Parthenolide and Its Soluble Analogues: Multitasking Compounds with Antitumor Properties
Abstract
:1. Introduction
2. The Effect of Parthenolide on Gene Expression Profile
2.1. Parthenolide and Nuclear Factor Kappa B (NF-κB)
2.2. Parthenolide and STAT Transcription Factors
2.3. Parthenolide Interplay with Other Transcription Factors and Epigenetic Effects
3. Parthenolide Can Specifically Modulate Signal Transduction Pathways
3.1. Modulation of PKC and MAPKs by Parthenolide
3.2. Effects of Parthenolide on Stress Kinases
4. Parthenolide and Cell Death
4.1. Classic Apoptosis
4.2. Caspase Independent Cell Death
4.3. Autophagy
5. Parthenolide and Oxidative Stress
6. Parthenolide Selectivity in Tumor Cells and Targeting Cancer Stem Cells
6.1. Selective Action of Parthenolide in Tumor Cells
6.2. Parthenolide and Cancer Stem Cells
7. Pathenolide Therapeutic Potential and Parthenolide Analogues
7.1. Synergistic Action of PN with Other Coumponds
7.2. Parthenolide Anologues
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| ABCB1 | ATP-binding cassette subfamily B member 1 |
| ABCG2 | ATP-binding cassette subfamily G member 2 |
| AEBP1 | Adipocyte enhancer-binding protein 1 |
| AIF | Apoptosis inducing factor |
| AML | Acute myeloid leukemia |
| ATM | Ataxia-telangiectasia mutated |
| B-Raf | v-Raf murine sarcoma viral oncogene homolog B1 |
| BSO | Buthionine sulfoximine |
| C/EBP-α | CCAAT enhancer-binding protein alpha |
| CaM kinases | Ca2+/calmodulin-dependent protein kinases |
| CDK | Cyclin-dependent kinase |
| c-FLIP | Cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein |
| CML | Chronic myeloid leukemia |
| c-MYB | Cellular myeloblastosis |
| COX-2 | Cyclooxygenase-2 |
| CSCs | Cancer stem cells |
| DMAPT | Dimethylaminoparthenolide |
| DNMT1 | DNA (cytosine-5)-methyltransferase 1 |
| ERK | Extracellular signal-regulated kinase |
| fGn | Carboxyl-functionalized nanographene |
| FRA-1 | Fos-related antigen 1 |
| GBM | Glioblastoma |
| GSCs | Glioma stem cells |
| GSH | Glutathione |
| HCC | Hepatocellular carcinoma |
| HDAC1 | Histone deacetylase 1 |
| HMEC | Human mammary epithelial cells |
| HOXA-4 | Homeobox A4 |
| ICAM | Intercellular adhesion molecule |
| IFN-γ | Interferon gamma |
| IkB | Inhibitor of NF-κB |
| IKC | IkB kinase complex |
| IKKα | IkappaB kinase alpha |
| IKKβ | IkappaB kinase beta |
| IL-1 | Interleukin-1 |
| IL-2 | Interleukin-2 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LEF1 | Lymphoid enhancer-binding factor 1 |
| LSCs | Leukemia stem cells |
| MAPKs | Mitogen-activated protein kinases |
| MCL-1 | Myeloid cell leukemia 1 |
| MDM2 | Murine double minute 2 |
| MDR1 | Multidrug resistance protein 1 |
| MITF | Melanocyte-inducing transcription factor |
| MM | Multiple myeloma |
| MM-CSCs | Multiple myeloma cancer stem cells |
| Mn-SOD | Manganese superoxide dismutase |
| NAC | N-acetyl-l-cysteine |
| NEMO | NF-κB essential modulator |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOX | NADPH oxidase |
| NPC | Nasopharyngeal carcinoma cells |
| PCD | Programmed cell death |
| PGE2 | Prostaglandin E2 |
| PI3-K | Phosphoinositide 3-kinase |
| PKC | Protein kinase C |
| PLGA | Polylactide co-glycolide |
| PN | Parthenolide |
| RIP1 | Receptor-interacting protein 1 |
| ROS | Reactive oxygen species |
| SAHA | Suberoylanilide hydroxamic acid |
| SP1 | Specificity protein 1 |
| SRF | Serum response factor |
| STAT | Signal transducer and activator of transcription |
| STAT-3 | Signal transducer and activator of transcription 3 |
| TCF4 | Transcription factor 4 |
| TLR4 | Toll-like receptor 4 |
| TNBC | Triple-negative breast cancer |
| TNF-α | Tumor necrosis factor alpha |
| TRAIL | TNF-related apoptosis-inducing ligand |
| UVB | Ultraviolet B-rays |
| XBP1 | X-box binding protein 1 |
| XIAP | X-linked inhibitor of apoptosis |
References
- Sur, R.; Martin, K.; Liebel, F.; Lyte, P.; Shapiro, S.; Southall, M. Anti-Inflammatory Activity of Parthenolide-Depleted Feverfew (Tanacetum Parthenium). Inflammopharmacology 2009, 17, 42–49. [Google Scholar] [CrossRef]
- Freund, R.R.A.; Gobrecht, P.; Fischer, D.; Arndt, H.-D. Advances in Chemistry and Bioactivity of Parthenolide. Nat. Prod. Rep. 2020, 37, 541–565. [Google Scholar] [CrossRef]
- Juliana, C.; Fernandes-Alnemri, T.; Wu, J.; Datta, P.; Solorzano, L.; Yu, J.-W.; Meng, R.; Quong, A.A.; Latz, E.; Scott, C.P.; et al. Anti-Inflammatory Compounds Parthenolide and Bay 11-7082 Are Direct Inhibitors of the Inflammasome. J. Biol. Chem. 2010, 285, 9792–9802. [Google Scholar] [CrossRef] [Green Version]
- Ghantous, A.; Sinjab, A.; Herceg, Z.; Darwiche, N. Parthenolide: From Plant Shoots to Cancer Roots. Drug Discov. Today 2013, 18, 894–905. [Google Scholar] [CrossRef]
- Pajak, B.; Gajkowska, B.; Orzechowski, A. Molecular Basis of Parthenolide-Dependent Proapoptotic Activity in Cancer Cells. Folia Histochem. Cytobiol. 2008, 46, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Li-Weber, M.; Palfi, K.; Giaisi, M.; Krammer, P.H. Dual Role of the Anti-Inflammatory Sesquiterpene Lactone: Regulation of Life and Death by Parthenolide. Cell Death Differ. 2005, 12, 408–409. [Google Scholar] [CrossRef]
- Zhang, S.; Ong, C.-N.; Shen, H.-M. Critical Roles of Intracellular Thiols and Calcium in Parthenolide-Induced Apoptosis in Human Colorectal Cancer Cells. Cancer Lett. 2004, 208, 143–153. [Google Scholar] [CrossRef]
- Skalska, J.; Brookes, P.S.; Nadtochiy, S.M.; Hilchey, S.P.; Jordan, C.T.; Guzman, M.L.; Maggirwar, S.B.; Briehl, M.M.; Bernstein, S.H. Modulation of Cell Surface Protein Free Thiols: A Potential Novel Mechanism of Action of the Sesquiterpene Lactone Parthenolide. PLoS ONE 2009, 4, e8115. [Google Scholar] [CrossRef]
- Dawood, M.; Ooko, E.; Efferth, T. Collateral Sensitivity of Parthenolide via NF-κB and HIF-α Inhibition and Epigenetic Changes in Drug-Resistant Cancer Cell Lines. Front. Pharmacol. 2019, 10, 542. [Google Scholar] [CrossRef] [Green Version]
- Nam, Y.J.; Lee, D.H.; Lee, M.S.; Lee, C.S. Sesquiterpene Lactone Parthenolide Attenuates Production of Inflammatory Mediators by Suppressing the Toll-like Receptor-4-Mediated Activation of the Akt, MTOR, and NF-κB Pathways. Naunyn-Schmiedeberg’s Arch. Pharm. 2015, 388, 921–930. [Google Scholar] [CrossRef]
- López-Franco, O.; Hernández-Vargas, P.; Ortiz-Muñoz, G.; Sanjuán, G.; Suzuki, Y.; Ortega, L.; Blanco, J.; Egido, J.; Gómez-Guerrero, C. Parthenolide Modulates the NF-κB–Mediated Inflammatory Responses in Experimental Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1864–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Mohamed, M.R.; Kim, M.; Smallwood, S.; McFadden, G. Co-Regulation of NF-κB and Inflammasome-Mediated Inflammatory Responses by Myxoma Virus Pyrin Domain-Containing Protein M013. PLoS Pathog. 2009, 5, e1000635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-κB) Signaling in Cancer Development and Immune Diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef]
