.7 | 39.4 ± 27.2 | * NAFL: 0.0001 * Ind-NASH: 0.0085 |
| γ-GTP, IU/L | 60.0 ± 54.0 | 75.0 ± 65.8 | 42.3 ± 21.2 | 39.5 ± 30.4 | NAFL: 0.2592 Ind-NASH: 0.1429 |
| Albumin, g/dL | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.2 ± 0.5 | NAFL: 0.7084 Ind-NASH: 0.7871 |
| T-Bil, mg/dL | 0.8 ± 0.4 | 0.8 ± 0.3 | 0.7 ± 0.4 | 1.0 ± 0.6 | NAFL: 0.3930 Ind-NASH: 0.7521 |
| Ferritin, ng/mL | 154.8 ± 129.8 | 171.2 ± 145.7 | 127.6 ± 84.2 | 140.1 ± 133.6 | NAFL: 0.7902 Ind-NASH: 0.7825 |
| Type-4 collagen, ng/mL | 4.8 ± 1.2 | 5.1 ± 1.3 | 4.5 ± 0.8 | 4.4 ± 1.2 | NAFL: 0.1940 Ind-NASH: 0.2673 |
| Hyaluronic acid, ng/mL | 31.7 ± 25.7 | 35.3 ± 30.8 | 27.1 ± 13.4 | 25.9 ± 19.5 | NAFL: 0.9997 Ind-NASH: 0.4679 |
| MDA-LDL, U/L | 140.4 ± 56.9 | 144.3 ± 54.4 | 123.9 ± 57.1 | 164.3 ± 66.9 | NAFL: 0.4414 Ind-NASH: 0.8297 |
| Transferrin, mg/dL | 270.0 ± 43.9 | 273.1 ± 42.9 | 264.3 ± 43.0 | 268.2 ± 53.2 | NAFL: 0.6456 Ind-NASH: 0.9510 |
| PAI-1, ng/mL | 53.7 ± 38.0 | 60.5 ± 36.5 | 39.8 ± 16.0 | 51.9 ± 62.4 | NAFL: 0.0982 Ind-NASH: 0.1388 |
| FIB-4 index, no unit | 0.9 ± 0.7 | 1.0 ± 0.9 | 0.7 ± 0.4 | 1.0 ± 0.4 | NAFL: 0.7834 Ind-NASH: 0.7107 |
| NAFIC score, no unit | 1.5 ± 1.1 | 1.8 ± 1.1 | 1.2 ± 0.8 | 0.9 ± 1.3 | NAFL: 0.1345 * Ind-NASH: 0.0369 |
| NAFLD fibrosis score, no unit | 0.1 ± 1.7 | 0.1 ± 1.9 | 0.8 ± 1.1 | 0.2 ± 1.4 | NAFL: 0.0595 Ind-NASH: 0.9989 |
| SFA, cm2 | 524.7 ± 133.5 | 515.9 ± 142.6 | 529.0 ± 132.6 | 554.2 ± 98.9 | NAFL: 0.9305 Ind-NASH: 0.5172 |
| VFA, cm2 | 272.8 ± 81.0 | 269.6 ± 81.4 | 267.2 ± 77.5 | 298.5 ± 92.3 | NAFL: 0.9140 Ind-NASH: 0.5796 |
| Waist, cm | 122.0 ± 9.5 | 121.0 ± 10.4 | 121.8 ± 9.2 | 126.6 ± 4.4 | NAFL: 0.9910 Ind-NASH: 0.1126 |
| Liver volume, mL | 2249.0 ± 542.7 | 2250.9 ± 578.6 | 2225.7 ± 443.1 | 2286.1 ± 625.2 | NAFL: 0.9949 Ind-NASH: 1.0000 |
| L/S ratio, no unit | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 | 1.0 ± 0.2 | NAFL: 0.2985 * Ind-NASH: 0.0037 |
| Initial | 6 Months after LSG | 12 Months after LSG | p-Value Initial vs. 12 Months | |
|---|---|---|---|---|
