Is Tissue Still the Issue? The Promise of Liquid Biopsy in Uveal Melanoma
Abstract
1. Introduction
2. Liquid Biopsy
2.1. Circulating Tumor Cells
2.1.1. CTC Enrichment and Enumeration
CellSearch
MACS
DynaBeads
2.1.2. CTC Characterization
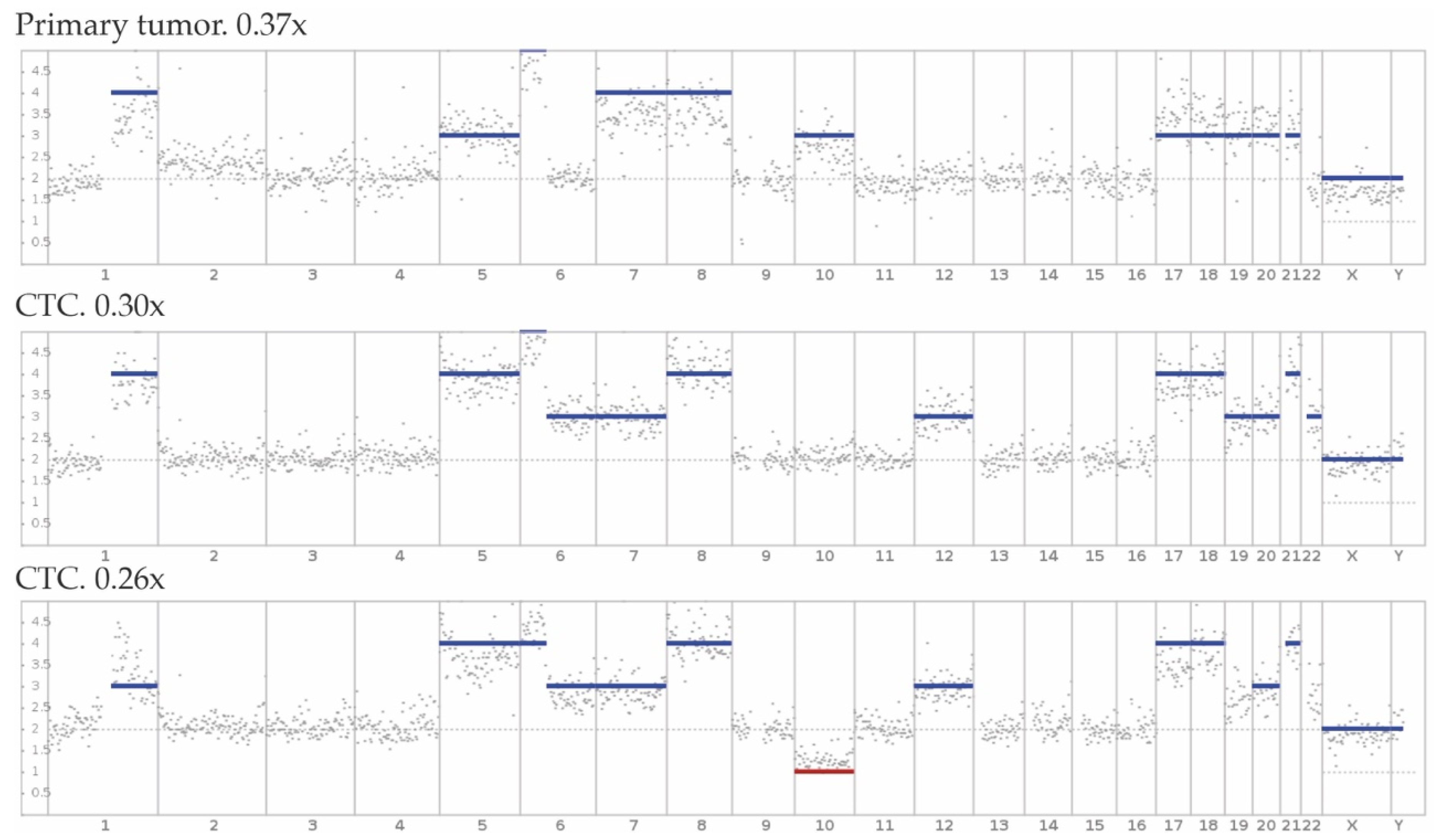
2.1.3. CTC Genotyping
2.2. Circulating Tumour DNA
2.2.1. Clinical Use of ctDNA in UM
2.2.2. ctDNA Genotyping
2.3. Extracellular Vesicles
2.4. Exosomes
2.5. Micro RNA
| microRNA | Expression | Found in Tissue Type | Sequencing Technique | Modulatory Effect |
|---|---|---|---|---|
| miR-20a [97] | Upregulated in UM-patients and metastatic patients | Plasma | RT-PCR | Promotes cell proliferation and migration by modulation of the cell cycle, focal adhesion and phosphoinositide 3-kinase (PI3K)-AKT signaling pathway [103,104]. |
| miR-20a [98] | Not DE between monosomy and disomy 3 | Plasma | RT-PCR | |
| miR-21 [94] | Upregulated in UM-patients | EVs: vitreous and FFPE UM-Tissue | TLDA | Promotes tumor growth, invasion, and metastasis, by regulation of tumorsuppressors (p53) [105] in CM and UM [106,107,108]. |
| miR-21 [98] | Not DE between monosomy and disomy 3 | Plasma | RT-PCR | |
| miR-34a [94] | Upregulated in UM-patients | EVs: vitreous and FFPE UM-Tissue | TLDA | PDL-1 is regulated by p53 via miR-34, causing immune evasion: UL16-binding protein 2 (ULBP2) is downregulated causing a diminished cell recognition by NK-cells [109,110]. |
| miR-92b [98] | Upregulated in monosomy 3 | Plasma | RT-PCR | Promotes proliferation and migration in hepatocellular carcinoma. No mechanistic information is known in (U)M [111]. |
| miR-107 [88] | Upregulated in UM-patients | EVs: isolated liver perfusate | RT2 array | Inhibits cell proliferation, migration, and invasion in CM. Highest expression is seen in metastatic melanoma [112]. |
| miR-124 [88] | Upregulated in UM-patients | EVs: isolated liver perfusate | RT2 array | Homeobox 11 (HOXA11)-antisense RNA promotes proliferation and invasion by inhibiting miR-124 in UM [113]. MiR-124 inhibits proliferation, migration, invasion and promotes apoptosis of melanoma cells [114]. |
| miR-125b [97] | Upregulated in metastatic disease | Plasma | RT-PCR | Induces apoptosis and inhibits proliferation and migration of CM cell line cells by targeting neural cell adhesion molecules (NCAM) [115]. |
| miR-146a [94,97] | Upregulated in UM-patients | EVs: vitreous, plasma and FFPE UM-Tissue; Plasma | TLDA/ RT-PCR | MiR-146 has a potential immunosuppressive role, when upregulated it causes NK-cell proliferation inhibition and apoptosis induction [103]. Additionally, miR-146 is regulated by microphtalmia-associated transcription factor (MITF) [94]. |
| miR-146a [97] | Upregulated in metastatic patients | Plasma | RT-PCR | |
| miR-155 [97] | Upregulated in metastatic patients | Plasma | RT-PCR | Is upregulated in UM-tumors and promotes invasion and proliferation by targeting Nedd4-family interactive protein 1 (NDFIP1). NDFIP1 is necessary for ubiquitination and translocation of, tumor suppressor, PTEN [116,117,118]. Upregulation is correlated to monosomy 3 status [118]. |
| miR-155 [94] | Downregulated in UM-patients | VH and VH EVs | TLDA | |
| miR-181a [97] | Downregulated in metastatic patients | Plasma | RT-PCR | Upregulation inhibits CTD small phosphatase like (CTDSPL) expression, which in turn promotes cell cycle progression in UM cells [119]. |
| miR-181a [94] | Downregulated in UM-patients | VH and VH EVs | TLDA | |
| miR-181a [97] | Upregulated in UM-patients | Plasma | RT-PCR | |
| miR-210 [88] | Upregulated in UM-patients | EVs: isolated liver perfusate | RT2 array | Targets vascular endothelial growth factor (VEGF)-dependent endothelial cell migration and tube formation factor ephrin A3 and subsequently promotes angiogenesis by formation of capillary like structures [120] and is induced by hypoxia in melanoma [121]. |
| miR-223 [98] | Upregulated in monosomy 3 | Plasma | RT-PCR/ qNPA | Regulates and suppresses myeloid derived suppressor cells, which expand during pathology and are related to UM [122,123,124]. |
| miR-223 [97] | Upregulated in UM and metastatic patients | Plasma | RT-PCR | |
| miR-320a [88] | Upregulated in UM-patients | EVs: isolated liver perfusate | RT2 array | Inhibits the epithelial to mesenchymal transition (EMT) by regulating the transforming growth factor (TGF)-β1/suppressor of mothers against decapentaplegic (SMAD) pathway [125,126]. |
| miR-370 [88] | Upregulated in UM-patients | EVs: isolated liver perfusate | RT2 array | Overexpression promotes cell growth and invasion of melanoma cells by regulation of pyruvate dehydrogenase E1 subunit Beta (PDHB) [127]. |
| miR-486a-5p [88] | Upregulated in UM-patients | EVs: isolated liver perfusate | RT2 array | Overexpression inhibits proliferation and migration in hepatocellular [128] and colorectal cancer [129]; however, no mechanistic information is available for (U)M. |
2.6. Proteins
2.7. Metabolites
2.8. Hepatic Biomarkers
3. Prognostic and Clinical Use of Medical Imaging
3.1. Ultrasonography
3.2. Optical Coherence Tomography
3.3. Magnetic Resonance Imaging
3.4. Computed Tomography
3.4.1. Computed Tomography
3.4.2. Positron Emission Tomography/Computed Tomography
4. Clinical Relevance and Added Value of Combining Minimally Invasive Modalities
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A-scan | A-mode ultrasonography |
| ABCB5 | ATP-binding cassette sub-family B member 5 |
| ADC | Apparent diffusion coefficient |
| AJCC | American Joint committee on cancer |
| AKT | Protein kinase B |
| ALIX | Programmed cell death 6-interacting protein |
| B-scan | B-mode ultrasonography |
| BAP1 | BRCA1-associated protein |
| CD | Cluster of differentiation |
| cfDNA | Cell free DNA |
| CM | Cutaneous melanoma |
| CNV | Copy number variation |
| CT | Computed tomography |
| CTC | Circulating tumor cell |
| ctDNA | Circulating tumor DNA |
| CTDSPL | CTD small phosphatase like |
| CTGF | Connective tissue growth factor |
| CTLA-1 | cytotoxic T-lymphocyte-associated antigen 4 |
| CYSLTR2 | Cysteinyl Leukotriene Receptor 2 |
| DCE-MRI | Dynamic contrast-enhanced magnetic resonance imaging |
| ddPCR | Droplet digital PCR |
| DJ-1 | Parkinson disease protein 7 |
| DNA | Deoxyribonucleic acid |
| dsDNA | Double stranded DNA |
| DW | Diffusion-weighted |