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer Cachexia, Mechanism and Treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef]
- Santos, J.M.O.; Moreira-Pais, A.; Neto, T.; Peixoto da Silva, S.; Oliveira, P.A.; Ferreira, R.; Mendes, J.; Bastos, M.M.S.M.; Lopes, C.; Casaca, F.; et al. Dimethylaminoparthenolide Reduces the Incidence of Dysplasia and Ameliorates a Wasting Syndrome in HPV16-Transgenic Mice. Drug Dev. Res. 2019, 80, 824–830. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Y.; Lv, J.; Guo, X.; Zhang, J.; Zhou, D.; Zhang, Z.; Xue, Z.; Yang, G.; Xi, Q.; et al. Parthenolide Regulates Oxidative Stress-Induced Mitophagy and Suppresses Apoptosis through P53 Signaling Pathway in C2C12 Myoblasts. J. Cell. Biochem. 2019, 120, 15695–15708. [Google Scholar] [CrossRef]
- Mathema, V.B.; Koh, Y.-S.; Thakuri, B.C.; Sillanpää, M. Parthenolide, a Sesquiterpene Lactone, Expresses Multiple Anti-Cancer and Anti-Inflammatory Activities. Inflammation 2012, 35, 560–565. [Google Scholar] [CrossRef]
- Wang, M.; Li, Q. Parthenolide Could Become a Promising and Stable Drug with Anti-Inflammatory Effects. Nat. Prod. Res. 2015, 29, 1092–1101. [Google Scholar] [CrossRef]
- Chen, C.-F.C.; Leung, A.Y.L.Y. Gene Response of Human Monocytic Cells for the Detection of Antimigraine Activity of Feverfew ExtractsThis Article Is One of a Selection of Papers Published in This Special Issue (Part 2 of 2) on the Safety and Efficacy of Natural Health Products. Can. J. Physiol. Pharmacol. 2007. [Google Scholar] [CrossRef]
- Brown, R.P.; Gerbarg, P.L. Herbs and Nutrients in the Treatment of Depression, Anxiety, Insomnia, Migraine, and Obesity. J. Psychiatr. Pract. 2001, 7, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Sztiller-Sikorska, M.; Czyz, M. Parthenolide as Cooperating Agent for Anti-Cancer Treatment of Various Malignancies. Pharmaceuticals 2020, 13, 194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Kim, S.L.; Park, Y.R.; Lee, S.-T.; Kim, S.W. Parthenolide Promotes Apoptotic Cell Death and Inhibits the Migration and Invasion of SW620 Cells. Intestig. Res. 2017, 15, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, Q.O.; Kong, Q.-J.; Yuan, W.; Yang, Y.-P.O. Parthenolide Induces Reactive Oxygen Species-Mediated Autophagic Cell Death in Human Osteosarcoma Cells. Cell. Physiol. Biochem. 2016, 40, 146–154. [Google Scholar] [CrossRef]
- Hexum, J.K.; Becker, C.M.; Kempema, A.M.; Ohlfest, J.R.; Largaespada, D.A.; Harki, D.A. Parthenolide Prodrug LC-1 Slows Growth of Intracranial Glioma. Bioorg. Med. Chem. Lett. 2015, 25, 2493–2495. [Google Scholar] [CrossRef]
- Guzman, M.L.; Rossi, R.M.; Neelakantan, S.; Li, X.; Corbett, C.A.; Hassane, D.C.; Becker, M.W.; Bennett, J.M.; Sullivan, E.; Lachowicz, J.L.; et al. An Orally Bioavailable Parthenolide Analog Selectively Eradicates Acute Myelogenous Leukemia Stem and Progenitor Cells. Blood 2007, 110, 4427–4435. [Google Scholar] [CrossRef]
- Shanmugam, R.; Kusumanchi, P.; Cheng, L.; Crooks, P.; Neelakantan, S.; Matthews, W.; Nakshatri, H.; Sweeney, C.J. A Water-Soluble Parthenolide Analogue Suppresses in Vivo Prostate Cancer Growth by Targeting NFκB and Generating Reactive Oxygen Species. Prostate 2010, 70, 1074–1086. [Google Scholar] [CrossRef]
- Avci, N.G.; Ebrahimzadeh-Pustchi, S.; Akay, Y.M.; Esquenazi, Y.; Tandon, N.; Zhu, J.-J.; Akay, M. NF-κB Inhibitor with Temozolomide Results in Significant Apoptosis in Glioblastoma via the NF-κB(P65) and Actin Cytoskeleton Regulatory Pathways. Sci. Rep. 2020, 10, 13352. [Google Scholar] [CrossRef]
- Arora, R.; Yates, C.; Gary, B.D.; McClellan, S.; Tan, M.; Xi, Y.; Reed, E.; Piazza, G.A.; Owen, L.B.; Dean-Colomb, W. Panepoxydone Targets NF-κB and FOXM1 to Inhibit Proliferation, Induce Apoptosis and Reverse Epithelial to Mesenchymal Transition in Breast Cancer. PLoS ONE 2014, 9, e98370. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Gupta, D.; Arora, R. NF-κB as a Key Player in Regulation of Cellular Radiation Responses and Identification of Radiation Countermeasures. Discoveries 2015, 3, e35. [Google Scholar] [CrossRef] [Green Version]
- Hellweg, C.E. The Nuclear Factor ΚB Pathway: A Link to the Immune System in the Radiation Response. Cancer Lett. 2015, 368, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, G.; Fredriksson, L.; Herpers, B.; Meerman, J.; van de Water, B.; de Graauw, M. TNF-α-Mediated NF-κB Survival Signaling Impairment by Cisplatin Enhances JNK Activation Allowing Synergistic Apoptosis of Renal Proximal Tubular Cells. Biochem. Pharmacol. 2013, 85, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.; Lutz, A.; Faletti, L.E.; Albrecht, U.; Thomas, M.; Bode, J.G.; Borner, C.; Sawodny, O.; Merfort, I. IL-1β and TNFα Differentially Influence NF-κB Activity and FasL-Induced Apoptosis in Primary Murine Hepatocytes during LPS-Induced Inflammation. Front. Physiol. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z. Crosstalk of Reactive Oxygen Species and NF-κB Signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Chaithongyot, S.; Jantaree, P.; Sokolova, O.; Naumann, M. NF-κB in Gastric Cancer Development and Therapy. Biomedicines 2021, 9, 870. [Google Scholar] [CrossRef]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Singh, T.G. Role of Nuclear Factor Kappa B (NF-κB) Signalling in Neurodegenerative Diseases: An Mechanistic Approach. Curr. Neuropharmacol. 2020, 18, 918–935. [Google Scholar] [CrossRef]
- Hehner, S.P.; Hofmann, T.G.; Dröge, W.; Schmitz, M.L. The Antiinflammatory Sesquiterpene Lactone Parthenolide Inhibits NF-κB by Targeting the IκB Kinase Complex. J. Immunol. 1999, 163, 5617–5623. [Google Scholar]
- Kwok, B.H.B.; Koh, B.; Ndubuisi, M.I.; Elofsson, M.; Crews, C.M. The Anti-Inflammatory Natural Product Parthenolide from the Medicinal Herb Feverfew Directly Binds to and Inhibits IκB Kinase. Chem. Biol. 2001, 8, 759–766. [Google Scholar] [CrossRef] [Green Version]
- Idris, A.I.; Libouban, H.; Nyangoga, H.; Landao-Bassonga, E.; Chappard, D.; Ralston, S.H. Pharmacologic Inhibitors of IκB Kinase Suppress Growth and Migration of Mammary Carcinosarcoma Cells in Vitro and Prevent Osteolytic Bone Metastasis in Vivo. Mol. Cancer 2009, 8, 2339–2347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, K.H.M.; Zheng, M.H.; Feng, H.T.; Steer, J.H.; Joyce, D.A.; Xu, J. Sesquiterpene Lactone Parthenolide Blocks Lipopolysaccharide-Induced Osteolysis Through the Suppression of NF-κB Activity. J. Bone Miner. Res. 2004, 19, 1905–1916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lin, Z.-N.; Yang, C.-F.; Shi, X.; Ong, C.-N.; Shen, H.-M. Suppressed NF-κB and Sustained JNK Activation Contribute to the Sensitization Effect of Parthenolide to TNF-α-Induced Apoptosis in Human Cancer Cells. Carcinogenesis 2004, 25, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Banik, K.; Kunnumakkara, A.B.; Kubatka, P.; Koklesova, L.; Shakibaei, M. Targeting NF-κB Signaling by Calebin A, a Compound of Turmeric, in Multicellular Tumor Microenvironment: Potential Role of Apoptosis Induction in CRC Cells. Biomedicines 2020, 8, 236. [Google Scholar] [CrossRef]