| All Patients (n = 63) | ||||
| Body weight, kg | 119.1 ± 20.5 | 89.4 ± 14.1 | 87.6 ± 15.7 | <0.0001 |
| BMI, kg/m2 | 43.6 ± 6.3 | 32.7 ± 4.0 | 31.9 ± 4.6 | <0.0001 |
| %TWL, % | - | 24.5 ± 6.5 | 26.7 ± 7.5 | - |
| FBG, mg/dL | 123.9 ± 42.1 | 91.0 ± 13.6 | 92.5 ± 16.5 | <0.0001 |
| IRI, μU/mL | 23.1 ± 18.8 | 8.1 ± 4.8 | 8.6 ± 6.1 | <0.0001 |
| HbA1c, % | 7.2 ± 1.7 | 5.7 ± 0.7 | 5.6 ± 0.7 | <0.0001 |
| HOMA-IR, no unit | 7.4 ± 7.6 | 1.8 ± 1.2 | 2.0 ± 1.9 | <0.0001 |
| HOMA-β, no unit | 172.7 ± 121.5 | 113.0 ± 89.3 | 115.9 ± 80.8 | 0.0053 |
| AST, IU/L | 47.7 ± 40.8 | 19.7 ± 13.3 | 18.5 ± 9.6 | <0.0001 |
| ALT, IU/L | 67.9 ± 62.3 | 22.5 ± 32.5 | 17.9 ± 8.8 | <0.0001 |
| γ-GTP, IU/L | 60.0 ± 54.0 | 31.1 ± 61.1 | 34.3 ± 69.3 | 0.0262 |
| LDL-C, mg/dL | 120.0 ± 30.9 | 111.4 ± 28.2 | 108.7 ± 30.1 | 0.0479 |
| HDL-C, mg/dL | 43.5 ± 10.3 | 52.5 ± 10.9 | 58.2 ± 13.1 | <0.0001 |
| TG, mg/dL | 138.9 ± 81.2 | 94.8 ± 52.5 | 90.3 ± 56.0 | 0.0002 |
| Ferritin, ng/mL | 154.8 ± 129.8 | 93.2 ± 70.6 | 84.4 ± 71.7 | 0.0006 |
| Type-4 collagen 7S, ng/mL | 4.8 ± 1.2 | 4.7 ± 0.9 | 6.1 ± 11.9 | 0.4225 |
| Hyaluronic acid, ng/mL | 31.7 ± 25.7 | 34.9 ± 28.4 | 37.3 ± 30.7 | 0.2973 |
| MDA-LDL, U/L | 140.4 ± 56.9 | 111.1 ± 40.5 | 106.9 ± 35.6 | 0.0004 |
| Transferrin, mg/dL | 270.0 ± 43.9 | 224.7 ± 46.7 | 257.6 ± 50.5 | 0.1621 |
| PAI-1, ng/mL | 53.7 ± 38.0 | 21.6 ± 15.1 | 20.0 ± 13.3 | <0.0001 |
| SFA, cm2 | 524.7 ± 133.5 | 339.2 ± 109.9 | 340.8 ± 133.4 | <0.0001 |
| VFA, cm2 | 272.8 ± 81.0 | 161.4 ± 70.0 | 145.6 ± 67.2 | <0.0001 |
| Waist, cm | 122.0 ± 9.5 | 104.8 ± 11.7 | 103.0 ± 13.0 | <0.0001 |
| Liver volume, mL | 2249.0 ± 542.7 | 1674.2 ± 332.0 | 1654.9 ± 316.2 | <0.0001 |
| L/S ratio, no unit | 0.8 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.2 | <0.0001 |
| NASH (n = 35) | ||||
| Body weight, kg | 114.7 ± 18.8 | 87.1 ± 13.5 | 86.2 ± 15.3 | <0.0001 |
| BMI, kg/m2 | 42.6 ± 5.2 | 32.2 ± 3.4 | 31.7 ± 4.0 | <0.0001 |
| %TWL, % | - | 23.7 ± 5.9 | 25.7 ± 6.7 | - |
| FBG, mg/dL | 135.8 ± 41.9 | 91.7 ± 17.9 | 94.7 ± 17.9 | <0.0001 |
