Concept of the Number Needed to Treat for the Analysis of Pain Relief Outcomes in Patients Treated with Spinal Cord Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Calculation and Interpretation of the NNT
2.2. NNT Calculation
2.3. SCS Literature Review
2.3.1. Data Sources
2.3.2. Eligibility Criteria
2.3.3. Data Extraction
3. NNT Applied to SCS Controlled Trials
4. Results
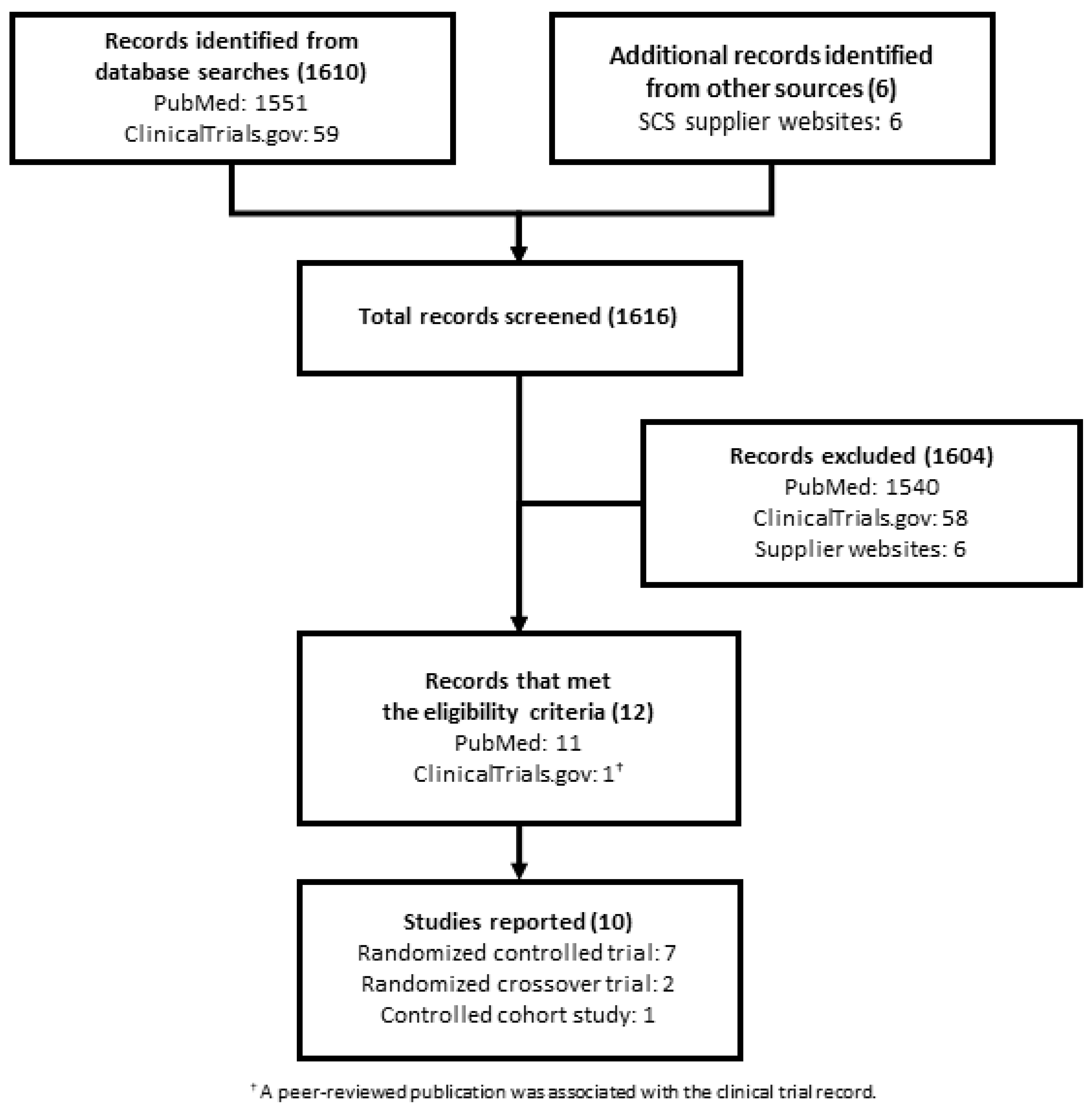
4.1. Literature Review Results
4.2. NNT Estimates for SCS Controlled Trials
5. Discussion
6. NNT Interpretation
6.1. Comparator Treatment
6.2. Baseline Risk
6.3. Time Frame
6.4. Outcome Variable
6.5. ITT and PP Populations
6.6. Uncertainty
6.7. Comparison Methodology
6.8. Number Needed to Harm
6.9. Analysis and Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.; Harker, J.; Bala, M.M.; Truyers, C.; Kellen, E.; Bekkering, G.; Kleijnen, J. Epidemiology of chronic non-cancer pain in Europe: Narrative review of prevalence, pain treatments and pain impact. Curr. Med. Res. Opin. 2010, 27, 449–462. [Google Scholar] [CrossRef]
- Majedi, H.; Dehghani, S.S.; Jahi, S.S.; Tafakhori, A.; Emami, S.A.; Mireskandari, M.; Hosseini, S.M. Assessment of Factors Predicting Inadequate Pain Management in Chronic Pain Patients. Anesthesiol. Pain Med. 2019, 9, 97229. [Google Scholar] [CrossRef]
- Cheung, C.W.; Choo, C.Y.; Kim, Y.-C.; Lin, F.S.; Moon, S.-H.; Osio-Salido, E.; Pan, S.-F.; Singh, V.A.; Yoon, S.H.; Moon, H.; et al. Inadequate Management of Chronic Non-cancer Pain and Treatment-Related Adverse Events in Asia: Perspectives from Patients from 10 Countries/Regions. SN Compr. Clin. Med. 2019, 1, 442–450. [Google Scholar] [CrossRef]
- Linderoth, B.; Foreman, R.D. Conventional and Novel Spinal Stimulation Algorithms: Hypothetical Mechanisms of Action and Comments on Outcomes. Neuromodul. Technol. Neural Interface 2017, 20, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, D.; Kagan, Z.; Wang, D.; Bradley, K. Differential Modulation of Dorsal Horn Neurons by Various Spinal Cord Stimulation Strategies. Biomedicines 2021, 9, 568. [Google Scholar] [CrossRef]
- Francio, V.T.; Polston, K.; Murphy, M.; Hagedorn, J.; Sayed, D. Management of Chronic and Neuropathic Pain with 10 kHz Spinal Cord Stimulation Technology: Summary of Findings from Preclinical and Clinical Studies. Biomedicines 2021, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Baranidharan, G.; Edgar, D.; Bretherton, B.; Crowther, T.; Lalkhen, A.-G.; Fritz, A.-K.; Vajramani, G. Efficacy and Safety of 10 kHz Spinal Cord Stimulation for the Treatment of Chronic Pain: A Systematic Review and Narrative Synthesis of Real-World Retrospective Studies. Biomedicines 2021, 9, 180. [Google Scholar] [CrossRef]
- Abdallat, M.; Saryyeva, A.; Blahak, C.; Wolf, M.; Weigel, R.; Loher, T.; Runge, J.; Heissler, H.; Kinfe, T.; Krauss, J. Centromedian–Parafascicular and Somatosensory Thalamic Deep Brain Stimulation for Treatment of Chronic Neuropathic Pain: A Contemporary Series of 40 Patients. Biomedicines 2021, 9, 731. [Google Scholar] [CrossRef]