- Park, S.H.; Riley, P.; Frisch, S.M. Regulation of Anoikis by Deleted in Breast Cancer-1 (DBC1) through NF-κB. Apoptosis 2013, 18, 949–962. [Google Scholar] [CrossRef] [Green Version]
- Verzella, D.; Pescatore, A.; Capece, D.; Vecchiotti, D.; Ursini, M.V.; Franzoso, G.; Alesse, E.; Zazzeroni, F. Life, Death, and Autophagy in Cancer: NF-κB Turns up Everywhere. Cell Death Dis. 2020, 11, 210. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an Active Player in Human Cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-L.; Kim, S.H.; Park, Y.R.; Liu, Y.-C.; Kim, E.-M.; Jeong, H.-J.; Kim, Y.N.; Seo, S.Y.; Kim, I.H.; Lee, S.O.; et al. Combined Parthenolide and Balsalazide Have Enhanced Antitumor Efficacy Through Blockade of NF-κB Activation. Mol. Cancer Res. 2017, 15, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-L.; Kim, S.H.; Trang, K.T.T.; Kim, I.H.; Lee, S.-O.; Lee, S.T.; Kim, D.G.; Kang, S.-B.; Kim, S.-W. Synergistic Antitumor Effect of 5-Fluorouracil in Combination with Parthenolide in Human Colorectal Cancer. Cancer Lett. 2013, 335, 479–486. [Google Scholar] [CrossRef]
- Mendonca, M.S.; Chin-Sinex, H.; Gomez-Millan, J.; Datzman, N.; Hardacre, M.; Comerford, K.; Nakshatri, H.; Nye, M.; Benjamin, L.; Mehta, S.; et al. Parthenolide Sensitizes Cells to X-Ray-Induced Cell Killing through Inhibition of NF-κB and Split-Dose Repair. Radiat. Res. 2007, 168, 689–697. [Google Scholar] [CrossRef]
- Sun, Y.; Clair, D.K.S.; Fang, F.; Warren, G.W.; Rangnekar, V.M.; Crooks, P.A.; Clair, W.H.S. The Radiosensitization Effect of Parthenolide in Prostate Cancer Cells Is Mediated by Nuclear Factor-ΚB Inhibition and Enhanced by the Presence of PTEN. Mol. Cancer 2007, 6, 2477–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, L.; Wu, L.; Zhou, D. Sensitization of Tumor Cells to Cancer Therapy by Molecularly Targeted Inhibition of the Inhibitor of Nuclear Factor ΚB Kinase. Transl. Cancer Res. 2012, 1, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Morel, K.L.; Ormsby, R.J.; Bezak, E.; Sweeney, C.J.; Sykes, P.J. Parthenolide Selectively Sensitizes Prostate Tumor Tissue to Radiotherapy While Protecting Healthy Tissues In Vivo. Radiat. Res. 2017, 187, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wang, L.; Yu, H.; Chen, D.; Zhu, W.; Sun, C. Pharmacological Effects of Polyphenol Phytochemicals on the JAK-STAT Signaling Pathway. Front. Pharmacol. 2021, 12, 2350. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, Y.; Tilborghs, S.; Jacobs, J.; De Waele, J.; Quatannens, D.; Deben, C.; Prenen, H.; Pauwels, P.; Trinh, X.B.; Wouters, A.; et al. The Potential and Controversy of Targeting STAT Family Members in Cancer. Semin. Cancer Biol. 2020, 60, 41–56. [Google Scholar] [CrossRef]
- Awasthi, N.; Liongue, C.; Ward, A.C. STAT Proteins: A Kaleidoscope of Canonical and Non-Canonical Functions in Immunity and Cancer. J. Hematol. Oncol. 2021, 14, 198. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. 2021, 6, 402. [Google Scholar] [CrossRef]
- Arumuggam, N.; Bhowmick, N.A.; Rupasinghe, H.P.V. A Review: Phytochemicals Targeting JAK/STAT Signaling and IDO Expression in Cancer. Phytother. Res. 2015, 29, 805–817. [Google Scholar] [CrossRef]
- Heppler, L.N.; Frank, D.A. Targeting Oncogenic Transcription Factors: Therapeutic Implications of Endogenous STAT Inhibitors. Trends Cancer 2017, 3, 816–827. [Google Scholar] [CrossRef]
- Tolomeo, M.; Cascio, A. The Multifaced Role of STAT3 in Cancer and Its Implication for Anticancer Therapy. Int. J. Mol. Sci. 2021, 22, 603. [Google Scholar] [CrossRef]
- Tošić, I.; Frank, D.A. STAT3 as a Mediator of Oncogenic Cellular Metabolism: Pathogenic and Therapeutic Implications. Neoplasia 2021, 23, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liao, S.; Bennett, S.; Tang, H.; Song, D.; Wood, D.; Zhan, X.; Xu, J. STAT3 and Its Targeting Inhibitors in Osteosarcoma. Cell Prolif. 2021, 54, e12974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, B.; Gaikwad, A.S.; Haridas, V.; Xu, Z.; Gutterman, J.U.; Duvic, M. Avicin D Selectively Induces Apoptosis and Downregulates P-STAT-3, Bcl-2, and Survivin in Cutaneous T-Cell Lymphoma Cells. J. Investig. Dermatol. 2008, 128, 2728–2735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, C.-Y.; Arya, A.; Naema, A.F.; Wong, W.F.; Sethi, G.; Looi, C.Y. Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication. Front. Oncol. 2019, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlisi, D.; D’Anneo, A.; Angileri, L.; Lauricella, M.; Emanuele, S.; Santulli, A.; Vento, R.; Tesoriere, G. Parthenolide Sensitizes Hepatocellular Carcinoma Cells to Trail by Inducing the Expression of Death Receptors through Inhibition of STAT3 Activation. J. Cell. Physiol. 2011, 226, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Sobota, R.; Szwed, M.; Kasza, A.; Bugno, M.; Kordula, T. Parthenolide Inhibits Activation of Signal Transducers and Activators of Transcription (STATs) Induced by Cytokines of the IL-6 Family. Biochem. Biophys. Res. Commun. 2000, 267, 329–333. [Google Scholar] [CrossRef]
- Liu, M.; Xiao, C.; Sun, M.; Tan, M.; Hu, L.; Yu, Q. Parthenolide Inhibits STAT3 Signaling by Covalently Targeting Janus Kinases. Molecules 2018, 23, 1478. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.-Y.; Wang, G.; Zhang, J.; Song, J.; Jiang, Y.-C.; Dou, J.-Y.; Lian, L.-H.; Nan, J.-X.; Wu, Y.-L. Parthenolide, Bioactive Compound of Chrysanthemum parthenium L., Ameliorates Fibrogenesis and Inflammation in Hepatic Fibrosis via Regulating the Crosstalk of TLR4 and STAT3 Signaling Pathway. Phytother. Res. 2021, 35, 5680–5693. [Google Scholar] [CrossRef]
- Li, H.; Lu, H.; Lv, M.; Wang, Q.; Sun, Y. Parthenolide Facilitates Apoptosis and Reverses Drug-Resistance of Human Gastric Carcinoma Cells by Inhibiting the STAT3 Signaling Pathway. Oncol. Lett. 2018, 15, 3572–3579. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, S.; Zahedpanah, M.; Nikbakht, M.; Shaiegan, M.; Hamidollah, G.; Nikugoftar, M.; Rahmani, B.; Hamedi Asl, D. Parthenolide Reduces Gene Transcription of Prosurvival Mediators in U937 Cells. Exp. Oncol. 2017, 39, 30–35. [Google Scholar] [CrossRef]
- Wu, Z.; Nicoll, M.; Ingham, R.J. AP-1 Family Transcription Factors: A Diverse Family of Proteins That Regulate Varied Cellular Activities in Classical Hodgkin Lymphoma and ALK+ ALCL. Exp. Hematol. Oncol. 2021, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Atsaves, V.; Leventaki, V.; Rassidakis, G.Z.; Claret, F.X. AP-1 Transcription Factors as Regulators of Immune Responses in Cancer. Cancers 2019, 11, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, M.L.; Talar, B.; Sztiller-Sikorska, M.; Nejc, D.; Czyz, M. Parthenolide Induces MITF-M Downregulation and Senescence in Patient-Derived MITF-M High Melanoma Cell Populations. Oncotarget 2016, 7, 9026–9040. [Google Scholar] [CrossRef]