| IRI, μU/mL | 25.6 ± 18.2 | 10.2 ± 5.0 | 10.5 ± 6.7 | <0.0001 |
| HbA1c, % | 7.7 ± 1.5 | 5.7 ± 0.6 | 5.8 ± 0.7 | <0.0001 |
| HOMA-IR, no unit | 8.7 ± 7.4 | 2.3 ± 1.3 | 2.7 ± 2.3 | 0.0001 |
| HOMA-β, no unit | 162.5 ± 119.4 | 138.9 ± 82.1 | 125.1 ± 61.9 | 0.1342 |
| AST, IU/L | 63.2 ± 47.7 | 21.7 ± 16.7 | 19.6 ± 12.0 | <0.0001 |
| ALT, IU/L | 90.4 ± 70.7 | 27.8 ± 42.6 | 18.7 ± 10.1 | <0.0001 |
| γ-GTP, IU/L | 75.0 ± 65.8 | 38.5 ± 80.4 | 45.5 ± 92.3 | 0.1456 |
| LDL-C, mg/dL | 114.9 ± 30.0 | 109.8 ± 27.7 | 102.4 ± 30.3 | 0.1038 |
| HDL-C, mg/dL | 45.2 ± 11.9 | 53.1 ± 12.2 | 59.3 ± 14.7 | <0.0001 |
| TG, mg/dL | 147.3 ± 94.3 | 104.5 ± 59.8 | 100.4 ± 68.3 | 0.0236 |
| Ferritin, ng/mL | 171.2 ± 145.7 | 86.5 ± 71.6 | 77.5 ± 68.9 | 0.0017 |
| Type-4 collagen, ng/mL | 5.1 ± 1.3 | 4.7 ± 1.0 | 7.4 ± 16.3 | 0.4578 |
| Hyaluronic acid, ng/mL | 35.3 ± 30.8 | 31.6 ± 28.9 | 33.3 ± 27.1 | 0.7775 |
| MDA-LDL, U/L | 144.3 ± 54.4 | 110.6 ± 31.9 | 103.5 ± 26.3 | 0.0005 |
| Transferrin, mg/dL | 273.1 ± 42.9 | 250.9 ± 43.3 | 264.2 ± 47.9 | 0.4357 |
| PAI-1, ng/mL | 60.5 ± 36.5 | 24.5 ± 18.2 | 23.5 ± 16.4 | <0.0001 |
| SFA, cm2 | 515.9 ± 142.6 | 337.3 ± 103.8 | 347.9 ± 117.5 | <0.0001 |
| VFA, cm2 | 269.6 ± 81.4 | 156.8 ± 63.3 | 152.3 ± 71.7 | <0.0001 |
| Waist, cm | 121.0 ± 10.4 | 103.8 ± 11.7 | 103.2 ± 12.6 | <0.0001 |
| Liver volume, mL | 2250.9 ± 578.6 | 1637.9 ± 328.9 | 1618.5 ± 347.8 | <0.0001 |
| L/S ratio, no unit | 0.8 ± 0.2 | 1.3 ± 0.3 | 1.3 ± 0.3 | <0.0001 |
| NAFL (n = 18) | ||||
| Body weight, kg | 119.0 ± 18.9 | 89.8 ± 13.7 | 88.0 ± 15.6 | <0.0001 |
| BMI, kg/m2 | 43.1 ± 5.6 | 32.5 ± 4.2 | 31.9 ± 5.0 | <0.0001 |
| %TWL, % | - | 24.2 ± 6.4 | 25.9 ± 7.3 | - |
| FBG, mg/dL | 112.1 ± 41.2 | 91.0 ± 17.2 | 87.3 ± 12.6 | 0.0283 |
| IRI, μU/mL | 19.0 ± 9.4 | 5.5 ± 2.4 | 6.9 ± 4.8 | 0.0001 |
| HbA1c, % | 6.6 ± 1.9 | 5.6 ± 0.7 | 5.5 ± 0.7 | 0.0342 |
| HOMA-IR, no unit | 4.9 ± 2.1 | 1.2 ± 0.7 | 1.4 ± 0.9 | <0.0001 |
| HOMA-β, no unit | 197.7 ± 123.1 | 62.7 ± 42.8 | 121.6 ± 106.4 | 0.0751 |