| ECM1 | Extracellular matrix protein 1 |
| EDI | Enhanced depth imaging |
| EIF1AX | Eukaryotic translation initiation factor 1A, X-linked |
| EMT | Epithelial to mesenchymal transition |
| EpCAM | Epithelial cell adhesion molecule |
| EV | Extracellular vesicles |
| FDA | Food and drug administration |
| FDG | Fluorodeoxyglucose |
| FFPE | Formalin-fixed paraffin-embedded |
| FISH | Fluorescence in situ hybridization |
| FNAB | Fine needle aspiration biopsy |
| fSRT | Fractionated stereotactic radiotherapy |
| GNA11 | G-protein α subunit 11 |
| GNAQ | G-protein α subunit Q |
| Gp100 | Glycoprotein 100 |
| HLA | Human leukocyte antigen |
| HOXA11 | Homeobox 11 |
| HTRA1 | Serine proteoase HTRA1 |
| IFN-γ | Interferon gamma |
| IGFBP1 | Insulin-like growth factor-binding protein 7 |
| IL | Interleukin |
| LAMA1 | Laminin subunit alpha-1 |
| LAMP1 | Lysosome-associated membrane glycoprotein 1 |
| LDH | Lactate dehydrogenase |
| MACS | Magnetic activated cell sorting |
| MAGED1 | Melanoma-associated antigen D1 |
| MAGED2 | Melanoma-associated antigen D2 |
| MCAM | Melanoma cell adhesion molecule |
| MCSP | Melanoma-associated chondroitin sulfate proteoglycan |
| MIA | Melanoma inhibitory activity |
| miR | MicroRNA |
| miRNA | MicroRNA |
| MITF | Microphthalmia-associated transcription factor |
| MLANA | Melanoma antigen recognized by T-cells 1 |
| MRI | Magnetic resonance imaging |
| mRNA | Messenger RNA |
| NCAM | Neural cell adhesion molecules |
| NDFIP1 | Nedd4-family interactive protein 1 |
| NHS | National Health Service |
| NRP2 | Neuropilin-2 |
| OCT | Optical coherence tomography |
| OS | Overall survival |
| OXPHOS | Oxidative phosphorylation |
| PAM | Peptidyl-glycine alpha-amidating monooxygenase |
| PBT | proton beam therapy |
| PD-1 | Programmed cell death protein 1 |
| PDHB | Pyruvate dehydrogenase E1 subunit Beta |
| PDT | photodynamic therapy |
| PET/CT | Positron emission tomography/computed tomography |
| PFS | Progression free survival |
| PI3K | Phosphoinositide 3-kinase |
| PLCB4 | Phospholipase C beta 4 |
| PTEN | Phosphatase and Tensin homolog |
| qNPA | Quantitative nuclease protection assay |
| RNA | Ribonucleic acid |
| ROMS | Rotterdam Ocular Melanoma Study group |
| RT-PCR | Reverse transcription polymerase chain reaction |
| RT2-array | Qiagen RT2 miRNA PCR array (brain cancer panel) |
| S-100β | S100 calcium binding protein beta |
| SDHA | Succinate dehydrogenase |
| SERPINE1 | Plasminogen activator inhibitor, type 1 |
| SF3B1 | Splicing factor 3B unit 1 |
| SMART-seq | Switching Mechanism at 5′ end of RNA Template |
| sWGS | Shallow whole genome sequencing |
| THBS2 | Thrombospondin 2 |
| TLDA | Taqman low density array |
| TOP2A | DNA topoisomerase IIa |
| TTT | Transpupillary thermotherapy |
| UK | United Kingdom |
| ULBP2 | UL16-binding protein 2 |
| UM | Uveal melanoma |
| US | Ultrasonography |
| V | Protein V homolog |
| VA | Visual acuity |
| VEGF | Vascular endothelial growth factor |
| VGF | VGF nerve growth factor inducible |
| VH | Vitreous humor |
| γGT | Gamma glutamyl transferase |
References
- Smidt-Nielsen, I.; Bagger, M.; Heegaard, S.; Andersen, K.K.; Kiilgaard, J.F. Posterior uveal melanoma incidence and survival by AJCC tumour size in a 70-year nationwide cohort. Acta Ophthalmol. 2021, 99, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.K.; Di Nicola, M. Ocular Oncology—Primary and Metastatic Malignancies. Med. Clin. N. Am. 2021, 105, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Amaro, A.; Gangemi, R.; Piaggio, F.; Angelini, G.; Barisione, G.; Ferrini, S.; Pfeffer, U. The biology of uveal melanoma. Cancer Metastasis Rev. 2017, 36, 109–140. [Google Scholar] [CrossRef] [PubMed]
- Smit, K.N.; Jager, M.J.; de Klein, A.; Kiliҫ, E. Uveal melanoma: Towards a molecular understanding. Prog. Retin. Eye Res. 2020, 75, 100800. [Google Scholar] [CrossRef]
- Bol, K.F.; Van den Bosch, T.; Schreibelt, G.; Mensink, H.W.; Keunen, J.E.; Kiliç, E.; Japing, W.J.; Geul, K.W.; Westdorp, H.; Boudewijns, S. Adjuvant dendritic cell vaccination in high-risk uveal melanoma. Ophthalmology 2016, 123, 2265–2267. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis Prim. 2020, 6, 38. [Google Scholar] [CrossRef]
- Pelster, M.S.; Gruschkus, S.K.; Bassett, R.; Gombos, D.S.; Shephard, M.; Posada, L.; Glover, M.S.; Simien, R.; Diab, A.; Hwu, P. Nivolumab and ipilimumab in metastatic uveal melanoma: Results from a single-arm phase II study. J. Clin. Oncol. 2021, 39, 599–607. [Google Scholar] [CrossRef]
- Hoefsmit, E.P.; Rozeman, E.A.; Van, T.M.; Dimitriadis, P.; Krijgsman, O.; Conway, J.W.; da Silva, I.P.; van der Wal, J.E.; Ketelaars, S.L.; Bresser, K. Comprehensive analysis of cutaneous and uveal melanoma liver metastases. J. Immunother. Cancer 2020, 8, e001501. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.-F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Vichitvejpaisal, P.; Dalvin, L.A.; Mazloumi, M.; Ewens, K.G.; Ganguly, A.; Shields, C.L. Genetic analysis of uveal melanoma in 658 patients using the cancer genome atlas classification of uveal melanoma as A, B, C, and D. Ophthalmology 2019, 126, 1445–1453. [Google Scholar] [CrossRef]
- Drabarek, W.; Yavuzyigitoglu, S.; Obulkasim, A.; van Riet, J.; Smit, K.N.; van Poppelen, N.M.; Vaarwater, J.; Brands, T.; Eussen, B.; Verdijk, R.M. Multi-modality analysis improves survival prediction in enucleated uveal melanoma patients. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3595–3605. [Google Scholar] [CrossRef] [PubMed]
- Yavuzyigitoglu, S.; Koopmans, A.E.; Verdijk, R.M.; Vaarwater, J.; Eussen, B.; Van Bodegom, A.; Paridaens, D.; Kiliç, E.; de Klein, A.; Group, R.O.M.S. Uveal melanomas with SF3B1 mutations: A distinct subclass associated with late-onset metastases. Ophthalmology 2016, 123, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, T.; Simpson, R.; Grossniklaus, H.E.; Jager, M.J.; Singh, A.D.; Caminal, J.M.; Pavlick, A.C.; Kujala, E.; Coupland, S.E.; Finger, P.T. Uveal Melanoma. In AJCC Cancer Staging Manual; Amin, M.B., Edge, S.B., Greene, F.L., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; Volume 8, pp. 805–831. [Google Scholar]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell 2017, 23, 204–220.e215. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Mashayekhi, A.; Shields, J.A. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina 2012, 32, 1363–1372. [Google Scholar] [CrossRef]
