Purinergic Receptor P2Y2 Stimulation Averts Aortic Valve Interstitial Cell Calcification and Myofibroblastic Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Aortic Valve Interstitial Cell Isolation and Culture
2.3. Calcification and Fibrosis Assays
2.4. Western Blot
2.5. Reverse Transcription and Real-Time PCR
2.6. Imaging Flow Cytometry Analyses
2.7. Capillary Western Blot
2.8. Statistical Analyses
3. Results
3.1. Valve Interstitial Cell Characterization
3.2. Extracellular Calcium Potential of VICs Treated with 2ThioUTP
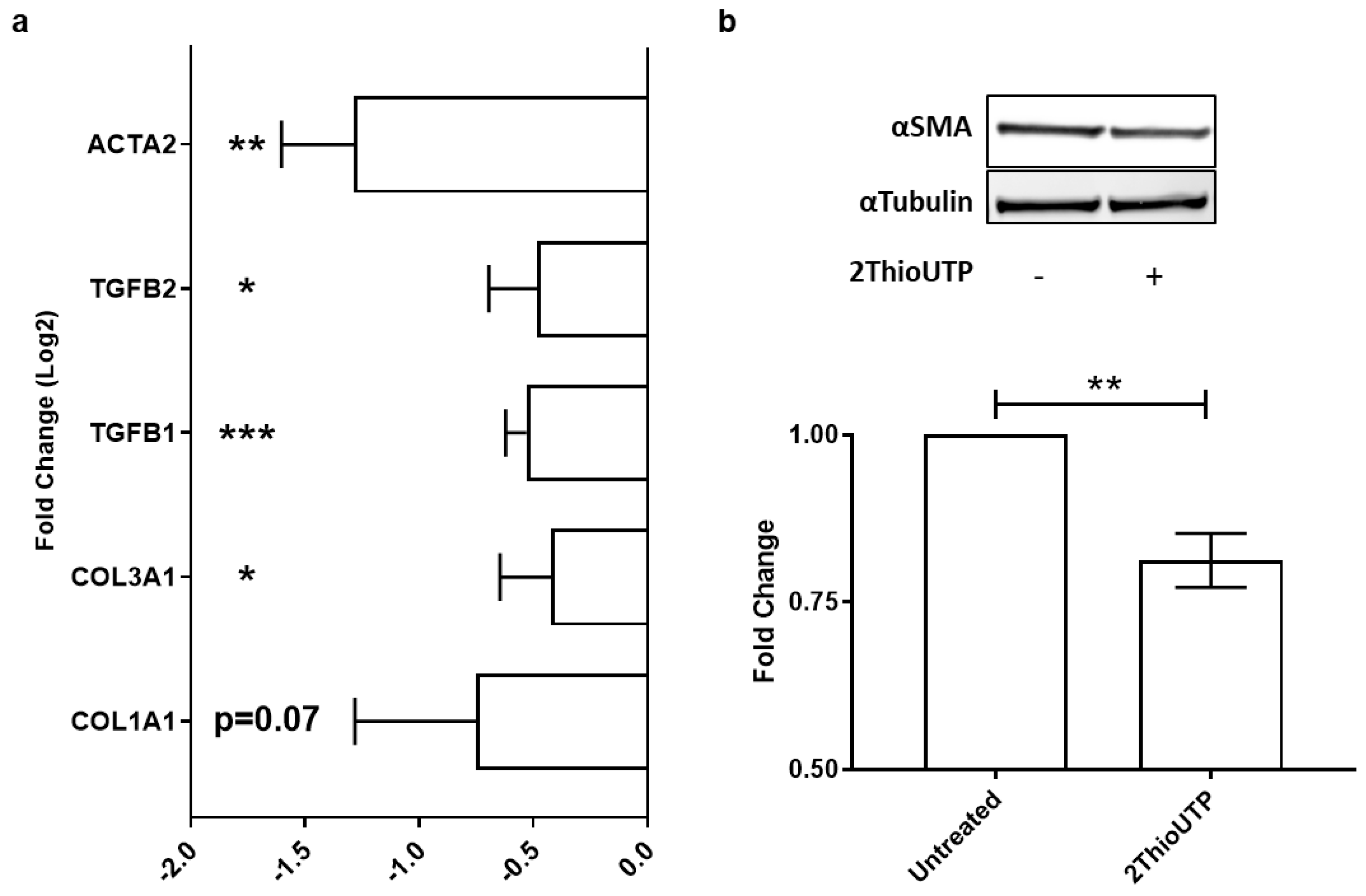
3.3. ThioUTP Effects on Myofibroblastic Activation of VICs
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Thorac. Cardiovasc. Surg. 2014, 148, e1–e132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e95–e1159. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.; Pellikka, P.A.; Quiñones, M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur. J. Echocardiogr. 2009, 10, 479. [Google Scholar] [CrossRef] [Green Version]
- Vahanian, A.; Otto, C.M. Risk stratification of patients with aortic stenosis. Eur. Heart J. 2010, 31, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Myasoedova, V.A.; Ravani, A.L.; Frigerio, B.; Valerio, V.; Moschetta, D.; Songia, P.; Poggio, P. Novel pharmacological targets for calcific aortic valve disease: Prevention and treatments. Pharmacol. Res. 2018, 136, 74–82. [Google Scholar] [CrossRef]
- Poggio, P.; Songia, P.; Moschetta, D.; Valerio, V.; Myasoedova, V.; Perrucci, G.L.; Pompilio, G. MiRNA profiling revealed enhanced susceptibility to oxidative stress of endothelial cells from bicuspid aortic valve. J. Mol. Cell. Cardiol. 2019, 131, 146–154. [Google Scholar] [CrossRef]
- Peeters, F.; Meex, S.J.R.; Dweck, M.R.; Aikawa, E.; Crijns, H.; Schurgers, L.J.; Kietselaer, B.L. Calcific aortic valve stenosis: Hard disease in the heart: A biomolecular approach towards diagnosis and treatment. Eur. Heart J. 2018, 39, 2618–2624. [Google Scholar] [CrossRef] [Green Version]
- Abdelbaky, A.; Corsini, E.; Figueroa, A.L.; Subramanian, S.; Fontanez, S.; Emami, H.; Hoffmann, U.; Narula, J.; Tawakol, A. Early aortic valve inflammation precedes calcification: A longitudinal FDG-PET/CT study. Atherosclerosis 2015, 238, 165–172. [Google Scholar] [CrossRef]
- Perrucci, G.L.; Zanobini, M.; Gripari, P.; Songia, P.; Alshaikh, B.; Tremoli, E.; Poggio, P. Pathophysiology of Aortic Stenosis and Mitral Regurgitation. Compr. Physiol. 2017, 7, 799–818. [Google Scholar]
- Xu, K.; Xie, S.; Huang, Y.; Zhou, T.; Liu, M.; Zhu, P.; Wang, C.; Shi, J.; Li, F.; Sellke, F.W.; et al. Cell-Type Transcriptome Atlas of Human Aortic Valves Reveal Cell Heterogeneity and Endothelial to Mesenchymal Transition Involved in Calcific Aortic Valve Disease. Arter. Thromb. Vasc. Biol. 2020, 40, 2910–2921. [Google Scholar] [CrossRef]