| AST, IU/L | 26.8 ± 16.3 | 16.8 ± 7.4 | 15.9 ± 4.3 | 0.0137 |
| ALT, IU/L | 39.9 ± 37.7 | 14.9 ± 7.6 | 15.1 ± 4.9 | 0.0124 |
| γ-GTP, IU/L | 42.3 ± 21.2 | 21.3 ± 17.5 | 18.1 ± 8.5 | 0.0002 |
| LDL-C, mg/dL | 127.2 ± 34.8 | 113.7 ± 27.6 | 115.4 ± 21.6 | 0.2349 |
| HDL-C, mg/dL | 42.9 ± 7.6 | 52.7 ± 8.0 | 56.9 ± 9.6 | <0.0001 |
| TG, mg/dL | 128.2 ± 68.2 | 82.5 ± 39.9 | 76.6 ± 27.0 | 0.0071 |
| Ferritin, ng/mL | 127.6 ± 84.2 | 91.4 ± 67.1 | 88.9 ± 84.8 | 0.2121 |
| Type-4 collagen, ng/mL | 4.5 ± 0.8 | 4.7 ± 0.8 | 4.5 ± 0.8 | 0.9823 |
| Hyaluronic acid, ng/mL | 27.1 ± 13.4 | 39.4 ± 27.4 | 39.1 ± 23.1 | 0.0869 |
| MDA-LDL, U/L | 123.9 ± 57.1 | 102.2 ± 41.7 | 99.0 ± 26.5 | 0.1277 |
| Transferrin, mg/dL | 264.3 ± 43.0 | 242.7 ± 51.6 | 255.5 ± 60.1 | 0.6394 |
| PAI-1, ng/mL | 39.8 ± 16.0 | 20.4 ± 10.8 | 16.1 ± 6.1 | <0.0001 |
| SFA, cm2 | 529.0 ± 132.6 | 340.2 ± 130.3 | 339.7 ± 160.6 | 0.0009 |
| VFA, cm2 | 267.2 ± 77.5 | 170.1 ± 85.5 | 143.9 ± 64.6 | <0.0001 |
| Waist, cm | 121.8 ± 9.2 | 104.8 ± 12.4 | 103.2 ± 14.6 | 0.0002 |
| Liver volume, mL | 2225.7 ± 443.1 | 1645.4 ± 307.8 | 1633.5 ± 236.2 | <0.0001 |
| L/S ratio, no unit | 0.9 ± 0.2 | 1.3 ± 0.1 | 1.3 ± 0.2 | <0.0001 |
| Ind-NASH (n = 10) | ||||
| Body weight, kg | 134.9 ± 22.9 | 96.8 ± 15.4 | 92.2 ± 18.1 | 0.0003 |
| BMI, kg/m2 | 48.1 ± 9.1 | 34.4 ± 5.5 | 32.9 ± 6.1 | 0.0006 |
| %TWL, % | - | 27.8 ± 7.8 | 32.0 ± 9.4 | - |
| FBG, mg/dL | 101.2 ± 30.5 | 88.8 ± 14.9 | 94.9 ± 17.7 | 0.5989 |
| IRI, μU/mL | 21.1 ± 32.2 | 4.8 ± 2.9 | 5.0 ± 3.5 | 0.2044 |
| HbA1c, % | 6.6 ± 1.5 | 5.5 ± 0.7 | 5.5 ± 0.7 | 0.0638 |
| HOMA-IR, no unit | 7.1 ± 13.5 | 1.1 ± 0.6 | 1.2 ± 0.8 | 0.2527 |
| HOMA-β, no unit | 162.5 ± 135.6 | 107.7 ± 133.7 | 79.2 ± 81.6 | 0.1590 |
| AST, IU/L | 31.0 ± 16.7 | 17.6 ± 4.6 | 19.6 ± 6.8 | 0.0692 |
| ALT, IU/L | 39.4 ± 27.2 | 17.3 ± 5.7 | 20.7 ± 9.5 | 0.0655 |
| γ-GTP, IU/L | 39.5 ± 30.4 | 22.4 ± 13.8 | 26.0 ± 19.3 | 0.2607 |
| LDL-C, mg/dL | 124.7 ± 25.1 | 113.2 ± 33.2 | 116.6 ± 40.9 | 0.6145 |
| HDL-C, mg/dL | 38.7 ± 6.7 | 50.3 ± 11.3 | 56.7 ± 13.9 | 0.0047 |