- Bagger, M.; Smidt-Nielsen, I.; Andersen, M.K.; Jensen, P.K.; Heegaard, S.; Andersen, K.K.; Friis, S.; Kiilgaard, J.F. Long-term metastatic risk after biopsy of posterior uveal melanoma. Ophthalmology 2018, 125, 1969–1976. [Google Scholar] [CrossRef]
- Caminal, J.M.; Sanz, S.; Carreras, M.; Català, I.; Arruga, J.; Roca, G. Epibulbar seeding at the site of a transvitreal fine-needle aspiration biopsy. Arch. Ophthalmol. 2006, 124, 587–589. [Google Scholar] [CrossRef]
- Schefler, A.C.; Gologorsky, D.; Marr, B.P.; Shields, C.L.; Zeolite, I.; Abramson, D.H. Extraocular extension of uveal melanoma after fine-needle aspiration, vitrectomy, and open biopsy. JAMA Ophthalmol. 2013, 131, 1220–1224. [Google Scholar] [CrossRef]
- Wilham, J. Fine Needle Aspiration Biopsy with Adjunct i Immunohistochemistry in Intraocular Tumor Management. Acta Cytol. 2005, 49, 297–308. [Google Scholar]
- Cohen, V.; Dinakaran, S.; Parsons, M.; Rennie, I. Transvitreal fine needle aspiration biopsy: The influence of intraocular lesion size on diagnostic biopsy result. Eye 2001, 15, 143–147. [Google Scholar] [CrossRef]
- Singh, A.D.; Medina, C.A.; Singh, N.; Aronow, M.E.; Biscotti, C.V.; Triozzi, P.L. Fine-needle aspiration biopsy of uveal melanoma: Outcomes and complications. Br. J. Ophthalmol. 2016, 100, 456–462. [Google Scholar] [CrossRef]
- Bagger, M.; Tebering, J.F.; Kiilgaard, J.F. The ocular consequences and applicability of minimally invasive 25-gauge transvitreal retinochoroidal biopsy. Ophthalmology 2013, 120, 2565–2572. [Google Scholar] [CrossRef] [PubMed]
- Eleuteri, A.; Rola, A.C.; Kalirai, H.; Hussain, R.; Sacco, J.; Damato, B.E.; Heimann, H.; Coupland, S.E.; Taktak, A.F. Cost-utility analysis of a decade of liver screening for metastases using the Liverpool Uveal Melanoma Prognosticator Online (LUMPO). Comput. Biol. Med. 2021, 130, 104221. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Roszik, J.; Gombos, D.; Upshaw, J.; Sarli, V.; Meas, S.; Lucci, A.; Hall, C.; Patel, S. Pilot study of circulating tumor cells in early-stage and metastatic uveal melanoma. Cancers 2019, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Mouliere, F.; Chandrananda, D.; Piskorz, A.M.; Moore, E.K.; Morris, J.; Ahlborn, L.B.; Mair, R.; Goranova, T.; Marass, F.; Heider, K. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl. Med. 2018, 10, eaat492. [Google Scholar] [CrossRef]
- Beasley, A.; Isaacs, T.; Khattak, M.A.; Freeman, J.B.; Allcock, R.; Chen, F.K.; Pereira, M.R.; Yau, K.; Bentel, J.; Vermeulen, T.; et al. Clinical application of circulating tumor cells and circulating tumor DNA in uveal melanoma. JCO Precis. Oncol. 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Wisser, D.; van Ackern, K.; Knoll, E.; Wisser, H.; Bertsch, T. Blood loss from laboratory tests. Clin. Chem. 2003, 49, 1651–1655. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Rao, C.; Bui, T.; Connelly, M.; Doyle, G.; Karydis, I.; Middleton, M.; Clack, G.; Malone, M.; Coumans, F.; Terstappen, L. Circulating melanoma cells and survival in metastatic melanoma. Int. J. Oncol. 2011, 38, 755–760. [Google Scholar]
- Salvianti, F.; Pazzagli, M.; Pinzani, P. Single circulating tumor cell sequencing as an advanced tool in cancer management. Expert Rev. Mol. Diagn. 2016, 16, 51–63. [Google Scholar] [CrossRef]
- Kozminsky, M.; Fouladdel, S.; Chung, J.S.; Wang, Y.; Smith, D.C.; Alva, A.; Azizi, E.; Morgan, T.; Nagrath, S. Detection of CTC clusters and a dedifferentiated RNA-expression survival signature in prostate cancer. Adv. Sci. 2019, 6, 1801254. [Google Scholar] [CrossRef]
- Schuster, R.; Bechrakis, N.E.; Stroux, A.; Busse, A.; Schmittel, A.; Thiel, E.; Foerster, M.H.; Keilholz, U. Prognostic relevance of circulating tumor cells in metastatic uveal melanoma. Oncology 2011, 80, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Speicher, M. The biology of circulating tumor cells. Oncogene 2016, 35, 1216–1224. [Google Scholar] [CrossRef]
- Tan, W.; Liang, G.; Xie, X.; Jiang, W.; Tan, L.; Sanders, A.J.; Liu, Z.; Ling, Y.; Zhong, W.; Tian, Z. Incorporating MicroRNA into molecular phenotypes of circulating tumor cells enhances the prognostic accuracy for patients with metastatic breast cancer. Oncologist 2019, 24, e1044. [Google Scholar] [CrossRef] [PubMed]
- Abouleila, Y.; Onidani, K.; Ali, A.; Shoji, H.; Kawai, T.; Lim, C.T.; Kumar, V.; Okaya, S.; Kato, K.; Hiyama, E. Live single cell mass spectrometry reveals cancer-specific metabolic profiles of circulating tumor cells. Cancer Sci. 2019, 110, 697–706. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 2019, 176, 98–112. [Google Scholar] [CrossRef]
- Foss, A.J.E.; Guille, M.J.; Occleston, N.L.; Hykin, P.G.; Hungerford, J.L.; Lightman, S. The detection of melanoma cells in peripheral blood by reverse transcription-polymerase chain reaction. Br. J. Cancer 1995, 72, 155–159. [Google Scholar] [CrossRef]
- Tobal, K.; Sherman, L.S.; Foss, A.J.E.; Lightman, S.L. Detection of melanocytes from uveal melanoma in peripheral blood using the polymerase chain reaction. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2622–2625. [Google Scholar]
- Bidard, F.C.; Madic, J.; Mariani, P.; Piperno-Neumann, S.; Rampanou, A.; Servois, V.; Cassoux, N.; Desjardins, L.; Milder, M.; Vaucher, I.; et al. Detection rate and prognostic value of circulating tumor cells and circulating tumor DNA in metastatic uveal melanoma. Int. J. Cancer 2014, 134, 1207–1213. [Google Scholar] [CrossRef]
- Bande, M.F.; Santiago, M.; Muinelo-Romay, L.; Blanco, M.J.; Mera, P.; Capeans, C.; Pardo, M.; Piñeiro, A. Detection of circulating melanoma cells in choroidal melanocytic lesions. BMC Res. Notes 2015, 8, 452. [Google Scholar] [CrossRef]
- Tura, A.; Lüke, J.; Merz, H.; Reinsberg, M.; Lüke, M.; Jager, M.J.; Grisanti, S. Identification of circulating melanoma cells in uveal melanoma patients by dual-marker immunoenrichment. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4395–4404. [Google Scholar] [CrossRef]
- Mazzini, C.; Pinzani, P.; Salvianti, F.; Scatena, C.; Paglierani, M.; Ucci, F.; Pazzagli, M.; Massi, D. Circulating tumor cells detection and counting in uveal melanomas by a filtration-based method. Cancers 2014, 6, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef]