- Balzani, E.; Fanelli, A.; Malafoglia, V.; Tenti, M.; Ilari, S.; Corraro, A.; Muscoli, C.; Raffaeli, W. A Review of the Clinical and Therapeutic Implications of Neuropathic Pain. Biomedicines 2021, 9, 1239. [Google Scholar] [CrossRef] [PubMed]
- Kirketeig, T.; Schultheis, C.; Zuidema, X.; Hunter, C.W.; Deer, T. Burst Spinal Cord Stimulation: A Clinical Review. Pain Med. 2019, 20, S31–S40. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, K.; Malayil, R.; Kirketeig, T.; Deer, T. Burst Spinal Cord Stimulation: A Systematic Review and Pooled Analysis of Real-World Evidence and Outcomes Data. Pain Med. 2019, 20, S47–S57. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Hunter, C.W.; Mehta, P.; Sayed, D.; Grider, J.S.; Lamer, T.J.; Pope, E.J.; Falowski, S.; Provenzano, A.D.; Esposito, M.F.; et al. A Systematic Literature Review of Dorsal Root Ganglion Neurostimulation for the Treatment of Pain. Pain Med. 2020, 21, 1581–1589. [Google Scholar] [CrossRef]
- Malinowski, M.N.; Jain, S.; Jassal, N.; Deer, T. Spinal cord stimulation for the treatment of neuropathic pain: Expert opinion and 5-year outlook. Expert Rev. Med. Devices 2020, 17, 1293–1302. [Google Scholar] [CrossRef]
- Laupacis, A.; Sackett, D.L.; Roberts, R.S. An Assessment of Clinically Useful Measures of the Consequences of Treatment. N. Engl. J. Med. 1988, 318, 1728–1733. [Google Scholar] [CrossRef]
- Altman, D.G. Confidence intervals for the number needed to treat. BMJ 1998, 317, 1309–1312. [Google Scholar] [CrossRef]
- Citrome, L.; Ketter, T.A. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int. J. Clin. Pr. 2013, 67, 407–411. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Vist, G.E.; Higgins, J.P.T.; Santesso, N.; Deeks, J.J.; Glasziou, P.; Akl, E.A.; Guyatt, G.H. Chapter 15: Interpreting results and drawing conclusions. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2019. [Google Scholar]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef]
- British Medical Journal (BMJ) Guidance for Authors (3 May 2018). Available online: https://www.bmj.com/about-bmj/resources-authors/article-types (accessed on 4 July 2021).
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Hasan, H.; Goddard, K.; Howard, A.F. Utility of the number needed to treat in paediatric haematological cancer randomised controlled treatment trials: A systematic review. BMJ Open 2019, 9, e022839. [Google Scholar] [CrossRef] [PubMed]
- Weeks, D.L.; Noteboom, J. Using the number needed to treat in clinical practice. Arch. Phys. Med. Rehabil. 2004, 85, 1729–1731. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Badgett, R.; Cornell, J. Number needed to treat: A descriptor for weighing therapeutic options. Am. J. Health Pharm. 2005, 62, 2031–2036. [Google Scholar] [CrossRef]
- Saver, J.L.; Lewis, R.J. Number Needed to Treat: Conveying the Likelihood of a Therapeutic Effect. JAMA 2019, 321, 798–799. [Google Scholar] [CrossRef] [PubMed]
- Bender, R. Number Needed to Treat (NNT). In Encyclopedia of Biostatistics; Wiley: Hooboken, NJ, USA, 2005. [Google Scholar]