- Zhang, J.; Mou, Y.; Gong, H.; Chen, H.; Xiao, H. Microphthalmia-Associated Transcription Factor in Senescence and Age-Related Diseases. Gerontology 2021, 67, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Bourseguin, J.; Bonet, C.; Renaud, E.; Pandiani, C.; Boncompagni, M.; Giuliano, S.; Pawlikowska, P.; Karmous-Benailly, H.; Ballotti, R.; Rosselli, F.; et al. FANCD2 Functions as a Critical Factor Downstream of MiTF to Maintain the Proliferation and Survival of Melanoma Cells. Sci. Rep. 2016, 6, 36539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trejo-Solis, C.; Escamilla-Ramirez, A.; Jimenez-Farfan, D.; Castillo-Rodriguez, R.A.; Flores-Najera, A.; Cruz-Salgado, A. Crosstalk of the Wnt/β-Catenin Signaling Pathway in the Induction of Apoptosis on Cancer Cells. Pharmaceuticals 2021, 14, 871. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-Catenin Signaling in Cancers and Targeted Therapies. Signal Transduct. Target. 2021, 6, 307. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, C.; Tian, C.; Li, C.; Nie, F.; Song, X.; Zeng, R.; Wu, D.; Hao, X.; Li, L. The Plant Sesquiterpene Lactone Parthenolide Inhibits Wnt/β-Catenin Signaling by Blocking Synthesis of the Transcriptional Regulators TCF4/LEF1. J. Biol. Chem. 2018, 293, 5335–5344. [Google Scholar] [CrossRef] [Green Version]
- Lees, A.; Sessler, T.; McDade, S. Dying to Survive—The P53 Paradox. Cancers 2021, 13, 3257. [Google Scholar] [CrossRef]
- Lukin, D.J.; Carvajal, L.A.; Liu, W.; Resnick-Silverman, L.; Manfredi, J.J. P53 Promotes Cell Survival Due to the Reversibility of Its Cell-Cycle Checkpoints. Mol. Cancer Res. 2015, 13, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Guadagno, J.; Swan, P.; Shaikh, R.; Cregan, S.P. Microglia-Derived IL-1β Triggers P53-Mediated Cell Cycle Arrest and Apoptosis in Neural Precursor Cells. Cell Death Dis. 2015, 6, e1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, H.; Sun, D.; Zhang, X. The Role of MDM2 Amplification and Overexpression in Therapeutic Resistance of Malignant Tumors. Cancer Cell Int. 2019, 19, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopal, Y.N.V.; Chanchorn, E.; Dyke, M.W.V. Parthenolide Promotes the Ubiquitination of MDM2 and Activates P53 Cellular Functions. Mol. Cancer 2009, 8, 552–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Fatlawi, A.A.; Al-Fatlawi, A.A.; Irshad, M.; Rahisuddin; Ahmad, A. Effect of parthenolide on growth and apoptosis regulatory genes of human cancer cell lines. Pharm. Biol. 2015, 53, 104–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talib, W.H.; Al Kury, L.T. Parthenolide Inhibits Tumor-Promoting Effects of Nicotine in Lung Cancer by Inducing P53—Dependent Apoptosis and Inhibiting VEGF Expression. Biomed. Pharmacother. 2018, 107, 1488–1495. [Google Scholar] [CrossRef]
- Steele, A.J.; Jones, D.T.; Ganeshaguru, K.; Duke, V.M.; Yogashangary, B.C.; North, J.M.; Lowdell, M.W.; Kottaridis, P.D.; Mehta, A.B.; Prentice, A.G.; et al. The Sesquiterpene Lactone Parthenolide Induces Selective Apoptosis of B-Chronic Lymphocytic Leukemia Cells in Vitro. Leukemia 2006, 20, 1073–1079. [Google Scholar] [CrossRef]
- Ghantous, A.; Saikali, M.; Rau, T.; Gali-Muhtasib, H.; Schneider-Stock, R.; Darwiche, N. Inhibition of Tumor Promotion by Parthenolide: Epigenetic Modulation of P21. Cancer Prev. Res. 2012, 5, 1298–1309. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.; Sarkar, M.; Giri, B. Anti-Inflammatory and Anti-Tumor Activities of Parthenolide: An Update. J. Chem. Biol. 2016, 1, 107. [Google Scholar] [CrossRef] [Green Version]
- Gopal, Y.N.V.; Arora, T.S.; Van Dyke, M.W. Parthenolide Specifically Depletes Histone Deacetylase 1 Protein and Induces Cell Death through Ataxia Telangiectasia Mutated. Chem. Biol. 2007, 14, 813–823. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Liu, S.; Xie, Z.; Pavlovicz, R.E.; Wu, J.; Chen, P.; Aimiuwu, J.; Pang, J.; Bhasin, D.; Neviani, P.; et al. Modulation of DNA Methylation by a Sesquiterpene Lactone Parthenolide. J. Pharm. Exp. 2009, 329, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Irshad, R.; Husain, M. Natural Products in the Reprogramming of Cancer Epigenetics. Toxicol. Appl. Pharmacol. 2021, 417, 115467. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.-K.; Ong, C.-N.; Shen, H.-M. Parthenolide Sensitizes Ultraviolet (UV)-B-Induced Apoptosis via Protein Kinase C-Dependent Pathways. Carcinogenesis 2005, 26, 2149–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Danilenko, M.; Kim, T.S. Differential Enhancement of Leukaemia Cell Differentiation without Elevation of Intracellular Calcium by Plant-Derived Sesquiterpene Lactone Compounds. Br. J. Pharm. 2008, 155, 814–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, B.T.; Hurt, E.M.; Kalathur, M.; Duhagon, M.A.; Milner, J.A.; Kim, Y.S.; Farrar, W.L. Effects of the Sesquiterpene Lactone Parthenolide on Prostate Tumor-Initiating Cells: An Integrated Molecular Profiling Approach. Prostate 2009, 69, 827–837. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.; Bi, H.; Yan, Y.; Huang, W.; Zhang, G.; Zhang, G.; Tang, S.; Liu, Y.; Zhang, L.; Ma, J.; et al. Parthenolide Suppresses Non-Small Cell Lung Cancer GLC-82 Cells Growth via B-Raf/MAPK/Erk Pathway. Oncotarget 2017, 8, 23436–23447. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Huang, R.; Li, M.; Zhu, Z.; Chen, Z.; Cui, L.; Luo, H.; Luo, L. Parthenolide Inhibits the Growth of Non-Small Cell Lung Cancer by Targeting Epidermal Growth Factor Receptor. Cancer Cell Int. 2020, 20, 561. [Google Scholar] [CrossRef]
- Berdan, C.A.; Ho, R.; Lehtola, H.S.; To, M.; Hu, X.; Huffman, T.R.; Petri, Y.; Altobelli, C.R.; Demeulenaere, S.G.; Olzmann, J.A.; et al. Parthenolide Covalently Targets and Inhibits Focal Adhesion Kinase in Breast Cancer Cells. Cell Chem. Biol. 2019, 26, 1027–1035. [Google Scholar] [CrossRef]
- Li, X.; Kong, L.; Yang, Q.; Duan, A.; Ju, X.; Cai, B.; Chen, L.; An, T.; Li, Y. Parthenolide Inhibits Ubiquitin-Specific Peptidase 7 (USP7), Wnt Signaling, and Colorectal Cancer Cell Growth. J. Biol. Chem. 2020, 295, 3576–3589. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [Green Version]
- Ki, Y.-W.; Park, J.H.; Lee, J.E.; Shin, I.C.; Koh, H.C. JNK and P38 MAPK Regulate Oxidative Stress and the Inflammatory Response in Chlorpyrifos-Induced Apoptosis. Toxicol. Lett. 2013, 218, 235–245. [Google Scholar] [CrossRef]
- Gao, H.-E.; Sun, Y.; Ding, Y.-H.; Long, J.; Liu, X.-L.; Yang, M.; Ji, Q.; Li, Y.-H.; Chen, Y.; Zhang, Q.; et al. Antineoplastic Effects of CPPTL via the ROS/JNK Pathway in Acute Myeloid Leukemia. Oncotarget 2017, 8, 38990–39000. [Google Scholar] [CrossRef] [Green Version]