- Uguen, A. Digital Pathology Slides-based Measurement of Tumor Cells and Lymphocytes Within Cytology Samples Supports the Relevance of the Separation by Size of Nonhematological Tumor and Hematological Nontumor Cells in Liquid Biopsies. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Huebner, H.; Fasching, P.A.; Gumbrecht, W.; Jud, S.; Rauh, C.; Matzas, M.; Paulicka, P.; Friedrich, K.; Lux, M.P.; Volz, B. Filtration based assessment of CTCs and CellSearch® based assessment are both powerful predictors of prognosis for metastatic breast cancer patients. BMC Cancer 2018, 18, 204. [Google Scholar] [CrossRef] [PubMed]
- Tura, A.; Merz, H.; Reinsberg, M.; Lüke, M.; Jager, M.J.; Grisanti, S.; Lüke, J. Analysis of monosomy-3 in immunomagnetically isolated circulating melanoma cells in uveal melanoma patients. Pigment Cell Melanoma Res. 2016, 29, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Huang, Y.-Y.; Lane, N.; Huebschman, M.; Uhr, J.W.; Frenkel, E.P.; Zhang, X. Microchip-based immunomagnetic detection of circulating tumor cells. Lab Chip 2011, 11, 3449–3457. [Google Scholar] [CrossRef] [PubMed]
- Marques, G.S.; Silva, Z.; Videira, P.A. Antitumor efficacy of human monocyte-derived dendritic cells: Comparing effects of two monocyte isolation methods. Biol. Proced. Online 2018, 20, 4. [Google Scholar] [CrossRef]
- Ulmer, A.; Beutel, J.; Süsskind, D.; Hilgers, R.D.; Ziemssen, F.; Lüke, M.; Röcken, M.; Rohrbach, M.; Fierlbeck, G.; Bartz-Schmidt, K.U.; et al. Visualization of circulating melanoma cells in peripheral blood of patients with primary uveal melanoma. Clin. Cancer Res. 2008, 14, 4469–4474. [Google Scholar] [CrossRef]
- Suesskind, D.; Ulmer, A.; Schiebel, U.; Fierlbeck, G.; Spitzer, B.; Spitzer, M.S.; Bartz-Schmidt, K.U.; Grisanti, S. Circulating melanoma cells in peripheral blood of patients with uveal melanoma before and after different therapies and association with prognostic parameters: A pilot study. Acta Ophthalmol. 2011, 89, 17–24. [Google Scholar] [CrossRef]
- Beasley, A.B.; Isaacs, T.W.; Vermeulen, T.; Freeman, J.; DeSousa, J.-L.; Bhikoo, R.; Hennessy, D.; Reid, A.; Chen, F.K.; Bentel, J.; et al. Analysis of Circulating Tumour Cells in Early-Stage Uveal Melanoma: Evaluation of Tumour Marker Expression to Increase Capture. Cancers 2021, 13, 5990. [Google Scholar] [CrossRef]
- Cools-Lartigue, J.J.; McCauley, C.S.; Marshall, J.-C.A.; Di Cesare, S.; Gregoire, F.; Antecka, E.; Logan, P.; Burnier, M.N., Jr. Immunomagnetic isolation and in vitro expansion of human uveal melanoma cell lines. Mol. Vis. 2008, 14, 50. [Google Scholar] [PubMed]
- Campos, M.; Prior, C.; Warleta, F.; Zudaire, I.; Ruiz-Mora, J.; Catena, R.; Calvo, A.; José, J.G. Phenotypic and genetic characterization of circulating tumor cells by combining immunomagnetic selection and FICTION techniques. J. Histochem. Cytochem. 2008, 56, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Brase, J.C.; Schmidt, M.; Fischbach, T.; Sültmann, H.; Bojar, H.; Koelbl, H.; Hellwig, B.; Rahnenführer, J.; Hengstler, J.G.; Gehrmann, M.C. ERBB2 and TOP2A in breast cancer: A comprehensive analysis of gene amplification, RNA levels, and protein expression and their influence on prognosis and prediction. Clin. Cancer Res. 2010, 16, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Wang, Z.; Ni, X.; Duan, J.; Gao, Y.; Zhuo, M.; Li, R.; Zhao, J.; Ma, Q.; Bai, H. Inferring the evolution and progression of small-cell lung cancer by single-cell sequencing of circulating tumor cells. Clin. Cancer Res. 2019, 25, 5049–5060. [Google Scholar] [CrossRef]
- Zong, C.; Lu, S.; Chapman, A.R.; Xie, X.S. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science 2012, 338, 1622–1626. [Google Scholar] [CrossRef]
- De Luca, G.; Cardinali, B.; Del Mastro, L.; Lastraioli, S.; Carli, F.; Ferrarini, M.; Calin, G.A.; Garuti, A.; Mazzitelli, C.; Zupo, S. Optimization of a WGA-Free Molecular Tagging-Based NGS Protocol for CTCs Mutational Profiling. Int. J. Mol. Sci. 2020, 21, 4364. [Google Scholar] [CrossRef]
- Ramsköld, D.; Luo, S.; Wang, Y.-C.; Li, R.; Deng, Q.; Faridani, O.R.; Daniels, G.A.; Khrebtukova, I.; Loring, J.F.; Laurent, L.C. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat. Biotechnol. 2012, 30, 777–782. [Google Scholar] [CrossRef]
- Shi, F.; Jia, F.; Wei, Z.; Ma, Y.; Fang, Z.; Zhang, W.; Hu, Z. A Microfluidic Chip for Efficient Circulating Tumor Cells Enrichment, Screening, and Single-Cell RNA Sequencing. Proteomics 2021, 21, 2000060. [Google Scholar] [CrossRef]
- Lang, J.E.; Ring, A.; Porras, T.; Kaur, P.; Forte, V.A.; Mineyev, N.; Tripathy, D.; Press, M.F.; Campo, D. RNA-Seq of circulating tumor cells in stage II–III breast cancer. Ann. Surg. Oncol. 2018, 25, 2261–2270. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Hoon, D.S.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Alborelli, I.; Generali, D.; Jermann, P.; Cappelletti, M.R.; Ferrero, G.; Scaggiante, B.; Bortul, M.; Zanconati, F.; Nicolet, S.; Haegele, J. Cell-free DNA analysis in healthy individuals by next-generation sequencing: A proof of concept and technical validation study. Cell Death Dis. 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Swarup, V.; Rajeswari, M. Circulating (cell-free) nucleic acids—A promising, non-invasive tool for early detection of several human diseases. FEBS Lett. 2007, 581, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Valle-Inclan, J.E.; Stangl, C.; de Jong, A.C.; van Dessel, L.F.; van Roosmalen, M.J.; Helmijr, J.C.; Renkens, I.; Janssen, R.; de Blank, S.; de Witte, C.J.; et al. Optimizing Nanopore sequencing-based detection of structural variants enables individualized circulating tumor DNA-based disease monitoring in cancer patients. Genome Med. 2021, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, M.; Cheung, M.; Hillman, J.; Rassekh, S.R.; Deyell, R.J.; Batist, G.; Karsan, A.; Wyatt, A.W.; Johnson, N.; Scott, D.W. Evaluating the quantity, quality and size distribution of cell-free DNA by multiplex droplet digital PCR. Sci. Rep. 2020, 10, 12564. [Google Scholar] [CrossRef] [PubMed]
- Volik, S.; Alcaide, M.; Morin, R.D.; Collins, C. Cell-free DNA (cfDNA): Clinical significance and utility in cancer shaped by emerging technologies. Mol. Cancer Res. 2016, 14, 898–908. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Su, Y.; Yue, Z.; Xing, T.; Zhao, W.; Zhao, Q.; Duan, C.; Huang, C.; Zhang, D. Plasma cell-free DNA quantification is highly correlated to tumor burden in children with neuroblastoma. Cancer Med. 2018, 7, 3022–3030. [Google Scholar] [CrossRef]