- McAlister, F.A. The “number needed to treat” turns 20—and continues to be used and misused. Can. Med. Assoc. J. 2008, 179, 549–553. [Google Scholar] [CrossRef]
- Daly, L.E. Confidence Limits Made Easy: Interval Estimation Using a Substitution Method. Am. J. Epidemiol. 1998, 147, 783–790. [Google Scholar] [CrossRef]
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; Milbouw, G.; et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007, 132, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Guyot, P.; Cheng, W.; Tremblay, G.; Copher, R.; Burnett, H.; Li, X.; Makin, C. Number needed to treat in indirect treatment comparison. J. Comp. Eff. Res. 2018, 7, 259–269. [Google Scholar] [CrossRef] [PubMed]
- North, R.B.; Kidd, D.H.; Farrokhi, F.; Piantadosi, S.A. Spinal Cord Stimulation versus Repeated Lumbosacral Spine Surgery for Chronic Pain: A Randomized, Controlled Trial. Neurosurgery 2005, 56, 98–107. [Google Scholar] [CrossRef]
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; Milbouw, G.; et al. The Effects of Spinal Cord Stimulation in Neuropathic Pain are Sustained: A 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery 2008, 63, 762–770. [Google Scholar] [CrossRef]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Brown, L.L.; Yearwood, T.L.; et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain. Anesthesiology 2015, 123, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Yearwood, T.L.; Bundschu, R.; et al. Comparison of 10-kHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-Month Results From a Multi-center, Randomized, Controlled Pivotal Trial. Neurosurgery 2016, 79, 667–677. [Google Scholar] [CrossRef]
- Bolash, R.; Creamer, M.; Rauck, R.; Vahedifar, P.; Calodney, A.; Fox, I.; Özaktay, C.; Panchal, S.; Vanquathem, N.; Yasin, M. Wireless High-Frequency Spinal Cord Stimulation (10 kHz) Compared with Multiwaveform Low-Frequency Spinal Cord Stimulation in the Management of Chronic Pain in Failed Back Surgery Syndrome Subjects: Preliminary Results of a Multicenter, Prospective Randomized Controlled Study. Pain Med. 2019, 20, 1971–1979. [Google Scholar] [CrossRef]
- Rigoard, P.; Basu, S.; Desai, M.; Taylor, R.; Annemans, L.; Tan, Y.; Johnson, M.J.; Abeele, C.V.D.; North, R.; PROMISE Study Group. Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: A multicenter randomized controlled trial. Pain 2019, 160, 1410–1420. [Google Scholar] [CrossRef]
- Mekhail, N.; Levy, R.M.; Deer, T.R.; Kapural, L.; Li, S.; Amirdelfan, K.; Hunter, C.W.; Rosen, S.M.; Costandi, S.J.; Falowski, S.M.; et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): A double-blind, randomised, controlled trial. Lancet Neurol. 2019, 19, 123–134. [Google Scholar] [CrossRef]
- Fishman, M.; Cordner, H.; Justiz, R.; Provenzano, D.; Merrell, C.; Shah, B.; Naranjo, J.; Kim, P.; Calodney, A.; Carlson, J.; et al. Twelve-Month results from multicenter, open-label, randomized controlled clinical trial comparing differential target multiplexed spinal cord stimulation and traditional spinal cord stimulation in subjects with chronic intractable back pain and leg pain. Pain Pract. 2021, 21, 912–923. [Google Scholar] [CrossRef]