- Nakshatri, H.; Rice, S.E.; Bhat-Nakshatri, P. Antitumor Agent Parthenolide Reverses Resistance of Breast Cancer Cells to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand through Sustained Activation of c-Jun N-Terminal Kinase. Oncogene 2004, 23, 7330–7344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. P38 and JNK MAPK Pathways Control the Balance of Apoptosis and Autophagy in Response to Chemotherapeutic Agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Achkar, I.W.; Abdulrahman, N.; Al-Sulaiti, H.; Joseph, J.M.; Uddin, S.; Mraiche, F. Cisplatin Based Therapy: The Role of the Mitogen Activated Protein Kinase Signaling Pathway. J. Transl. Med. 2018, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, E.F.; Nebreda, Á.R. Signal Integration by JNK and P38 MAPK Pathways in Cancer Development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Auh, S.; Blokh, L.; Long, C.; Gagnon, I.; Hamann, K.J. TNF-α Induces Transient Resistance to Fas-Induced Apoptosis in Eosinophilic Acute Myeloid Leukemia Cells. Mol. Immunol. 2007, 4, 10. [Google Scholar]
- Chen, Y.-T.; Lin, C.-W.; Su, C.-W.; Yang, W.-E.; Chuang, C.-Y.; Su, S.-C.; Hsieh, M.-J.; Yang, S.-F. Magnolol Triggers Caspase-Mediated Apoptotic Cell Death in Human Oral Cancer Cells through JNK1/2 and P38 Pathways. Biomedicines 2021, 9, 1295. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell Death: A Review of the Major Forms of Apoptosis, Necrosis and Autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Chen, Q.; Kang, J.; Fu, C. The Independence of and Associations among Apoptosis, Autophagy, and Necrosis. Signal Transduct. Target. 2018, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Liu, X.; Su, L. Parthenolide Induces Apoptosis via TNFRSF10B and PMAIP1 Pathways in Human Lung Cancer Cells. J. Exp. Clin. Cancer Res. 2014, 33, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suvannasankha, A.; Crean, C.D.; Shanmugam, R.; Farag, S.S.; Abonour, R.; Boswell, H.S.; Nakshatri, H. Antimyeloma Effects of a Sesquiterpene Lactone Parthenolide. Clin. Cancer Res. 2008, 14, 1814–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karam, L.; Abou Staiteieh, S.; Chaaban, R.; Hayar, B.; Ismail, B.; Neipel, F.; Darwiche, N.; Abou Merhi, R. Anticancer Activities of Parthenolide in Primary Effusion Lymphoma Preclinical Models. Mol. Carcinog. 2021, 60, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, S.; Oddo, E.; D’Anneo, A.; Notaro, A.; Calvaruso, G.; Lauricella, M.; Giuliano, M. Routes to Cell Death in Animal and Plant Kingdoms: From Classic Apoptosis to Alternative Ways to Die—A Review. Rend. Lincei. Sci. Fis. Nat. 2018, 29, 397–409. [Google Scholar] [CrossRef]
- Pozarowski, P.; Halicka, D.H.; Darzynkiewicz, Z. Cell Cycle Effects and Caspase-Dependent and Independent Death of HL-60 and Jurkat Cells Treated with the Inhibitor of NF-KappaB Parthenolide. Cell Cycle 2003, 2, 377–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Anneo, A.; Carlisi, D.; Lauricella, M.; Puleio, R.; Martinez, R.; Di Bella, S.; Di Marco, P.; Emanuele, S.; Di Fiore, R.; Guercio, A.; et al. Parthenolide Generates Reactive Oxygen Species and Autophagy in MDA-MB231 Cells. A Soluble Parthenolide Analogue Inhibits Tumour Growth and Metastasis in a Xenograft Model of Breast Cancer. Cell Death Dis. 2013, 4, e891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Anneo, A.; Carlisi, D.; Lauricella, M.; Emanuele, S.; Di Fiore, R.; Vento, R.; Tesoriere, G. Parthenolide Induces Caspase-Independent and AIF-Mediated Cell Death in Human Osteosarcoma and Melanoma Cells. J. Cell. Physiol. 2013, 228, 952–967. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Tang, X.; Shao, X.; Feng, L.; Wu, N.; Yao, K. Parthenolide Protects Human Lens Epithelial Cells from Oxidative Stress-Induced Apoptosis via Inhibition of Activation of Caspase-3 and Caspase-9. Cell Res. 2007, 17, 565–571. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [Green Version]
- Emanuele, S.; Lauricella, M.; D’Anneo, A.; Carlisi, D.; De Blasio, A.; Di Liberto, D.; Giuliano, M. P62: Friend or Foe? Evidences for OncoJanus and NeuroJanus Roles. Int. J. Mol. Sci. 2020, 21, 5029. [Google Scholar] [CrossRef]
- Lei, Y.; Klionsky, D.J. The Emerging Roles of Autophagy in Human Diseases. Biomedicines 2021, 9, 1651. [Google Scholar] [CrossRef] [PubMed]
- Kriel, J.; Loos, B. The Good, the Bad and the Autophagosome: Exploring Unanswered Questions of Autophagy-Dependent Cell Death. Cell Death Differ. 2019, 26, 640–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellerito, C.; Emanuele, S.; Ferrante, F.; Celesia, A.; Giuliano, M.; Fiore, T. Tributyltin(IV) Ferulate, a Novel Synthetic Ferulic Acid Derivative, Induces Autophagic Cell Death in Colon Cancer Cells: From Chemical Synthesis to Biochemical Effects. J. Inorg. Biochem. 2020, 205, 110999. [Google Scholar] [CrossRef] [PubMed]
- Kocaturk, N.M.; Akkoc, Y.; Kig, C.; Bayraktar, O.; Gozuacik, D.; Kutlu, O. Autophagy as a Molecular Target for Cancer Treatment. Eur. J. Pharm. Sci. 2019, 134, 116–137. [Google Scholar] [CrossRef]
- Lan, B.; Wan, Y.-J.; Pan, S.; Wang, Y.; Yang, Y.; Leng, Q.-L.; Jia, H.; Liu, Y.; Zhang, C.-Z.; Cao, Y. Parthenolide Induces Autophagy via the Depletion of 4E-BP1. Biochem. Biophys. Res. Commun. 2015, 456, 434–439. [Google Scholar] [CrossRef]
- Eramo, A.; Ricci-Vitiani, L.; Zeuner, A.; Pallini, R.; Lotti, F.; Sette, G.; Pilozzi, E.; Larocca, L.M.; Peschle, C.; De Maria, R. Chemotherapy Resistance of Glioblastoma Stem Cells. Cell Death Differ. 2006, 13, 1238–1241. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.K.; Chiu, S.C.; Lin, C.W.; Su, M.J.; Liao, M.H. Induction of survivin inhibition, G2/M cell cycle arrest and autophagic on cell death in human malignant glioblastoma cells. Chin. J. Physiol. 2015, 58, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Gào, X.; Schöttker, B. Reduction–Oxidation Pathways Involved in Cancer Development: A Systematic Review of Literature Reviews. Oncotarget 2017, 8, 51888–51906. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Yun, B.-R.; Lee, M.-J.; Kim, J.-H.; Kim, I.-H.; Yu, G.-R.; Kim, D.-G. Enhancement of Parthenolide-Induced Apoptosis by a PKC-Alpha Inhibition through Heme Oxygenase-1 Blockage in Cholangiocarcinoma Cells. Exp. Mol. Med. 2010, 42, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Zhang, J.; Yao, J.; Liu, Y.; Fang, J. Targeting Thioredoxin Reductase by Parthenolide Contributes to Inducing Apoptosis of HeLa Cells. J. Biol. Chem. 2016, 291, 10021–10031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.; You, K.-R.; Lee, S.-Y.; Song, C.-H.; Kim, D.-G. Oxidative Stress-Mediated Apoptosis: The anticancer effect of the sesquiterpene lactone parthenolide. J. Biol. Chem. 2002, 277, 38954–38964. [Google Scholar] [CrossRef] [Green Version]
- Emanuele, S.; D’Anneo, A.; Calvaruso, G.; Cernigliaro, C.; Giuliano, M.; Lauricella, M. The Double-Edged Sword Profile of Redox Signaling: Oxidative Events as Molecular Switches in the Balance between Cell Physiology and Cancer. Chem. Res. Toxicol. 2018, 31, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-L.; Trang, K.T.T.; Kim, S.H.; Kim, I.H.; Lee, S.O.; Lee, S.T.; Kim, D.G.; Kim, S.-W. Parthenolide Suppresses Tumor Growth in a Xenograft Model of Colorectal Cancer Cells by Inducing Mitochondrial Dysfunction and Apoptosis. Int. J. Oncol. 2012, 41, 1547–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-H.; Liu, L.; Lee, S.-O.; Kim, Y.-T.; You, K.-R.; Kim, D.-G. Susceptibility of Cholangiocarcinoma Cells to Parthenolide-Induced Apoptosis. Cancer Res. 2005, 65, 6312–6320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Adachi, M.; Kawamura, R.; Sakamoto, H.; Hayashi, T.; Ishida, T.; Imai, K.; Shinomura, Y. Parthenolide-Induced Apoptosis in Multiple Myeloma Cells Involves Reactive Oxygen Species Generation and Cell Sensitivity Depends on Catalase Activity. Apoptosis 2006, 11, 2225–2235. [Google Scholar] [CrossRef]