- Park, J.J.; Diefenbach, R.J.; Byrne, N.; Long, G.V.; Scolyer, R.A.; Gray, E.S.; Carlino, M.S.; Rizos, H. Circulating Tumor DNA Reflects Uveal Melanoma Responses to Protein Kinase C Inhibition. Cancers 2021, 13, 1740. [Google Scholar] [CrossRef]
- Ma, J.; Weng, L.; Bastian, B.C.; Chen, X. Functional characterization of uveal melanoma oncogenes. Oncogene 2021, 40, 806–820. [Google Scholar] [CrossRef]
- Cabel, L.; Riva, F.; Servois, V.; Livartowski, A.; Daniel, C.; Rampanou, A.; Lantz, O.; Romano, E.; Milder, M.; Buecher, B.; et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: A proof-of-concept study. Ann. Oncol. 2017, 28, 1996–2001. [Google Scholar] [CrossRef]
- Nguyen, J.Q.N.; Drabarek, W.; Yavuzyigitoglu, S.; Medico Salsench, E.; Verdijk, R.M.; Naus, N.C.; de Klein, A.; Kiliç, E.; Brosens, E. Spliceosome Mutations in Uveal Melanoma. Int. J. Mol. Sci. 2020, 21, 9546. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Berman, E.L.; Zahler, J.D.; Hoberman, D.M.; Dinh, D.H.; Mashayekhi, A.; Shields, J.A. Choroidal nevus transformation into melanoma: Analysis of 2514 consecutive cases. Arch. Ophthalmol. 2009, 127, 981–987. [Google Scholar] [CrossRef]
- Bustamante, P.; Tsering, T.; Coblentz, J.; Mastromonaco, C.; Abdouh, M.; Fonseca, C.; Proença, R.P.; Blanchard, N.; Dugé, C.L.; Andujar, R.A.S. Circulating tumor DNA tracking through driver mutations as a liquid biopsy-based biomarker for uveal melanoma. J. Exp. Clin. Cancer Res. 2021, 40, 196. [Google Scholar] [CrossRef]
- Le Guin, C.H.; Bornfeld, N.; Bechrakis, N.E.; Jabbarli, L.; Richly, H.; Lohmann, D.R.; Zeschnigk, M. Early detection of metastatic uveal melanoma by the analysis of tumor-specific mutations in cell-free plasma DNA. Cancer Med. 2021, 10, 5974–5982. [Google Scholar] [CrossRef]
- Peneder, P.; Stutz, A.M.; Surdez, D.; Krumbholz, M.; Semper, S.; Chicard, M.; Sheffield, N.C.; Pierron, G.; Lapouble, E.; Totzl, M.; et al. Multimodal analysis of cell-free DNA whole-genome sequencing for pediatric cancers with low mutational burden. Nat. Commun. 2021, 12, 3230. [Google Scholar] [CrossRef]
- Adalsteinsson, V.A.; Ha, G.; Freeman, S.S.; Choudhury, A.D.; Stover, D.G.; Parsons, H.A.; Gydush, G.; Reed, S.C.; Rotem, D.; Rhoades, J. Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat. Commun. 2017, 8, 1324. [Google Scholar] [CrossRef]
- Stover, D.G.; Parsons, H.A.; Ha, G.; Freeman, S.S.; Barry, W.T.; Guo, H.; Choudhury, A.D.; Gydush, G.; Reed, S.C.; Rhoades, J. Association of cell-free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer. J. Clin. Oncol. 2018, 36, 543. [Google Scholar] [CrossRef]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.-J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef]
- Tsering, T.; Laskaris, A.; Abdouh, M.; Bustamante, P.; Parent, S.; Jin, E.; Ferrier, S.T.; Arena, G.; Burnier, J.V. Uveal Melanoma-Derived Extracellular Vesicles Display Transforming Potential and Carry Protein Cargo Involved in Metastatic Niche Preparation. Cancers 2020, 12, 2923. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S.; Takasugi, M.; Watanabe, S.; Kanemaki, M.T.; Obuse, C. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R. Reassessment of exosome composition. Cell 2019, 177, 428–445.e418. [Google Scholar] [CrossRef]
- Eldh, M.; Olofsson Bagge, R.; Lässer, C.; Svanvik, J.; Sjöstrand, M.; Mattsson, J.; Lindnér, P.; Choi, D.S.; Gho, Y.S.; Lötvall, J. MicroRNA in exosomes isolated directly from the liver circulation in patients with metastatic uveal melanoma. BMC Cancer 2014, 14, 962. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- An, M.; Wu, J.; Zhu, J.; Lubman, D.M. Comparison of an optimized ultracentrifugation method versus size-exclusion chromatography for isolation of exosomes from human serum. J. Proteome Res. 2018, 17, 3599–3605. [Google Scholar] [CrossRef]
- Baranyai, T.; Herczeg, K.; Onódi, Z.; Voszka, I.; Módos, K.; Marton, N.; Nagy, G.; Mäger, I.; Wood, M.J.; El Andaloussi, S. Isolation of exosomes from blood plasma: Qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS ONE 2015, 10, e0145686. [Google Scholar] [CrossRef]
- Wróblewska, J.P.; Lach, M.S.; Kulcenty, K.; Galus, Ł.; Suchorska, W.M.; Rösel, D.; Brábek, J.; Marszałek, A. The Analysis of Inflammation-Related Proteins in a Cargo of Exosomes Derived from the Serum of Uveal Melanoma Patients Reveals Potential Biomarkers of Disease Progression. Cancers 2021, 13, 3334. [Google Scholar] [CrossRef]
- Iorio, M.V.; Croce, C.M. MicroRNAs in cancer: Small molecules with a huge impact. J. Clin. Oncol. 2009, 27, 5848. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, M.; Barbagallo, C.; Statello, L.; Caltabiano, R.; Russo, A.; Puzzo, L.; Avitabile, T.; Longo, A.; Toro, M.D.; Barbagallo, D.; et al. miRNA profiling in vitreous humor, vitreal exosomes and serum from uveal melanoma patients: Pathological and diagnostic implications. Cancer Biol. Ther. 2015, 16, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, D.; Zucknick, M.; Wallwiener, M.; Cuk, K.; Modugno, C.; Scharpff, M.; Schott, S.; Heil, J.; Turchinovich, A.; Yang, R. Circulating miRNAs as surrogate markers for circulating tumor cells and prognostic markers in metastatic breast cancer. Clin. Cancer Res. 2012, 18, 5972–5982. [Google Scholar] [CrossRef]
- Smit, K.N.; Chang, J.; Derks, K.; Vaarwater, J.; Brands, T.; Verdijk, R.M.; Wiemer, E.A.; Mensink, H.W.; Pothof, J.; de Klein, A. Aberrant MicroRNA expression and its implications for uveal melanoma metastasis. Cancers 2019, 11, 815. [Google Scholar] [CrossRef]
- Achberger, S.; Aldrich, W.; Tubbs, R.; Crabb, J.W.; Singh, A.D.; Triozzi, P.L. Circulating immune cell and microRNA in patients with uveal melanoma developing metastatic disease. Mol. Immunol. 2014, 58, 182–186. [Google Scholar] [CrossRef]
- Triozzi, P.L.; Achberger, S.; Aldrich, W.; Crabb, J.W.; Saunthararajah, Y.; Singh, A.D. Association of tumor and plasma microRNA expression with tumor monosomy-3 in patients with uveal melanoma. Clin. Epigenetics 2016, 8, 80. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef]