- Deer, T.; Slavin, K.V.; Amirdelfan, K.; North, R.B.; Burton, A.W.; Yearwood, T.L.; Tavel, E.; Staats, P.; Falowski, S.; Pope, J.; et al. Success Using Neuromodulation With BURST (SUNBURST) Study: Results From a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodul. Technol. Neural Interface 2018, 21, 56–66. [Google Scholar] [CrossRef] [PubMed]
- North, J.; Loudermilk, E.; Lee, A.; Sachdeva, H.; Kaiafas, D.; Washabaugh, E.; Sheth, S.; Scowcroft, J.; Mekhail, N.; Lampert, B.; et al. Outcomes of a Multicenter, Prospective, Crossover, Randomized Controlled Trial Evaluating Subperception Spinal Cord Stimulation at ≤1.2 kHz in Previously Implanted Subjects. Neuromodul. Technol. Neural Interface 2019, 23, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A.; Hollingworth, W.; Comstock, B.A.; Deyo, R.A. Spinal cord stimulation for failed back surgery syndrome: Outcomes in a workers’ compensation setting. Pain 2010, 148, 14–25. [Google Scholar] [CrossRef]
- Schober, P.; Bossers, S.; Schwarte, L.A. Statistical Significance Versus Clinical Importance of Observed Effect Sizes: What Do P Values and Confidence Intervals Really Represent? Anesthesia Analg. 2018, 126, 1068–1072. [Google Scholar] [CrossRef]
- Garg, V.; Shen, X.; Cheng, Y.; Nawarskas, J.J.; Raisch, D.W. Use of Number Needed to Treat in Cost-Effectiveness Analyses. Ann. Pharmacother. 2013, 47, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Oxman, A.D.; Glenton, C.; Rosenbaum, S.; Lewin, S.; Gülmezoglu, A.M.; Souza, J.P. Policymakers’ and other stakeholders’ perceptions of key considerations for health system decisions and the presentation of evidence to inform those considerations: An international survey. Health Res. Policy Syst. 2013, 11, 19. [Google Scholar] [CrossRef]
- Citrome, L. Number Needed to Treat: What It Is and What It Isn’t, and Why Every Clinician Should Know How to Calculate It. J. Clin. Psychiatry 2011, 72, 412–413. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.P.; Khalid, J.M.; Smyth, M.D.; Patel, H. The number needed to treat and relevant between-trial comparisons of competing interventions. Clin. Outcomes Res. 2018, 10, 865–871. [Google Scholar] [CrossRef]
- Harris, I.; Mulford, J.; Solomon, M.; Van Gelder, J.M.; Young, J. Association Between Compensation Status and Outcome After Surgery: A meta-analysis. JAMA 2005, 293, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; De Salvatore, S.; Ambrosio, L.; Vadalà, G.; Fontana, L.; Papalia, R.; Rantanen, J.; Iavicoli, S.; Denaro, V. Does Workers’ Compensation Status Affect Outcomes after Lumbar Spine Surgery? A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 6165. [Google Scholar] [CrossRef]
- Wilson, C.A.; Roffey, D.; Chow, D.; Alkherayf, F.; Wai, E.K. A systematic review of preoperative predictors for postoperative clinical outcomes following lumbar discectomy. Spine J. 2016, 16, 1413–1422. [Google Scholar] [CrossRef]