| TG, mg/dL | 128.8 ± 49.1 | 83.0 ± 40.6 | 82.8 ± 48.2 | 0.0551 |
| Ferritin, ng/mL | 140.1 ± 133.6 | 119.2 ± 74.5 | 100.8 ± 60.1 | 0.4377 |
| Type-4 collagen, ng/mL | 4.4 ± 1.2 | 5.0 ± 0.7 | 5.1 ± 0.9 | 0.1827 |
| Hyaluronic acid, ng/mL | 25.9 ± 19.5 | 38.7 ± 29.3 | 47.4 ± 50.1 | 0.2567 |
| MDA-LDL, U/L | 164.3 ± 66.9 | 128.9 ± 57.6 | 131.6 ± 61.7 | 0.3605 |
| Transferrin, mg/dL | 268.2 ± 53.2 | 227.7 ± 48.5 | 239.0 ± 39.7 | 0.1034 |
| PAI-1, ng/mL | 51.9 ± 62.4 | 14.0 ± 4.5 | 15.1 ± 5.7 | 0.1161 |
| SFA, cm2 | 554.2 ± 98.9 | 343.8 ± 100.5 | 320.5 ± 141.5 | 0.0014 |
| VFA, cm2 | 298.5 ± 92.3 | 160.6 ± 66.3 | 127.6 ± 60.2 | 0.0008 |
| Waist, cm | 126.6 ± 4.4 | 108.1 ± 11.3 | 101.8 ± 13.1 | 0.0007 |
| Liver volume, mL | 2286.1 ± 625.2 | 1846.0 ± 363.0 | 1805.9 ± 325.7 | 0.0872 |
| L/S ratio, no unit | 1.0 ± 0.2 | 1.2 ± 0.1 | 1.3 ± 0.3 | 0.0109 |
| Parameters | Improvement (n = 23) | Non-Improvement (n = 9) | Odds Ratio (95% Confidence Interval) | p-Value | |
|---|---|---|---|---|---|
| Preoperative Parameters | |||||
| Male, n (%) | 12 (52.2%) | 6 (66.7%) | 1.833 (0.382–10.404) | 0.4541 | |
| BMI, kg/m2 | 44.1 ± 5.5 | 42.0 ± 5.1 | 1.083 (0.933–1.286) | 0.3014 | |
| FBG, mg/dL | 127.5 ± 45.4 | 147.8 ± 33.1 | 0.989 (0.970–1.007) | 0.2330 | |
| IRI, μU/mL | 26.8 ± 20.7 | 37.1 ± 29.2 | 0.982 (0.945–1.017) | 0.3037 | |
| HbA1c, % | 7.1 ± 1.5 | 8.4 ± 1.3 | 0.532 (0.279–0.909) | 0.0206 * | |
| HOMA-IR, no unit | 8.8 ± 9.1 | 13.9 ± 10.9 | 0.950 (0.865–1.032) | 0.2215 | |
| AST, IU/L | 52.3 ± 38.4 | 88.1 ± 64.1 | 0.986 (0.968–1.001) | 0.0695 | |
| ALT, IU/L | 81.4 ± 60.6 | 115.2 ± 96.5 | 0.994 (0.983–1.004) | 0.2471 | |
| γ-GTP, IU/L | 54.7 ± 47.5 | 126.6 ± 88.1 | 0.984 (0.968–0.996) | 0.0082 * | |
| Type-4 collagen 7S, ng/mL | 4.6 ± 1.2 | 5.8 ± 1.5 | 0.508 (0.235–0.968) | 0.0263 * | |
| Hyaluronic acid, ng/mL | 27.9 ± 22.5 | 45.4 ± 45.0 | 0.982 (0.955–1.008) | 0.1708 | |
| MDA-LDL, U/L | 133.6 ± 47.4 | 166.1 ± 79.3 | 0.990 (0.973–1.005) | 0.1837 | |