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N. Serum microRNAs are promising novel biomarkers. PLoS ONE 2008, 3, e3148. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef]
- Kroh, E.M.; Parkin, R.K.; Mitchell, P.S.; Tewari, M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 2010, 50, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Aksenenko, M.; Palkina, N.; Komina, A.; Tashireva, L.; Ruksha, T. Differences in microRNA expression between melanoma and healthy adjacent skin. BMC Dermatol. 2019, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Cheng, Y. Inhibition of miR-20 promotes proliferation and autophagy in articular chondrocytes by PI3K/AKT/mTOR signaling pathway. Biomed. Pharmacother. 2018, 97, 607–615. [Google Scholar] [CrossRef]
- White, N.M.; Fatoohi, E.; Metias, M.; Jung, K.; Stephan, C.; Yousef, G.M. Metastamirs: A stepping stone towards improved cancer management. Nat. Rev. Clin. Oncol. 2011, 8, 75. [Google Scholar] [CrossRef]
- Wang, Y.C.; Yang, X.; Wei, W.B.; Xu, X.L. Role of microRNA-21 in uveal melanoma cell invasion and metastasis by regulating p53 and its downstream protein. Int. J. Ophthalmol. 2018, 11, 1258–1268. [Google Scholar]
- Saldanha, G.; Potter, L.; Lee, Y.S.; Watson, S.; Shendge, P.; Pringle, J.H. MicroRNA-21 expression and its pathogenetic significance in cutaneous melanoma. Melanoma Res. 2016, 26, 21–28. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, L.; Li, Y.; Zheng, Z.; Lin, X.; Yang, C. LncRNA MEG3 promotes melanoma growth, metastasis and formation through modulating miR-21/E-cadherin axis. Cancer Cell Int. 2020, 20, 12. [Google Scholar] [CrossRef]
- Cortez, M.A.; Ivan, C.; Valdecanas, D.; Wang, X.; Peltier, H.J.; Ye, Y.; Araujo, L.; Carbone, D.P.; Shilo, K.; Giri, D.K. PDL1 Regulation by p53 via miR-34. J. Natl. Cancer Inst. 2016, 108, djv303. [Google Scholar] [CrossRef]
- Heinemann, A.; Zhao, F.; Pechlivanis, S.; Eberle, J.; Steinle, A.; Diederichs, S.; Schadendorf, D.; Paschen, A. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Cancer Res. 2012, 72, 460–471. [Google Scholar] [CrossRef]
- Zhuang, L.; Yang, Y.; Ma, X.; Han, B.; Wang, Z.; Zhao, Q.; Wu, L.; Qu, Z. MicroRNA-92b promotes hepatocellular carcinoma progression by targeting Smad7 and is mediated by long non-coding RNA XIST. Cell Death Dis. 2016, 7, e2203. [Google Scholar] [CrossRef]
- Zhao, G.; Wei, Z.; Guo, Y. MicroRNA-107 is a novel tumor suppressor targeting POU3F2 in melanoma. Biol. Res. 2020, 53, 1. [Google Scholar] [CrossRef]
- Lu, Q.; Zhao, N.; Zha, G.; Wang, H.; Tong, Q.; Xin, S. LncRNA HOXA11-AS exerts oncogenic functions by repressing p21 and miR-124 in uveal melanoma. DNA Cell Biol. 2017, 36, 837–844. [Google Scholar] [CrossRef]
- Shen, C.; Hua, H.; Gu, L.; Cao, S.; Cai, H.; Yao, X.; Chen, X. miR-124 functions as a melanoma tumor suppressor by targeting RACK1. OncoTargets Ther. 2019, 12, 9975. [Google Scholar] [CrossRef]
- Wang, T.; Li, W.; Yin, S.; Chen, W. MiR-125b acts as a tumor suppressor of melanoma by targeting NCAM. J. BU ON 2021, 26, 182–188. [Google Scholar]
- Peng, J.; Liu, H.; Liu, C. MiR-155 promotes uveal melanoma cell proliferation and invasion by regulating NDFIP1 expression. Technol. Cancer Res. Treat. 2017, 16, 1160–1167. [Google Scholar] [CrossRef]
- Howitt, J.; Low, L.-H.; Putz, U.; Doan, A.; Lackovic, J.; Goh, C.-P.; Gunnersen, J.; Silke, J.; Tan, S.-S. Ndfip1 represses cell proliferation by controlling Pten localization and signaling specificity. J. Mol. Cell. Biol. 2015, 7, 119–131. [Google Scholar] [CrossRef]
- Souri, Z.; Wierenga, A.; Kiliç, E.; Brosens, E.; Böhringer, S.; Kroes, W.G.; Verdijk, R.M.; van Der Velden, P.A.; Luyten, G.P.; Jager, M.J. MiRNAs Correlate with HLA Expression in Uveal Melanoma: Both Up-and Downregulation Are Related to Monosomy 3. Cancers 2021, 13, 4020. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.; Li, F.; Pan, H.; Huang, X.; Wen, X.; Zhang, H.; Li, B.; Ge, S.; Xu, X. The miR-181 family promotes cell cycle by targeting CTDSPL, a phosphatase-like tumor suppressor in uveal melanoma. J. Exp. Clin. Cancer Res. 2018, 37, 15. [Google Scholar] [CrossRef]
- Fasanaro, P.; D’Alessandra, Y.; Di Stefano, V.; Melchionna, R.; Romani, S.; Pompilio, G.; Capogrossi, M.C.; Martelli, F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J. Biol. Chem. 2008, 283, 15878–15883. [Google Scholar] [CrossRef]
- Walbrecq, G.; Lecha, O.; Gaigneaux, A.; Fougeras, M.R.; Philippidou, D.; Margue, C.; Tetsi Nomigni, M.; Bernardin, F.; Dittmar, G.; Behrmann, I. Hypoxia-induced adaptations of miRNomes and proteomes in melanoma cells and their secreted extracellular vesicles. Cancers 2020, 12, 692. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, M.; Jiang, X.; Zhang, Z.; Dai, L.; Min, S.; Wu, X.; He, Q.; Liu, J.; Zhang, Y. miR-223 suppresses differentiation of tumor-induced CD11b+ Gr1+ myeloid-derived suppressor cells from bone marrow cells. Int. J. Cancer 2011, 129, 2662–2673. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- McKenna, K.C.; Beatty, K.M.; Bilonick, R.A.; Schoenfield, L.; Lathrop, K.L.; Singh, A.D. Activated CD11b+ CD15+ granulocytes increase in the blood of patients with uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4295–4303. [Google Scholar] [CrossRef] [PubMed]
- Sugano, T.; Masuda, M.; Takeshita, F.; Motoi, N.; Hirozane, T.; Goto, N.; Kashimoto, S.; Uno, Y.; Moriyama, H.; Sawa, M. Pharmacological blockage of transforming growth factor-β signalling by a Traf2-and Nck-interacting kinase inhibitor, NCB-0846. Br. J. Cancer 2021, 124, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Li, R.; Li, Y.J.; Yu, X.X.; Sun, Q.N.; Li, A.Y.; Kong, Y. eIF4E-related miR-320a and miR-340-5p inhibit endometrial carcinoma cell metastatic capability by preventing TGF-β1-induced epithelial-mesenchymal transition. Oncol. Rep. 2020, 43, 447–460. [Google Scholar] [CrossRef]
- Wei, S.; Ma, W. MiR-370 functions as oncogene in melanoma by direct targeting pyruvate dehydrogenase B. Biomed. Pharmacother. 2017, 90, 278–286. [Google Scholar] [CrossRef]
- He, J.; Xiao, B.; Li, X.; He, Y.; Li, L.; Sun, Z. MiR-486-5p suppresses proliferation and migration of hepatocellular carcinoma cells through downregulation of the E3 ubiquitin ligase CBL. BioMed Res. Int. 2019, 2019, 2732057. [Google Scholar] [CrossRef]