- Kumar, K.; Wilson, J.R. Factors affecting spinal cord stimulation outcome in chronic benign pain with suggestions to improve success rate. Acta Neurochir. Suppl. 2007, 97, 91–99. [Google Scholar] [CrossRef]
- Guthrie, B.; Thompson, A.; Dumbreck, S.; Flynn, A.; Alderson, P.; Nairn, M.; Treweek, S.; Payne, K. Better guidelines for better care: Accounting for multimorbidity in clinical guidelines—Structured examination of exemplar guidelines and health economic modelling. Health Serv. Deliv. Res. 2017, 5. [Google Scholar] [CrossRef]
- McCoy, C.E. Understanding the Intention-to-treat Principle in Randomized Controlled Trials. West. J. Emerg. Med. 2017, 18, 1075–1078. [Google Scholar] [CrossRef]
- Bender, R. Calculating Confidence Intervals for the Number Needed to Treat. Control. Clin. Trials 2001, 22, 102–110. [Google Scholar] [CrossRef]
- North, R.; Eldabe, S. Neuromodulation Device Comparison Studies: Coming of Age Revisited. Pain Med. 2018, 19, 2096–2097. [Google Scholar] [CrossRef] [PubMed]
| Study Name/ID | Design | Single or Multicenter | Study Group(s) | Indication | Pain Relief Measure | Proportion with FBSS | Definition of Response |
|---|---|---|---|---|---|---|---|
| North 2005 [32] | RCT (open-label) | Single-center | t-SCS vs. Reoperation | Predominant leg pain | VAS | 100% | ≥50% pain relief + patient satisfaction |
| PROCESS: Kumar 2007 [30], Kumar 2008 [33] | RCT (open-label) | Multicenter | t-SCS vs. CMM | Predominant leg pain | VAS | 100% | ≥50% pain relief |
| Turner 2010 [42] | Controlled cohort (open-label) | Multicenter | t-SCS vs. PCM | Predominant leg pain | NRS | 100% | ≥50% pain relief |
| SENZA-RCT: Kapural 2015 [34], Kapural 2016 [35] | RCT (open-label) | Multicenter | 10 kHz SCS vs. t-SCS | Back and leg pain | VAS | 87% | ≥50% pain relief without stimulation-related neurological deficit |
| SUNBURST: Deer 2018 [40] | RCOT (open-label) | Multicenter | Burst stim. vs. t-SCS | Trunk and/or limb pain | VAS | 42% | ≥50% pain relief * |
| SURF: Bolash 2019 [36] | RCT (open-label) | Multicenter | Externally powered 10 kHz SCS vs.
10–1500 Hz SCS | Back ± leg pain | VAS | 100% | ≥50% pain relief |
| PROMISE: Rigoard 2019 [37] | RCT (open-label) | Multicenter | t-SCS + OMM vs. CMM | Predominant back pain | NRS | 100% | ≥50% pain relief |
| WHISPER: North 2020 [41] | RCOT (open-label) | Multicenter | ≤1.2 kHz subperc. SCS vs. t-SCS | Trunk and/or limb pain | VRS | 46% | ≥50% pain relief + no increase in pain medication intake |
| EVOKE: Mekhail 2020 [38] | RCT (double-blind) | Multicenter | Closed-loop SCS vs. t-SCS | Back and leg pain | VAS | 60% | 50% threshold + no increase in pain medication intake |
| Fishman 2021 [39] | RCT (open-label) | Multicenter | DTM SCS vs. t-SCS | Back and leg pain | VAS | 59% | ≥50% pain relief |
| Study/Article ID | Design | Treatment Group | Control Group | Follow-up Time | Pain Area | NNT for ITT Treatment Group vs.