- Flores-Lopez, G.; Moreno-Lorenzana, D.; Ayala-Sanchez, M.; Aviles-Vazquez, S.; Torres-Martinez, H.; Crooks, P.A.; Guzman, M.L.; Mayani, H.; Chávez-González, A. Parthenolide and DMAPT Induce Cell Death in Primitive CML Cells through Reactive Oxygen Species. J. Cell. Mol. Med. 2018, 22, 4899–4912. [Google Scholar] [CrossRef]
- Sun, Y.; Clair, D.K.S.; Xu, Y.; Crooks, P.A.; Clair, W.H.S. A NADPH Oxidase–Dependent Redox Signaling Pathway Mediates the Selective Radiosensitization Effect of Parthenolide in Prostate Cancer Cells. Cancer Res. 2010, 70, 2880–2890. [Google Scholar] [CrossRef] [Green Version]
- Carlisi, D.; D’Anneo, A.; Martinez, R.; Emanuele, S.; Buttitta, G.; Di Fiore, R.; Vento, R.; Tesoriere, G.; Lauricella, M. The Oxygen Radicals Involved in the Toxicity Induced by Parthenolide in MDA-MB-231 Cells. Oncol. Rep. 2014, 32, 167–172. [Google Scholar] [CrossRef] [Green Version]
- D’Anneo, A.; Carlisi, D.; Emanuele, S.; Buttitta, G.; Di Fiore, R.; Vento, R.; Tesoriere, G.; Lauricella, M. Parthenolide Induces Superoxide Anion Production by Stimulating EGF Receptor in MDA-MB-231 Breast Cancer Cells. Int. J. Oncol. 2013, 43, 1895–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurdi, M.; Bowers, M.C.; Dado, J.; Booz, G.W. Parthenolide Induces a Distinct Pattern of Oxidative Stress in Cardiac Myocytes. Free Radic. Biol. Med. 2007, 42, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Dauer, P.; Sharma, N.S.; Gupta, V.K.; Nomura, A.; Dudeja, V.; Saluja, A.; Banerjee, S. GRP78-mediated Antioxidant Response and ABC Transporter Activity Confers Chemoresistance to Pancreatic Cancer Cells. Mol. Oncol. 2018, 12, 1498–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chio, I.I.C.; Jafarnejad, S.M.; Ponz-Sarvise, M.; Park, Y.; Rivera, K.; Palm, W.; Wilson, J.; Sangar, V.; Hao, Y.; Öhlund, D.; et al. NRF2 Promotes Tumor Maintenance by Modulating MRNA Translation in Pancreatic Cancer. Cell 2016, 166, 963–976. [Google Scholar] [CrossRef] [Green Version]
- Emanuele, S.; Celesia, A.; D’Anneo, A.; Lauricella, M.; Carlisi, D.; De Blasio, A.; Giuliano, M. The Good and Bad of Nrf2: An Update in Cancer and New Perspectives in COVID-19. Int. J. Mol. Sci. 2021, 22, 7963. [Google Scholar] [CrossRef]
- Carlisi, D.; De Blasio, A.; Drago-Ferrante, R.; Di Fiore, R.; Buttitta, G.; Morreale, M.; Scerri, C.; Vento, R.; Tesoriere, G. Parthenolide Prevents Resistance of MDA-MB231 Cells to Doxorubicin and Mitoxantrone: The Role of Nrf2. Cell Death Discov. 2017, 3, 17078. [Google Scholar] [CrossRef] [Green Version]
- De Blasio, A.; Di Fiore, R.; Pratelli, G.; Drago-Ferrante, R.; Saliba, C.; Baldacchino, S.; Grech, G.; Scerri, C.; Vento, R.; Tesoriere, G. A Loop Involving NRF2, MiR-29b-1-5p and AKT, Regulates Cell Fate of MDA-MB-231 Triple-Negative Breast Cancer Cells. J. Cell. Physiol. 2020, 235, 629–637. [Google Scholar] [CrossRef]
- Drago-Ferrante, R.; Pentimalli, F.; Carlisi, D.; Blasio, A.D.; Saliba, C.; Baldacchino, S.; Degaetano, J.; Debono, J.; Caruana-Dingli, G.; Grech, G.; et al. Suppressive Role Exerted by MicroRNA-29b-1-5p in Triple Negative Breast Cancer through SPIN1 Regulation. Oncotarget 2017, 8, 28939–28958. [Google Scholar] [CrossRef] [Green Version]
- Pei, S.; Minhajuddin, M.; D’Alessandro, A.; Nemkov, T.; Stevens, B.M.; Adane, B.; Khan, N.; Hagen, F.K.; Yadav, V.K.; De, S.; et al. Rational Design of a Parthenolide-Based Drug Regimen That Selectively Eradicates Acute Myelogenous Leukemia Stem Cells. J. Biol. Chem. 2016, 291, 21984–22000. [Google Scholar] [CrossRef] [Green Version]
- Carlisi, D.; Buttitta, G.; Di Fiore, R.; Scerri, C.; Drago-Ferrante, R.; Vento, R.; Tesoriere, G. Parthenolide and DMAPT Exert Cytotoxic Effects on Breast Cancer Stem-like Cells by Inducing Oxidative Stress, Mitochondrial Dysfunction and Necrosis. Cell Death Dis. 2016, 7, e2194. [Google Scholar] [CrossRef] [Green Version]
- Mendonca, M.S.; Turchan, W.T.; Alpuche, M.E.; Watson, C.N.; Estabrook, N.C.; Chin-Sinex, H.; Shapiro, J.B.; Imasuen-Williams, I.E.; Rangel, G.; Gilley, D.P.; et al. DMAPT Inhibits NF-κB Activity and Increases Sensitivity of Prostate Cancer Cells to X-Rays In Vitro and in Tumor Xenografts In Vivo. Free Radic. Biol. Med. 2017, 112, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Oka, D.; Nishimura, K.; Shiba, M.; Nakai, Y.; Arai, Y.; Nakayama, M.; Takayama, H.; Inoue, H.; Okuyama, A.; Nonomura, N. Sesquiterpene Lactone Parthenolide Suppresses Tumor Growth in a Xenograft Model of Renal Cell Carcinoma by Inhibiting the Activation of NF-κB. Int. J. Cancer 2007, 120, 2576–2581. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Mehrotra, S.; Sadaria, M.R.; Kumar, S.; Shortle, N.H.; Roman, Y.; Sheridan, C.; Campbell, R.A.; Murry, D.J.; Badve, S.; et al. The Sesquiterpene Lactone Parthenolide in Combination with Docetaxel Reduces Metastasis and Improves Survival in a Xenograft Model of Breast Cancer. Mol. Cancer 2005, 4, 1004–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curry, E.A.; Murry, D.J.; Yoder, C.; Fife, K.; Armstrong, V.; Nakshatri, H.; O’Connell, M.; Sweeney, C.J. Phase I Dose Escalation Trial of Feverfew with Standardized Doses of Parthenolide in Patients with Cancer. Investig. New Drugs 2004, 22, 299–305. [Google Scholar] [CrossRef]
- Wan Kamarul Zaman, W.S.; Nurul, A.A.; Nordin, F. Stem Cells and Cancer Stem Cells: The Jekyll and Hyde Scenario and Their Implications in Stem Cell Therapy. Biomedicines 2021, 9, 1245. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.-K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.-R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells—Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Kuşoğlu, A.; Biray Avcı, Ç. Cancer Stem Cells: A Brief Review of the Current Status. Gene 2019, 681, 80–85. [Google Scholar] [CrossRef]
- Zhang, S.; Ju, X.; Yang, Q.; Zhu, Y.; Fan, D.; Su, G.; Kong, L.; Li, Y. USP47 maintains the stemness of colorectal cancer cells and is inhibited by parthenolide. Biochem. Biophys. Res. Commun. 2021, 562, 21–28. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting Cancer Stem Cells and Signaling Pathways by Phytochemicals: Novel Approach for Breast Cancer Therapy. Semin. Cancer Biol. 2016, 40–41, 192–208. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.P.; Almeida, F.S.; Chi, A.; Nguyen, L.M.; Cohen, D.; Karlsson, U.; Vinh-Hung, V. Molecular biology of breast cancer stem cells: Potential clinical applications. Cancer Treat. Rev. 2010, 36, 485–491. [Google Scholar] [CrossRef]