| SFA, cm2 | 563.3 ± 158.7 | 495.2 ± 86.0 | 1.004 (0.998–1.010) | 0.2133 | |
| VFA, cm2 | 261.5 ± 76.3 | 292.2 ± 78.2 | 0.995 (0.983–1.005) | 0.3012 | |
| Liver volume, mL | 2167.8 ± 506.4 | 2437.5 ± 712.2 | 0.999 (0.998–1.001) | 0.2349 | |
| L/S ratio, no unit | 0.7 ± 0.2 | 0.9 ± 0.1 | 0.061 (0.0006–2.434) | 0.1464 | |
| Multivariate Analysis | |||||
| Parameters | Odds Ratio | 95% Confidence Interval | p-Value | ||
| HbA1c, % | 0.435 | 0.130–1.040 | 0.0620 | ||
| AST, IU/L | 0.994 | 0.964–1.023 | 0.6756 | ||
| γ-GTP, IU/L | 0.979 | 0.939–1.001 | 0.0645 | ||
| Type-4 collagen 7S, ng/mL | 1.411 | 1.411–6.909 | 0.6081 | ||
| L/S ratio, no unit | 0.002 | <0.001–0.592 | 0.0310 | ||
| Parameters | Improvement (n = 23) | Non-Improvement (n = 9) | Odds Ratio (95% Confidence Interval) | p-Value | |
|---|---|---|---|---|---|
| Postoperative Parameters (12 Months after LSG) | |||||
| Body weight, kg | 86.9 ± 16.6 | 92.7 ± 13.9 | 0.977 (0.927–1.026) | 0.3293 | |
| %TWL, % | 27.5 ± 7.6 | 21.8 ± 5.9 | 1.150 (0.991–1.336) | 0.0331 * | |
| FBG, mg/dL | 90.5 ± 18.4 | 101.0 ± 14.9 | 0.969 (0.918–1.011) | 0.1457 | |
| IRI, μU/mL | 8.5 ± 4.3 | 15.4 ± 9.9 | 0.847 (0.683–0.968) | 0.0122 * | |
| HbA1c, % | 5.5 ± 0.4 | 6.1 ± 0.7 | 0.136 (0.017–0.636) | 0.0094 * | |
| HOMA-IR, no unit | 1.8 ± 1.2 | 4.2 ± 3.3 | 0.547 (0.221–0.910) | 0.0153 * | |
| AST, IU/L | 16.5 ± 4.4 | 27.7 ± 19.9 | 0.887 (0.751–0.982) | 0.0149 * | |
| ALT, IU/L | 15.4 ± 6.8 | 29.2 ± 11.3 | 0.840 (0.714–0.936) | 0.0004 * | |
| γ-GTP, IU/L | 23.8 ± 32.4 | 86.3 ± 153.5 | 0.987 (0.956–1.001) | 0.0666 | |
| LDL-C, mg/dL | 101.8 ± 34.1 | 112.9 ± 33.0 | 0.990 (0.962–1.015) | 0.4137 | |
| TG, mg/dL | 85.2 ± 43.3 | 135.9 ± 103.4 | 0.988 (0.969–1.001) | 0.0642 | |
| Hyaluronic acid, ng/mL | 30.4 ± 18.0 | 38.4 ± 42.6 | 0.989 (0.960–1.019) | 0.4535 | |
| MDA-LDL, U/L | 96.9 ± 24.4 | 123.2 ± 17.9 | 0.939 (0.882–0.982) | 0.0032 * | |
| PAI-1, ng/mL | 20.3 ± 13.9 | 25.1 ± 19.9 | 0.982 (0.932–1.032) | 0.4468 | |
| VFA, cm2 | 140.6 ± 79.9 | 195.7 ± 28.0 | 0.989 (0.975–1.001) | 0.0810 | |