- Pisano, A.; Griñan-Lison, C.; Farace, C.; Fiorito, G.; Fenu, G.; Jiménez, G.; Scognamillo, F.; Peña-Martin, J.; Naccarati, A.; Pröll, J. The inhibitory role of mir-486-5p on csc phenotype has diagnostic and prognostic potential in colorectal cancer. Cancers 2020, 12, 3432. [Google Scholar] [CrossRef]
- Pardo, M.; García, Á.; Antrobus, R.; Blanco, M.J.; Dwek, R.A.; Zitzmann, N. Biomarker discovery from uveal melanoma secretomes: Identification of gp100 and cathepsin D in patient serum. J. Proteome Res. 2007, 6, 2802–2811. [Google Scholar] [CrossRef]
- Bande, M.F.; Santiago, M.; Mera, P. ME20-S as a Potential Biomarker for the Evaluation of Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2015, 12, 7007–7011. [Google Scholar] [CrossRef][Green Version]
- Angi, M.; Kalirai, H.; Prendergast, S.; Simpson, D.; Hammond, D.E.; Madigan, M.C.; Beynon, R.J.; Coupland, S.E. In-depth proteomic profiling of the uveal melanoma secretome. Oncotarget 2016, 7, 49623–49635. [Google Scholar] [CrossRef] [PubMed]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Barisione, G.; Fabbi, M.; Gino, A.; Queirolo, P.; Orgiano, L.; Spano, L.; Picasso, V.; Pfeffer, U.; Mosci, C.; Jager, M.J. Potential role of soluble c-Met as a new candidate biomarker of metastatic uveal melanoma. JAMA Ophthalmol. 2015, 133, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Tian, J.J.; Su, L.; Jing, Y.; Zhang, S.C.; Zhang, H.X.; Wang, X.Q.; Zhu, C.B. DJ-1: A promising marker in metastatic uveal melanoma. J. Cancer Res. Clin. Oncol. 2015, 141, 315–321. [Google Scholar] [CrossRef]
- Barak, V.; Frenkel, S.; Kalickman, I.; Maniotis, A.J.; Folberg, R.; Pe’er, J. Serum markers to detect metastatic uveal melanoma. Anticancer Res. 2007, 27, 1897–1900. [Google Scholar]
- Song, J.; Merbs, S.L.; Sokoll, L.J. A multiplex immunoassay of serum biomarkers for the detection of uveal melanoma. Clin. Proteomics 2019, 16, 10. [Google Scholar] [CrossRef]
- Chattopadhyay, C.; Oba, J.; Roszik, J.; Marszalek, J.R.; Chen, K.; Qi, Y.; Eterovic, K.; Gordon Robertson, A.; Burks, J.K.; McCannel, T.A.; et al. Elevated endogenous SDHA drives pathological metabolism in highly metastatic uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4187–4195. [Google Scholar] [CrossRef]
- Han, A.; Purwin, T.J.; Bechtel, N.; Liao, C.; Chua, V.; Seifert, E.; Sato, T.; Schug, Z.T.; Speicher, D.W.; Harbour, J.W. BAP1 mutant uveal melanoma is stratified by metabolic phenotypes with distinct vulnerability to metabolic inhibitors. Oncogene 2021, 40, 618–632. [Google Scholar] [CrossRef]
- Huang, L.; Wang, L.; Hu, X.; Chen, S.; Tao, Y.; Su, H.; Yang, J.; Xu, W.; Vedarethinam, V.; Wu, S. Machine learning of serum metabolic patterns encodes early-stage lung adenocarcinoma. Nat. Commun. 2020, 11, 3556. [Google Scholar] [CrossRef]
- Lorenzo, D.; Piulats, J.M.; Ochoa, M.; Arias, L.; Gutiérrez, C.; Català, J.; Cobos, E.; Garcia-Bru, P.; Dias, B.; Padrón-Pérez, N. Clinical predictors of survival in metastatic uveal melanoma. Jpn. J. Ophthalmol. 2019, 63, 197–209. [Google Scholar] [CrossRef]
- Diener-West, M.; Reynolds, S.M.; Agugliaro, D.J.; Caldwell, R.; Cumming, K.; Earle, J.D.; Green, D.L.; Hawkins, B.S.; Hayman, J.; Jaiyesimi, I. Screening for metastasis from choroidal melanoma: The collaborative ocular melanoma study group report 23. J. Clin. Oncol. 2004, 22, 2438–2444. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.; Foss, A.J.; Hungerford, J.L. Predictive power of screening tests for metastasis in uveal melanoma. Eye 1998, 12, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, T. Diagnosis of uveal melanoma. Dev. Ophthalmol. 2012, 49, 1–15. [Google Scholar] [PubMed]
- Grisanti, S.; Tura, A. Uveal Melanoma. In Noncutaneous Melanoma; Scott, J.F., Gerstenblith, M.R., Eds.; Codon Publications: Brisbane, Australia, 2018; pp. 1–18. [Google Scholar]
- Coleman, D.J.; Silverman, R.H.; Rondeau, M.J.; Boldt, H.C.; Lloyd, H.O.; Lizzi, F.L.; Weingeist, T.A.; Chen, X.; Vangveeravong, S.; Folberg, R. Noninvasive in vivo detection of prognostic indicators for high-risk uveal melanoma: Ultrasound parameter imaging. Ophthalmology 2004, 111, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.A.; Jaarsma-Coes, M.G.; Marinkovic, M.; Verbist, B.; Verdijk, R.M.; Jager, M.J.; Luyten, G.P.; Beenakker, J.-W.M. MR imaging characteristics of uveal melanoma with histopathological validation. Neuroradiology 2022, 64, 171–184. [Google Scholar] [CrossRef]
- Hee, M.R.; Izatt, J.A.; Swanson, E.A.; Huang, D.; Schuman, J.S.; Lin, C.P.; Puliafito, C.A.; Fujimoto, J.G. Optical coherence tomography of the human retina. Arch. Ophthalmol. 1995, 113, 325–332. [Google Scholar] [CrossRef]
- Yeung, L.; Lima, V.C.; Garcia, P.; Landa, G.; Rosen, R.B. Correlation between spectral domain optical coherence tomography findings and fluorescein angiography patterns in diabetic macular edema. Ophthalmology 2009, 116, 1158–1167. [Google Scholar] [CrossRef]
- Cennamo, G.; Romano, M.R.; Breve, M.; Velotti, N.; Reibaldi, M.; De Crecchio, G. Evaluation of choroidal tumors with optical coherence tomography: Enhanced depth imaging and OCT-angiography features. Eye 2017, 31, 906–915. [Google Scholar] [CrossRef]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, A.; Omae, T.; Tani, T.; Sogawa, K.; Yokota, H.; Yoshida, A. Optical coherence tomography angiography in diabetic retinopathy: A prospective pilot study. Am. J. Ophthalmol. 2015, 160, 35–44.e1. [Google Scholar] [CrossRef]
- Ali, Z.C.; Gray, J.; Balaskas, K. Features of choroidal naevi on swept source optical coherence tomography angiography and structural reverse flow optical coherence tomography. Graefe Arch. Clin. Exp. Ophthalmol. 2018, 256, 1319–1323. [Google Scholar] [CrossRef]
- Li, Y.; Say, E.A.; Ferenczy, S.; Agni, M.; Shields, C.L. Altered parafoveal microvasculature in treatment-naive choroidal melanoma eyes detected by optical coherence tomography angiography. Retina 2017, 37, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Greig, E.C.; Laver, N.V.; Mendonca, L.S.; Levine, E.S.; Baumal, C.R.; Waheed, N.K.; Duker, J.S. Swept-Source Optical Coherence Tomography Angiography in Small Choroidal Melanomas and Choroidal Nevi. Retina 2021, 41, 1182. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Corvi, F.; Invernizzi, A.; Ravera, V.; Cereda, M.G.; Staurenghi, G. Swept-source optical coherence tomography angiography in choroidal melanoma: An analysis of 22 consecutive cases. Retina 2019, 39, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.A.; Grech Fonk, L.; Jaarsma-Coes, M.G.; van Haren, G.G.; Marinkovic, M.; Beenakker, J.-W.M. MRI of uveal melanoma. Cancers 2019, 11, 377. [Google Scholar] [CrossRef]