- Yi, J.; Wang, L.; Wang, X.-Y.; Sun, J.; Yin, X.-Y.; Hou, J.-X.; Chen, J.; Xie, B.; Wei, H.-L. Suppression Of Aberrant Activation Of NF-κB Pathway in Drug-Resistant Leukemia Stem Cells Contributes To Parthenolide-Potentiated Reversal Of Drug Resistance in Leukemia. J. Cancer 2021, 12, 5519–5529. [Google Scholar] [CrossRef] [PubMed]
- Gunn, E.J.; Williams, J.T.; Huynh, D.T.; Iannotti, M.J.; Han, C.; Barrios, F.J.; Kendall, S.; Glackin, C.A.; Colby, D.A.; Kirshner, J. The Natural Products Parthenolide and Andrographolide Exhibit Anti-Cancer Stem Cell Activity in Multiple Myeloma. Leuk. Lymphoma 2011, 52, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, H.; Gu, P.; Bai, J.; Margolick, J.B.; Zhang, Y. NF-κB Pathway Inhibitors Preferentially Inhibit Breast Cancer Stem-like Cells. Breast Cancer Res. Treat 2008, 111, 419–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Lu, W.-L.; Guo, J.; Du, J.; Li, T.; Wu, J.-W.; Wang, G.-L.; Wang, J.-C.; Zhang, X.; Zhang, Q. A Potential Target Associated with Both Cancer and Cancer Stem Cells: A Combination Therapy for Eradication of Breast Cancer Using Vinorelbine Stealthy Liposomes plus Parthenolide Stealthy Liposomes. J. Control. Release 2008, 129, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, L.N.; Chow, E.K.-H. Mechanisms of Chemoresistance in Cancer Stem Cells. Clin. Transl. Med. 2013, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Liao, K.; Xia, B.; Zhuang, Q.-Y.; Hou, M.-J.; Zhang, Y.-J.; Luo, B.; Qiu, Y.; Gao, Y.-F.; Li, X.-J.; Chen, H.-F.; et al. Parthenolide Inhibits Cancer Stem-Like Side Population of Nasopharyngeal Carcinoma Cells via Suppression of the NF-κB/COX-2 Pathway. Theranostics 2015, 5, 302–321. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Qiu, L.; Jin, X.; Guo, Z.; Guo, C. Nuclear Factor-ΚB Inhibition by Parthenolide Potentiates the Efficacy of Taxol in Non–Small Cell Lung Cancer In Vitro and In Vivo. Mol. Cancer Res. 2009, 7, 1139–1149. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, R.; Jayaprakasan, V.; Gokmen-Polar, Y.; Kelich, S.; Miller, K.D.; Yip-Schneider, M.; Cheng, L.; Bhat-Nakshatri, P.; Sledge, G.W., Jr.; Nakshatri, H.; et al. Restoring Chemotherapy and Hormone Therapy Sensitivity by Parthenolide in a Xenograft Hormone Refractory Prostate Cancer Model. Prostate 2006, 66, 1498–1511. [Google Scholar] [CrossRef]
- deGraffenried, L.A.; Chandrasekar, B.; Friedrichs, W.E.; Donzis, E.; Silva, J.; Hidalgo, M.; Freeman, J.W.; Weiss, G.R. NF-κB Inhibition Markedly Enhances Sensitivity of Resistant Breast Cancer Tumor Cells to Tamoxifen. Ann. Oncol. 2004, 15, 885–890. [Google Scholar] [CrossRef]
- Riggins, R.B.; Zwart, A.; Nehra, R.; Clarke, R. The Nuclear Factor ΚB Inhibitor Parthenolide Restores ICI 182,780 (Faslodex; Fulvestrant)–Induced Apoptosis in Antiestrogen-Resistant Breast Cancer Cells. Mol. Cancer 2005, 4, 33–41. [Google Scholar]
- Carlisi, D.; Lauricella, M.; D’Anneo, A.; Buttitta, G.; Emanuele, S.; di Fiore, R.; Martinez, R.; Rolfo, C.; Vento, R.; Tesoriere, G. The Synergistic Effect of SAHA and Parthenolide in MDA-MB231 Breast Cancer Cells. J. Cell. Physiol. 2015, 230, 1276–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.-J.; Shao, X.-T.; Wang, S.; Lu, G.-H.; Xu, T.; Zhou, J.-Y. Sesquiterpene Lactone Parthenolide Markedly Enhances Sensitivity of Human A549 Cells to Low-Dose Oxaliplatin via Inhibition of NF-κB Activation and Induction of Apoptosis. Planta Med. 2010, 76, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Francescato, H.D.C.; Costa, R.S.; Scavone, C.; Coimbra, T.M. Parthenolide Reduces Cisplatin-Induced Renal Damage. Toxicology 2007, 230, 64–75. [Google Scholar] [CrossRef]
- Ghorbani-Abdi-Saedabad, A.; Hanafi-Bojd, M.Y.; Parsamanesh, N.; Tayarani-Najaran, Z.; Mollaei, H.; Hoshyar, R. Anticancer and Apoptotic Activities of Parthenolide in Combination with Epirubicin in Mda-Mb-468 Breast Cancer Cells. Mol. Biol Rep. 2020, 47, 5807–5815. [Google Scholar] [CrossRef]
- Ralstin, M.C.; Gage, E.A.; Yip-Schneider, M.T.; Klein, P.J.; Wiebke, E.A.; Schmidt, C.M. Parthenolide Cooperates with NS398 to Inhibit Growth of Human Hepatocellular Carcinoma Cells through Effects on Apoptosis and G0-G1 Cell Cycle Arrest. Mol. Cancer Res. 2006, 4, 387–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip-Schneider, M.T.; Wu, H.; Ralstin, M.; Yiannoutsos, C.; Crooks, P.A.; Neelakantan, S.; Noble, S.; Nakshatri, H.; Sweeney, C.J.; Schmidt, C.M. Suppression of Pancreatic Tumor Growth by Combination Chemotherapy with Sulindac and LC-1 Is Associated with Cyclin D1 Inhibition in Vivo. Mol. Cancer 2007, 6, 1736–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, T.; Takao, T.; Iwasaki, Y.; Nishiyama, M.; Asaba, K.; Hashimoto, K. Suppressive Effects of Dehydroepiandrosterone and the Nuclear Factor-ΚB Inhibitor Parthenolide on Corticotroph Tumor Cell Growth and Function In Vitro and In Vivo. J. Endocrinol. 2006, 188, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Duechler, M.; Stańczyk, M.; Czyż, M.; Stępnik, M. Potentiation of Arsenic Trioxide Cytotoxicity by Parthenolide and Buthionine Sulfoximine in Murine and Human Leukemic Cells. Cancer Chemother. Pharm. 2008, 61, 727–737. [Google Scholar] [CrossRef]
- Watson, C.; Miller, D.A.; Chin-Sinex, H.; Losch, A.; Hughes, W.; Sweeney, C.; Mendonca, M.S. Suppression of NF-κB Activity by Parthenolide Induces X-Ray Sensitivity through Inhibition of Split-Dose Repair in TP53 Null Prostate Cancer Cells. Radiat. Res. 2009, 171, 389–396. [Google Scholar] [CrossRef]
- Hayashi, S.; Hatashita, M.; Hayashi, A.; Matsumoto, H.; Shioura, H.; Kitai, R. Thermosensitization by Parthenolide in Human Lung Adenocarcinoma A549 Cells and P53- and Hsp72-Independent Apoptosis Induction via the Nuclear Factor-ΚB Signal Pathway. Int. J. Mol. Med. 2008, 21, 585–592. [Google Scholar] [CrossRef]
- Hayashi, S.; Sakurai, H.; Hayashi, A.; Tanaka, Y.; Hatashita, M.; Shioura, H. Inhibition of NF-κB by Combination Therapy with Parthenolide and Hyperthermia and Kinetics of Apoptosis Induction and Cell Cycle Arrest in Human Lung Adenocarcinoma Cells. Int. J. Mol. Med. 2010, 25, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Liu, Q.; Wang, S.; Zeng, B.; Du, G.; Zhang, C.; Li, Y. Synthesis, Cytotoxicity, and in Vivo Antitumor Activity Study of Parthenolide Semicarbazones and Thiosemicarbazones. Bioorg. Med. Chem. 2020, 28, 115557. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Xu, Y.; Mustafa, T.; Kannarpady, G.; Bratton, S.M.; Radominska-Pandya, A.; Crooks, P.A.; Biris, A.S. Nanodelivery of Parthenolide Using Functionalized Nanographene Enhances Its Anticancer Activity. RSC Adv. 2015, 5, 2411–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwish, N.H.E.; Sudha, T.; Godugu, K.; Bharali, D.J.; Elbaz, O.; El-ghaffar, H.A.A.; Azmy, E.; Anber, N.; Mousa, S.A. Novel Targeted Nano-Parthenolide Molecule against NF-κB in Acute Myeloid Leukemia. Molecules 2019, 24, 2103. [Google Scholar] [CrossRef] [Green Version]