| Waist, cm | 102.3 ± 13.8 | 108.8 ± 6.9 | 0.958 (0.886–1.027) | 0.2320 | |
| Liver volume, mL | 1551.9 ± 291.7 | 1921.7 ± 300.9 | 0.996 (0.992–0.999) | 0.0071 * | |
| L/S ratio, no unit | 1.3 ± 0.2 | 1.2 ± 0.5 | 6.745 (0.275–544.575) | 0.2461 | |
| Multivariate Analysis | |||||
| Parameters | Odds Ratio | 95% Confidence Interval | p-Value | ||
| HbA1c, % | 0.023 | <0.001–9.391 | 0.2550 | ||
| ALT, IU/L | 0.717 | 0.178–0.953 | 0.0081 | ||
| MDA-LDL, U/L | 0.953 | 0.821–1.055 | 0.3843 | ||
| Liver volume, mL | 0.990 | 0.951–1.000 | 0.0566 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, A.; Umemura, A.; Ishida, K.; Takahashi, N.; Nikai, H.; Nitta, H.; Takikawa, Y.; Kakisaka, K.; Abe, T.; Nishiya, M.; et al. The Concept of Indeterminable NASH Inducted by Preoperative Diet and Metabolic Surgery: Analyses of Histopathological and Clinical Features. Biomedicines 2022, 10, 453. https://doi.org/10.3390/biomedicines10020453
Sasaki A, Umemura A, Ishida K, Takahashi N, Nikai H, Nitta H, Takikawa Y, Kakisaka K, Abe T, Nishiya M, et al. The Concept of Indeterminable NASH Inducted by Preoperative Diet and Metabolic Surgery: Analyses of Histopathological and Clinical Features. Biomedicines. 2022; 10(2):453. https://doi.org/10.3390/biomedicines10020453
Chicago/Turabian StyleSasaki, Akira, Akira Umemura, Kazuyuki Ishida, Naoto Takahashi, Haruka Nikai, Hiroyuki Nitta, Yasuhiro Takikawa, Keisuke Kakisaka, Tamami Abe, Masao Nishiya, and et al. 2022. "The Concept of Indeterminable NASH Inducted by Preoperative Diet and Metabolic Surgery: Analyses of Histopathological and Clinical Features" Biomedicines 10, no. 2: 453. https://doi.org/10.3390/biomedicines10020453
APA StyleSasaki, A., Umemura, A., Ishida, K., Takahashi, N., Nikai, H., Nitta, H., Takikawa, Y., Kakisaka, K., Abe, T., Nishiya, M., & Sugai, T. (2022). The Concept of Indeterminable NASH Inducted by Preoperative Diet and Metabolic Surgery: Analyses of Histopathological and Clinical Features. Biomedicines, 10(2), 453. https://doi.org/10.3390/biomedicines10020453