- Wei, W.; Jia, G.; von Tengg-Kobligk, H.; Heverhagen, J.T.; Abdel-Rahman, M.; Wei, L.; Christoforidis, J.B.; Davidorf, F.; Knopp, M.V. Dynamic contrast-enhanced magnetic resonance imaging of ocular melanoma as a tool to predict metastatic potential. J. Comput. Assist. Tomogr. 2017, 41, 823–827. [Google Scholar] [CrossRef]
- Foti, P.V.; Longo, A.; Reibaldi, M.; Russo, A.; Privitera, G.; Spatola, C.; Raffaele, L.; Salamone, V.; Farina, R.; Palmucci, S. Uveal melanoma: Quantitative evaluation of diffusion-weighted MR imaging in the response assessment after proton-beam therapy, long-term follow-up. Radiol. Med. 2017, 122, 131–139. [Google Scholar] [CrossRef]
- Russo, A.; Mariotti, C.; Longo, A.; Foti, P.V.; Avitabile, T.; Uva, M.G.; Franco, L.M.; Bonfiglio, V.; Milone, P.; Ettorre, G.C. Diffusion-weighted magnetic resonance imaging and ultrasound evaluation of choroidal melanomas after proton-beam therapy. Radiol. Med. 2015, 120, 634–640. [Google Scholar] [CrossRef]
- Gordon, Y.; Partovi, S.; Müller-Eschner, M.; Amarteifio, E.; Bäuerle, T.; Weber, M.-A.; Kauczor, H.-U.; Rengier, F. Dynamic contrast-enhanced magnetic resonance imaging: Fundamentals and application to the evaluation of the peripheral perfusion. Cardiovasc. Diagn. Ther. 2014, 4, 147. [Google Scholar]
- Francis, J.H.; Patel, S.P.; Gombos, D.S.; Carvajal, R.D. Surveillance options for patients with uveal melanoma following definitive management. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 382–387. [Google Scholar] [CrossRef]
- Yavuzyigitoglu, S.; Tang, M.C.; Jansen, M.; Geul, K.W.; Dwarkasing, R.S.; Vaarwater, J.; Drabarek, W.; Verdijk, R.M.; Paridaens, D.; Naus, N.C. Radiological Patterns of Uveal Melanoma Liver Metastases in Correlation to Genetic Status. Cancers 2021, 13, 5316. [Google Scholar] [CrossRef]
- Papastefanou, V.P.; Islam, S.; Szyszko, T.; Grantham, M.; Sagoo, M.S.; Cohen, V.M. Metabolic activity of primary uveal melanoma on PET/CT scan and its relationship with monosomy 3 and other prognostic factors. Br. J. Ophthalmol. 2014, 98, 1659–1665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCANNEL, T.A.; Reddy, S.; Burgess, B.L.; Auerbach, M. Association of positive dual-modality positron emission tomography/computed tomography imaging of primary choroidal melanoma with chromosome 3 loss and tumor size. Retina 2010, 30, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Strobel, K.; Bode, B.; Dummer, R.; Veit-Haibach, P.; Fischer, D.; Imhof, L.; Goldinger, S.; Steinert, H.C.; Von Schulthess, G. Limited value of 18 F-FDG PET/CT and S-100B tumour marker in the detection of liver metastases from uveal melanoma compared to liver metastases from cutaneous melanoma. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1774–1782. [Google Scholar] [CrossRef]
- Orcurto, V.; Denys, A.; Voelter, V.; Schalenbourg, A.; Schnyder, P.; Zografos, L.; Leyvraz, S.; Delaloye, A.B.; Prior, J.O. 18F-fluorodeoxyglucose positron emission tomography/computed tomography and magnetic resonance imaging in patients with liver metastases from uveal melanoma: Results from a pilot study. Melanoma Res. 2012, 22, 63–69. [Google Scholar] [CrossRef]
- Ruhen, O.; Mirzai, B.; Clark, M.E.; Nguyen, B.; Salomon, C.; Erber, W.; Meehan, K. Comparison of circulating tumour dna and extracellular vesicle dna by low-pass whole-genome sequencing reveals molecular drivers of disease in a breast cancer patient. Biomedicines 2021, 9, 14. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Vincent, J.J.; Mortimer, S.; Vowles, J.V.; Ulrich, B.C.; Banks, K.C.; Fairclough, S.R.; Zill, O.A.; Sikora, M.; Mokhtari, R. Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue-and plasma-based methodologies. Clin. Cancer Res. 2018, 24, 3539–3549. [Google Scholar] [CrossRef]
- Lohr, J.G.; Adalsteinsson, V.A.; Cibulskis, K.; Choudhury, A.D.; Rosenberg, M.; Cruz-Gordillo, P.; Francis, J.M.; Zhang, C.-Z.; Shalek, A.K.; Satija, R. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat. Biotechnol. 2014, 32, 479–484. [Google Scholar] [CrossRef]

| Bead-Bound Antibody Used | Disease Status | CTC Count Median (Range) | Detection Rate | |
|---|---|---|---|---|
| n. | % | |||
| MCSP | Localized | 2.5 (1–5)/50 mL | 10/52 | 19% [49] |
| Localized | 1 (1–8)/50 mL | 13/94 | 14% [50] | |
| Localized | 2 (1–37)/8 mL * | 18/26 | 69% [26] | |
| ABCB5, gp100, MCAM, MCSP | Localized | 3 (1–89)/8 mL | 37/43 | 86% [51] |
| CD63 and gp100 | Localized | 3.5 (1–10)/10 mL | 29/31 | 94% [41] |
| MCAM (CellSearch) | Localized | 2 (1–3)/7.5 mL | 4/8 | 50% [40] |
| Localized | 1.5 (1–3)/10 mL * | 8/20 | 40% [24] | |
| Metastatic | 2 (1–38)/10 mL * | 13/19 | 68% [24] | |
| Metastatic | 3 (1–20)/7.5 mL * | 12/40 | 30% [39] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Bruyn, D.P.; Beasley, A.B.; Verdijk, R.M.; van Poppelen, N.M.; Paridaens, D.; de Keizer, R.O.B.; Naus, N.C.; Gray, E.S.; de Klein, A.; Brosens, E.; et al. Is Tissue Still the Issue? The Promise of Liquid Biopsy in Uveal Melanoma. Biomedicines 2022, 10, 506. https://doi.org/10.3390/biomedicines10020506
de Bruyn DP, Beasley AB, Verdijk RM, van Poppelen NM, Paridaens D, de Keizer ROB, Naus NC, Gray ES, de Klein A, Brosens E, et al. Is Tissue Still the Issue? The Promise of Liquid Biopsy in Uveal Melanoma. Biomedicines. 2022; 10(2):506. https://doi.org/10.3390/biomedicines10020506
Chicago/Turabian Stylede Bruyn, Daniël P., Aaron B. Beasley, Robert M. Verdijk, Natasha M. van Poppelen, Dion Paridaens, Ronald O. B. de Keizer, Nicole C. Naus, Elin S. Gray, Annelies de Klein, Erwin Brosens, and et al. 2022. "Is Tissue Still the Issue? The Promise of Liquid Biopsy in Uveal Melanoma" Biomedicines 10, no. 2: 506. https://doi.org/10.3390/biomedicines10020506
APA Stylede Bruyn, D. P., Beasley, A. B., Verdijk, R. M., van Poppelen, N. M., Paridaens, D., de Keizer, R. O. B., Naus, N. C., Gray, E. S., de Klein, A., Brosens, E., & Kiliç, E., on behalf of the Rotterdam Ocular Melanoma Study Group. (2022). Is Tissue Still the Issue? The Promise of Liquid Biopsy in Uveal Melanoma. Biomedicines, 10(2), 506. https://doi.org/10.3390/biomedicines10020506