- Zhai, J.-D.; Li, D.; Long, J.; Zhang, H.-L.; Lin, J.-P.; Qiu, C.-J.; Zhang, Q.; Chen, Y. Biomimetic Semisynthesis of Arglabin from Parthenolide. J. Org. Chem. 2012, 77, 7103–7107. [Google Scholar] [CrossRef]
- Lone, S.H.; Bhat, K.A.; Khuroo, M.A. Arglabin: From Isolation to Antitumor Evaluation. Chem.-Biol. Interact. 2015, 240, 180–198. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y.; Ding, Y.; Zhai, J.; Ji, Q.; Ma, W.; Yang, M.; Fan, H.; Long, J.; Tong, Z.; et al. Guaianolide Sesquiterpene Lactones, a Source to Discover Agents That Selectively Inhibit Acute Myelogenous Leukemia Stem and Progenitor Cells. J. Med. Chem. 2012, 55, 8757–8769. [Google Scholar] [CrossRef]
- He, W.; Lai, R.; Lin, Q.; Huang, Y.; Wang, L. Arglabin is a plant sesquiterpene lactone that exerts potent anticancer effects on human oral squamous cancer cells via mitochondrial apoptosis and downregulation of the mTOR/PI3K/Akt signaling pathway to inhibit tumor growth in vivo. J. Buon 2018, 23, 1679–1685. [Google Scholar]
- Yu, L.; Chen, W.; Tang, Q.; Ji, K.-Y. Micheliolide Inhibits Liver Cancer Cell Growth Via Inducing Apoptosis And Perturbing Actin Cytoskeleton. Cancer Manag. Res. 2019, 11, 9203–9212. [Google Scholar] [CrossRef] [Green Version]
- Viennois, E.; Xiao, B.; Ayyadurai, S.; Wang, L.; Wang, P.G.; Zhang, Q.; Chen, Y.; Merlin, D. Micheliolide, a New Sesquiterpene Lactone That Inhibits Intestinal Inflammation and Colitis-Associated Cancer. Lab. Investig. 2014, 94, 950–965. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Gong, W.; Chen, J.; Qing, Y.; Wu, S.; Li, H.; Huang, C.; Chen, Y.; Wang, Y.; Xu, Z.; et al. Micheliolide Alleviates Hepatic Steatosis in Db/Db Mice by Inhibiting Inflammation and Promoting Autophagy via PPAR-γ-Mediated NF-κB and AMPK/MTOR Signaling. Int. Immunopharmacol. 2018, 59, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ding, Q.; Chen, C.; Chen, F.; Zhou, X.; Hong, C.J.; Pan, W. Micheliolide Inhibits Gastric Cancer Growth in Vitro and in Vivo via Blockade of the IL-6/STAT3 Pathway. Die Pharm. Int. J. Pharm. Sci. 2019, 74, 175–178. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Guo, J.; Li, Q.; Long, J.; Ma, C.; Ding, Y.; Yan, C.; Li, L.; Wu, Z.; et al. Natural Product Micheliolide (MCL) Irreversibly Activates Pyruvate Kinase M2 and Suppresses Leukemia. J. Med. Chem. 2018, 61, 4155–4164. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Guo, W.; Li, L.; Xu, C.; Yang, D.; Wang, S.; Lu, Y.; Zhang, Q.; Zhai, J.; Fan, H.; et al. Micheliolide Derivative DMAMCL Inhibits Glioma Cell Growth In Vitro and In Vivo. PLoS ONE 2015, 10, e0116202. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, L.; Liu, J.; Xie, X.; Hu, H.; Luo, F. Anticancer Effects of ACT001 via NF-κB Suppression in Murine Triple-Negative Breast Cancer Cell Line 4T1. Cancer Manag. Res. 2020, 12, 5131–5139. [Google Scholar] [CrossRef]
- Jin, X.-H.; Jia, Y.-S.; Shi, Y.-H.; Li, Q.-Y.; Bao, S.-Q.; Lu, W.-P.; Tong, Z.-S. ACT001 Can Prevent and Reverse Tamoxifen Resistance in Human Breast Cancer Cell Lines by Inhibiting NF-κB Activation. J. Cell. Biochem. 2019, 120, 1386–1397. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, B.; Liu, W.; Yu, B.; Shi, Q.; Luo, F.; Bai, Y.; Feng, H. Targeting of Glioma Stem-like Cells with a Parthenolide Derivative ACT001 through Inhibition of AEBP1/PI3K/AKT Signaling. Theranostics 2021, 11, 555–566. [Google Scholar] [CrossRef]




| Synergistic Action of PN | |||
|---|---|---|---|
| Coumpounds | Class of Compound | Tested on | Ref. |
| Taxol, Docetaxel, Vinorelbine | Antimicrotubule agents | Lung cancer, prostate cancer, breast cancer cell lines | [164,167,168] |
| Tamoxifen, Fulvestrant | Antiestrogen agents | Breast cancer cell lines | [169,170] |
| SAHA | Histone deacetylase inhibitor | Breast cancer cell lines | [171] |
| Oxaliplatin | Antineoplastic platinum drugs | Lung cancer cell lines | [172] |
| Epirubicin | Anthracycline doxorubicin analog | Breast cancer cell lines | [174] |
| NS398, sulindac | COX-2 inhibitors | Hepatocellular carcinoma cell lines | [175,176] |
| Dehydroepiandrosterone | Steroid anti-inflammatory agents | Pituitary tumor cell lines | [177] |
| Retinoic acid | Active metabolite of vitamin A | Leukaemia cell lines | [93] |
| Arsenic trioxide | Toxic metalloid | Leukaemia cell lines | [178] |
| TRAIL | Tumor necrosis factor family | Hepatocellular carcinoma and breast cancer cell lines | [50,65,179] |
| Radiotherapy | X-ray | Lung adenocarcinoma, prostate cancer cell lines | [50,179,180,181] |
| PN Anologues | |||
|---|---|---|---|
| Coumpounds | Structure | Tested on | Ref. |
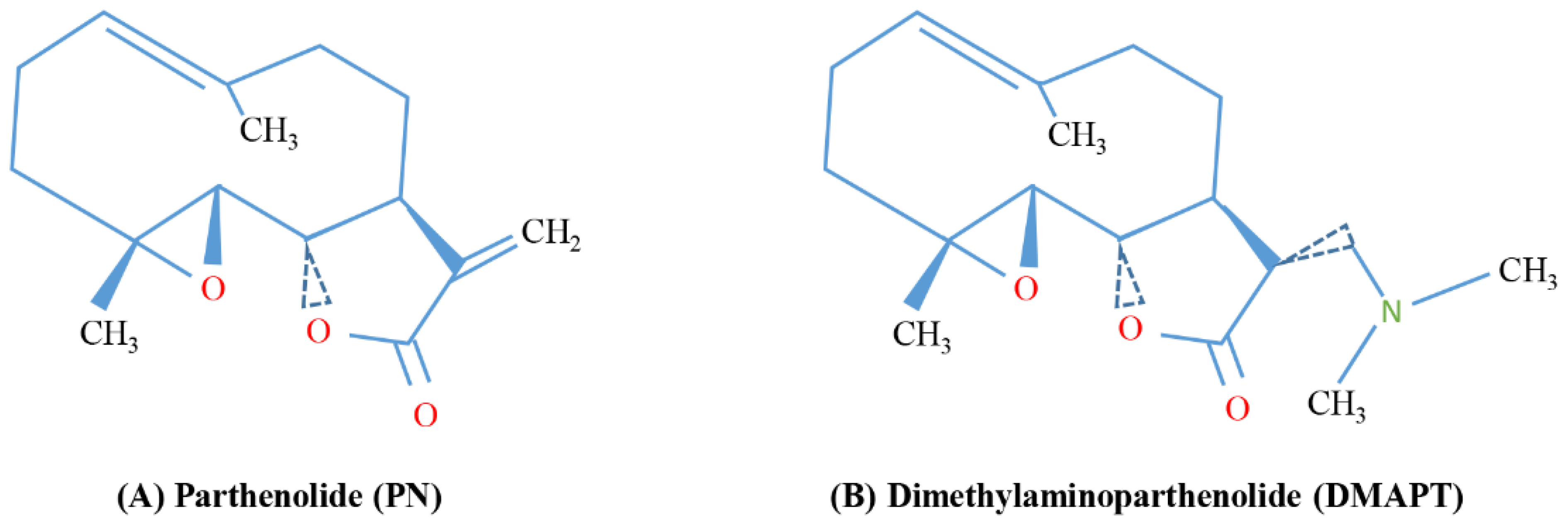
| Dimethylaminoparthenolide (DMAPT), also known as LC-1 | PN with a methyl group also in the form of fumarate salt | Leukemia, prostate cancer, breast cancer | [26,27,116] |
| PN semicarbazone or thiosemicarbazone | PN with semicarbazone/thiosemi-carbazone groups | Colorectal carcinoma, glioblastoma, liver carcinoma, gastric cancer and lung cancer cell lines | [182] |
| PN-fGn or DMAPT-fGn | Carboxyl-functionalized nanographene | Pancreatic cancer cell lines | [183] |
| PLGA-anti CD44-PN nanoparticles | Polylactide coglycolide (PLGA) nanoparticles conjugated with antiCD44 | Acute myeloid leukemia | [184] |
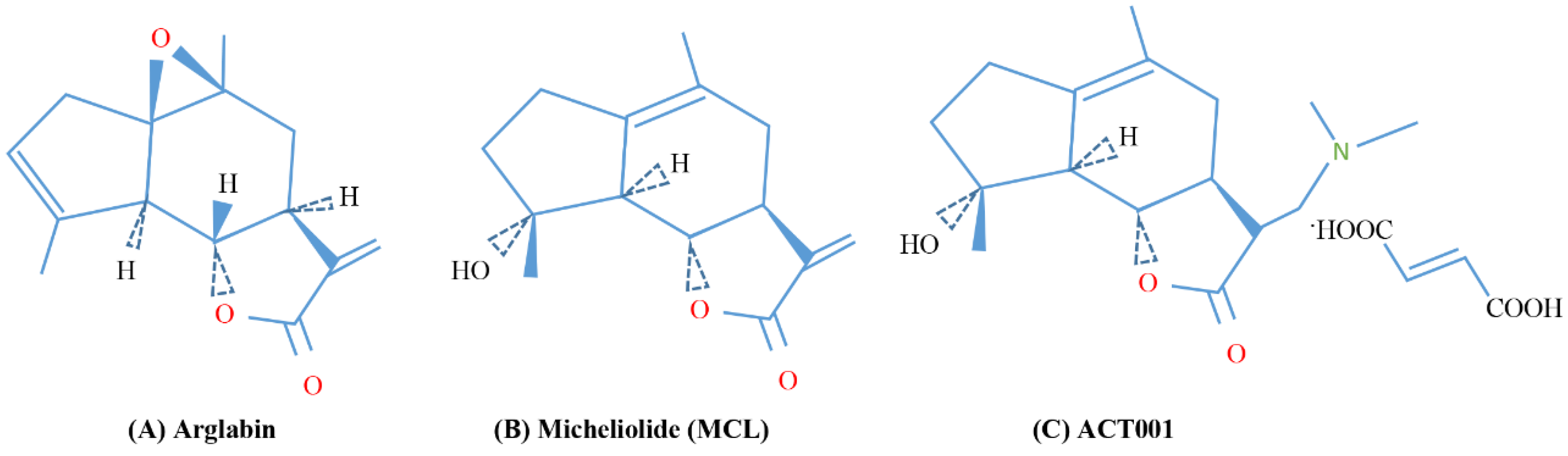
| Arglabin | Guaianolide sesquiterpene lactone | Leukemia, human oral squamous and lung cancer cell lines | [185,186,187,188] |
| Micheliolide (MCL) | Guaianolide sesquiterpene lactone | Hepatocellular carcinoma, leukemia cells | [185,189,190,191,192,193] |
| ACT001 | Fumarate salt of dimethylaminomicheliolide | Breast cancer cells, glioma stem cells | [194,195,196,197] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlisi, D.; Lauricella, M.; D’Anneo, A.; De Blasio, A.; Celesia, A.; Pratelli, G.; Notaro, A.; Calvaruso, G.; Giuliano, M.; Emanuele, S. Parthenolide and Its Soluble Analogues: Multitasking Compounds with Antitumor Properties. Biomedicines 2022, 10, 514. https://doi.org/10.3390/biomedicines10020514
Carlisi D, Lauricella M, D’Anneo A, De Blasio A, Celesia A, Pratelli G, Notaro A, Calvaruso G, Giuliano M, Emanuele S. Parthenolide and Its Soluble Analogues: Multitasking Compounds with Antitumor Properties. Biomedicines. 2022; 10(2):514. https://doi.org/10.3390/biomedicines10020514
Chicago/Turabian StyleCarlisi, Daniela, Marianna Lauricella, Antonella D’Anneo, Anna De Blasio, Adriana Celesia, Giovanni Pratelli, Antonietta Notaro, Giuseppe Calvaruso, Michela Giuliano, and Sonia Emanuele. 2022. "Parthenolide and Its Soluble Analogues: Multitasking Compounds with Antitumor Properties" Biomedicines 10, no. 2: 514. https://doi.org/10.3390/biomedicines10020514
APA StyleCarlisi, D., Lauricella, M., D’Anneo, A., De Blasio, A., Celesia, A., Pratelli, G., Notaro, A., Calvaruso, G., Giuliano, M., & Emanuele, S. (2022). Parthenolide and Its Soluble Analogues: Multitasking Compounds with Antitumor Properties. Biomedicines, 10(2), 514. https://doi.org/10.3390/biomedicines10020514