- Goody, P.R.; Hosen, M.R.; Christmann, D.; Niepmann, S.T.; Zietzer, A.; Adam, M.; Bönner, F.; Zimmer, S.; Nickenig, G.; Jansen, F. Aortic Valve Stenosis: From Basic Mechanisms to Novel Therapeutic Targets. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.S.; Stadiotti, I.; Pilato, C.A.; Perrucci, G.L.; Saverio, V.; Catto, V.; Vettor, G.; Casella, M.; Guarino, A.; Polvani, G.; et al. Excess TGF-beta1 Drives Cardiac Mesenchymal Stromal Cells to a Pro-Fibrotic Commitment in Arrhythmogenic Cardiomyopathy. Int. J. Mol. Sci. 2021, 22, 2673. [Google Scholar] [CrossRef] [PubMed]
- Buttner, P.; Feistner, L.; Lurz, P.; Thiele, H.; Hutcheson, J.D.; Schlotter, F. Dissecting Calcific Aortic Valve Disease-The Role, Etiology, and Drivers of Valvular Fibrosis. Front. Cardiovasc. Med. 2021, 8, 660797. [Google Scholar] [CrossRef]
- Zhou, T.; Han, D.; Liu, J.; Shi, J.; Zhu, P.; Wang, Y.; Dong, N. Factors influencing osteogenic differentiation of human aortic valve interstitial cells. J. Thorac. Cardiovasc. Surg. 2021, 161, e85–e163. [Google Scholar] [CrossRef] [PubMed]
- Santoro, R.; Scaini, D.; Severino, L.U.; Amadeo, F.; Ferrari, S.; Bernava, G.; Garoffolo, G.; Agrifoglio, M.; Casalis, L.; Pesce, M. Activation of human aortic valve interstitial cells by local stiffness involves YAP-dependent transcriptional signaling. Biomaterials 2018, 181, 268–279. [Google Scholar] [CrossRef]
- Blaser, M.C.; Kraler, S.; Luscher, T.F.; Aikawa, E. Multi-Omics Approaches to Define Calcific Aortic Valve Disease Pathogenesis. Circ. Res. 2021, 128, 1371–1397. [Google Scholar] [CrossRef]
- Cote, N.; El Husseini, D.; Pepin, A.; Guauque-Olarte, S.; Ducharme, V.; Bouchard-Cannon, P.; Audet, A.; Fournier, D.; Gaudreault, N.; Derbali, H.; et al. ATP acts as a survival signal and prevents the mineralization of aortic valve. J. Mol. Cell. Cardiol. 2012, 52, 1191–1202. [Google Scholar] [CrossRef]
- Bouchareb, R.; Côté, N.; Boulanger, M.C.; Le Quang, K.; El Husseini, D.; Asselin, J.; Hadji, F.; Lachance, D.; Shayhidin, E.E.; Mahmut, A.; et al. Carbonic anhydrase XII in valve interstitial cells promotes the regression of calcific aortic valve stenosis. J. Mol. Cell. Cardiol. 2015, 82, 104–115. [Google Scholar] [CrossRef]
- El Husseini, D.; Boulanger, M.C.; Mahmut, A.; Bouchareb, R.; Laflamme, M.H.; Fournier, D.; Pibarot, P.; Bossè, Y.; Mathieu, P. P2Y2 receptor represses IL-6 expression by valve interstitial cells through Akt: Implication for calcific aortic valve disease. J. Mol. Cell. Cardiol. 2014, 72, 146–156. [Google Scholar] [CrossRef]