ITT Control Group (95% CI) | NNT for PP Treatment Group vs. PP Control Group (95% CI) |
|---|---|---|---|---|---|---|---|
| North 2005 [32] | RCT (open-label) | t-SCS | Reoperation | 2.9 Yr | Comb. pain | 5.00 (2.53, 250.00) | 2.79 (1.63, 9.77) |
| PROCESS: Kumar 2007 [30] | RCT (open-label) | t-SCS | CMM | 3 Mo | Leg pain | 2.20 (1.64, 3.35) | 2.13 (1.59, 3.25) |
| PROCESS: Kumar 2007 [30] | RCT (open-label) | t-SCS | CMM | 6 Mo | Leg pain | 2.64 (1.87, 4.51) | 2.57 (1.81, 4.41) |
| PROCESS: Kumar 2007 [30] | RCT (open-label) | t-SCS | CMM | 12 Mo | Leg pain | 4.08 (2.58, 9.78) | 3.74 (2.36, 9.09) |
| PROCESS: Kumar 2008 [33] | RCT (open-label) | t-SCS | CMM | 24 Mo | Leg pain | 3.27 (2.27, 5.80) | 2.90 (2.03, 5.05) |
| Turner 2010 [42] | Controlled cohort (open-label) | t-SCS | PCM | 6 Mo | Leg pain | 7.99 (−3732.39, 3.99) † | 8.08 (−384.11, 4.00) † |
| Turner 2010 [42] | Controlled cohort (open-label) | t-SCS | PCM | 12 Mo | Leg pain | 16.58 (−15.19, 5.36) † | 15.24 (−14.19, 4.96) † |
| Turner 2010 [42] | Controlled cohort (open-label) | t-SCS | PCM | 24 Mo | Leg pain | 110.50 (−7.57, 6.66) † | 63.57 (−6.82, 5.62) † |
| SENZA-RCT: Kapural 2015 [34] | RCT (open-label) | 10 kHz SCS | t-SCS | 3 Mo | Back pain | 2.62 (1.96, 3.95) | 2.47 (1.86, 3.67) |
| SENZA-RCT: Kapural 2015 [34] | RCT (open-label) | 10 kHz SCS | t-SCS | 3 Mo | Leg pain | 3.58 (2.44, 6.77) | 3.55 (2.41, 6.78) |
| SENZA-RCT: Kapural 2015 [34] | RCT (open-label) | 10 kHz SCS | t-SCS | 6 Mo | Back pain | 4.08 (2.63, 9.02) | 4.08 (2.59, 9.57) |
| SENZA-RCT: Kapural 2015 [34] | RCT (open-label) | 10 kHz SCS | t-SCS | 6 Mo | Leg pain | 3.78 (2.52, 7.60) | 3.77 (2.49, 7.77) |
| SENZA-RCT: Kapural 2015 [34] | RCT (open-label) | 10 kHz SCS | t-SCS | 12 Mo | Back pain | 3.70 (2.48, 7.31) | 3.66 (2.43, 7.42) |
| SENZA-RCT: Kapural 2015 [34] | RCT (open-label) | 10 kHz SCS | t-SCS | 12 Mo | Leg pain | 3.70 (2.48, 7.31) | 3.66 (2.43, 7.42) |
| SENZA-RCT: Kapural 2016 [35] | RCT (open-label) | 10 kHz SCS | t-SCS | 24 Mo | Back pain | 3.54 (2.40, 6.71) | 3.68 (2.39, 8.03) |
| SENZA-RCT: Kapural 2016 [35] | RCT (open-label) | 10 kHz SCS | t-SCS | 24 Mo | Leg pain | 3.95 (2.58, 8.45) | 4.23 (2.59, 11.54) |
| SUNBURST: Deer 2018 [40] | RCOT (open-label) | Burst stim. | t-SCS | 3 Mo | Comb. pain | 14.29 (−16.06, 4.94) † | 13.71 (−15.81, 4.78) † |
| SURF: Bolash 2019 [36] | RCT (open-label) | Externally powered 10 kHz SCS | 10–1500 Hz SCS | 6 Mo | Back pain | 7.78 (−16.83, 3.16) † | 10.25 (−17.65, 3.97) † |