- Lu, D.; Insel, P.A. Cellular mechanisms of tissue fibrosis. 6. Purinergic signaling and response in fibroblasts and tissue fibrosis. Am. J. Physiol. Cell Physiol. 2014, 306, C779–C788. [Google Scholar] [CrossRef] [PubMed]
- Poggio, P.; Branchetti, E.; Grau, J.B.; Lai, E.K.; Gorman, R.C.; Gorman, J.H., 3rd; Sacks, M.S.; Bavaria, J.E.; Ferrari, G. Osteopontin-CD44v6 interaction mediates calcium deposition via phospho-Akt in valve interstitial cells from patients with noncalcified aortic valve sclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2086–2094. [Google Scholar] [CrossRef] [Green Version]
- Branchetti, E.; Sainger, R.; Poggio, P.; Grau, J.B.; Patterson-Fortin, J.; Bavaria, J.E.; Chorny, M.; Lai, E.; Gorman, R.C.; Levy, R.J.; et al. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, e66–e74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, T.; Takahashi, C.; Matsuzaki, H.; Takeyama, S.; Sato, S.; Sato, A.; Kuroda, Y.; Higashi, H. N-glycan-dependent cell-surface expression of the P2Y 2 receptor and N -glycan-independent distribution to lipid rafts. Biochem. Biophys. Res. Commun. 2017, 485, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Towler, D.A. Molecular and cellular aspects of calcific aortic valve disease. Circ. Res. 2013, 113, 198–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myasoedova, V.A.; Genovese, S.; Cavallotti, L.; Bonomi, A.; Chiesa, M.; Campodonico, J.; Rondinelli, M.; Cosentino, N.; Baldassarre, D.; Veglia, F.; et al. Aortic Valve Sclerosis in High-Risk Coronary Artery Disease Patients. Front. Cardiovasc. Med. 2021, 8, 711899. [Google Scholar] [CrossRef]
- Gharacholou, S.M.; Karon, B.L.; Shub, C.; Pellikka, P.A. Aortic valve sclerosis and clinical outcomes: Moving toward a definition. Am. J. Med. 2011, 124, 103–110. [Google Scholar] [CrossRef]
- Myasoedova, V.A.; Saccu, C.; Chiesa, M.; Songia, P.; Alfieri, V.; Massaiu, I.; Valerio, V.; Moschetta, D.; Gripari, P.; Naliato, M.; et al. Aortic Valve Sclerosis as an Important Predictor of Long-Term Mortality in Patients with Carotid Atheromatous Plaque Requiring Carotid Endarterectomy. Front. Cardiovasc. Med. 2021, 8, 653991. [Google Scholar] [CrossRef]
- Massaiu, I.; Songia, P.; Chiesa, M.; Valerio, V.; Moschetta, D.; Alfieri, V.; Myasoedova, V.; Schmid, M.; Cassetta, L.; Colombo, G.; et al. Evaluation of Oxford Nanopore MinION RNA-Seq Performance for Human Primary Cells. Int. J. Mol. Sci. 2021, 22, 6317. [Google Scholar] [CrossRef]
- Summerhill, V.I.; Moschetta, D.; Orekhov, A.N.; Poggio, P.; Myasoedova, V.A. Sex-Specific Features of Calcific Aortic Valve Disease. Int. J. Mol. Sci. 2020, 21, 5620. [Google Scholar] [CrossRef]


| Variables | Patients (n = 33) | AVSc (n = 13) | AS (n = 20) | p-Value |
|---|---|---|---|---|
| Male sex, n (%) | 25 (75.5) | 10 (76.9) | 15 (75.0) | >0.99 |
| Age (years), mean ± SD | 68.7 ± 9.4 | 64.1 ± 11.7 | 71.2 ± 6.8 | 0.06 |
| Height (m), mean ± SD | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 0.45 |
| Weight (kg), mean ± SD | 79.5 ± 14.2 | 77.3 ± 15.0 | 81.0 ± 13.8 | 0.48 |
| BMI (kg/m2), mean ± SD | 27.3 ± 4.1 | 26.0 ± 3.9 | 28.2 ± 4.0 | 0.13 |
| Diabetes, n (%) | 2 (6.1) | 0 (0) | 2 (10) | >0.99 |
| Hypertension, n (%) | 23 (69.7) | 9 (69.2) | 14 (70.0) | >0.99 |
| Dyslipidemia, n (%) | 19 (57.6) | 8 (61.5) | 11 (55.0) | 0.74 |
| Smokers, n (%) | 5 (15.1) | 2 (15.4) | 3 (15.0) | >0.99 |
| CAD, n (%) | 11 (33.3) | 5 (38.5) | 6 (30.0) | 0.71 |
| PAD, n (%) | 6 (18.2) | 1 (7.7) | 5 (25.0) | 0.36 |
| TAV morphology, n (%) | 27 (81.8) | 11 (84.6) | 16 (80.0) | >0.99 |
| LVEF (%), mean ± SD | 59.5 ± 9.3 | 55.8 ± 9.5 | 61.9 ± 8.7 | 0.07 |
| AV Velocity max (m/s), mean ± SD | 3.5 ± 1.2 | 2.2 ± 0.50 | 4.2 ± 0.8 | <0.0001 |
| AV Gradient max (mmHg), mean ± SD | 53.1 ± 33.1 | 19.8 ± 9.0 | 73.1 ± 24.9 | <0.0001 |
| AV Gradient med (mmHg), mean ± SD | 35.3 ± 18.5 | 12.0 ± 5.0 | 42.3 ± 14.9 | <0.0001 |
| Area (cm2), mean ± SD | 1.1 ± 0.5 | 2.0 ± 0.2 | 0.9 ± 0.2 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moschetta, D.; Di Maria, E.; Valerio, V.; Massaiu, I.; Bozzi, M.; Songia, P.; D’alessandra, Y.; Myasoedova, V.A.; Poggio, P. Purinergic Receptor P2Y2 Stimulation Averts Aortic Valve Interstitial Cell Calcification and Myofibroblastic Activation. Biomedicines 2022, 10, 457. https://doi.org/10.3390/biomedicines10020457
Moschetta D, Di Maria E, Valerio V, Massaiu I, Bozzi M, Songia P, D’alessandra Y, Myasoedova VA, Poggio P. Purinergic Receptor P2Y2 Stimulation Averts Aortic Valve Interstitial Cell Calcification and Myofibroblastic Activation. Biomedicines. 2022; 10(2):457. https://doi.org/10.3390/biomedicines10020457
Chicago/Turabian StyleMoschetta, Donato, Enrico Di Maria, Vincenza Valerio, Ilaria Massaiu, Michele Bozzi, Paola Songia, Yuri D’alessandra, Veronika A. Myasoedova, and Paolo Poggio. 2022. "Purinergic Receptor P2Y2 Stimulation Averts Aortic Valve Interstitial Cell Calcification and Myofibroblastic Activation" Biomedicines 10, no. 2: 457. https://doi.org/10.3390/biomedicines10020457
APA StyleMoschetta, D., Di Maria, E., Valerio, V., Massaiu, I., Bozzi, M., Songia, P., D’alessandra, Y., Myasoedova, V. A., & Poggio, P. (2022). Purinergic Receptor P2Y2 Stimulation Averts Aortic Valve Interstitial Cell Calcification and Myofibroblastic Activation. Biomedicines, 10(2), 457. https://doi.org/10.3390/biomedicines10020457