| PROMISE: Rigoard 2019 [37] | RCT (open-label) | t-SCS + OMM | OMM | 6 Mo | Back pain | 11.10 (6.04, 68.13) | 8.70 (4.98, 34.47) |
| PROMISE: Rigoard 2019 [37] | RCT (open-label) | t-SCS + OMM | OMM | 6 Mo | Leg pain | 4.62 (3.16, 8.59) | 3.67 (2.60, 6.24) |
| WHISPER: North 2019 [41] | RCOT (open-label) | ≤1.2 kHz subperc. SCS | t-SCS | 3 Mo | Comb. pain | 10.00 (−17.99, 3.91) † | - * |
| EVOKE: Mekhail 2019 [38] | RCT (double-blind) | Closed-loop SCS | t-SCS | 3 Mo | Comb. pain | 5.15 (2.85, 26.66) | 6.16 (3.23, 67.14) |
| EVOKE: Mekhail 2019 [38] | RCT (double-blind) | Closed-loop SCS | t-SCS | 3 Mo | Back pain | 4.79 (2.72, 19.81) | 5.47 (2.97, 34.51) |
| EVOKE: Mekhail 2019 [38] | RCT (double-blind) | Closed-loop SCS | t-SCS | 3 Mo | Leg pain | 9.57 (−19.78, 3.85) † | 19.71 (−11.50, 5.31) † |
| EVOKE: Mekhail 2019 [38] | RCT (double-blind) | Closed-loop SCS | t-SCS | 12 Mo | Comb. pain | 5.15 (2.83, 29.17) | 7.10 (−148.78, 3.47) † |
| EVOKE: Mekhail 2019 [38] | RCT (double-blind) | Closed-loop SCS | t-SCS | 12 Mo | Back pain | 5.15 (2.81, 31.50) | 6.84 (−79.46, 3.28) † |
| EVOKE: Mekhail 2019 [38] | RCT (double-blind) | Closed-loop SCS | t-SCS | 12 Mo | Leg pain | 5.15 (2.83, 29.17) | 7.10 (−148.78, 3.47) † |
| Fishman 2021 [39] | RCT (open-label) | DTM SCS | t-SCS | 12 Mo | Back pain | 6.24 (−98.36, 3.02) † | 4.25 (2.36, 21.37) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailey-Classen, A.; Parikh, A.; Adimi, N.; Edgar, D.; Yan, A.; Rotte, A.; Caraway, D. Concept of the Number Needed to Treat for the Analysis of Pain Relief Outcomes in Patients Treated with Spinal Cord Stimulation. Biomedicines 2022, 10, 497. https://doi.org/10.3390/biomedicines10020497
Bailey-Classen A, Parikh A, Adimi N, Edgar D, Yan A, Rotte A, Caraway D. Concept of the Number Needed to Treat for the Analysis of Pain Relief Outcomes in Patients Treated with Spinal Cord Stimulation. Biomedicines. 2022; 10(2):497. https://doi.org/10.3390/biomedicines10020497
Chicago/Turabian StyleBailey-Classen, Ashley, Amar Parikh, Nima Adimi, Deborah Edgar, Alice Yan, Anand Rotte, and David Caraway. 2022. "Concept of the Number Needed to Treat for the Analysis of Pain Relief Outcomes in Patients Treated with Spinal Cord Stimulation" Biomedicines 10, no. 2: 497. https://doi.org/10.3390/biomedicines10020497
APA StyleBailey-Classen, A., Parikh, A., Adimi, N., Edgar, D., Yan, A., Rotte, A., & Caraway, D. (2022). Concept of the Number Needed to Treat for the Analysis of Pain Relief Outcomes in Patients Treated with Spinal Cord Stimulation. Biomedicines, 10(2), 497. https://doi.org/10.3390/biomedicines10020497






