Decreased Plasma Level of Cytokeratin 20 (KRT20) Is Indicative of the Emergence and Severity of Acute GvHD Irrespective to the Type of Organ Involvement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design
2.3. Sample Collection
2.4. ELISA
2.5. Statistical Analysis
3. Results
3.1. Marker Selection
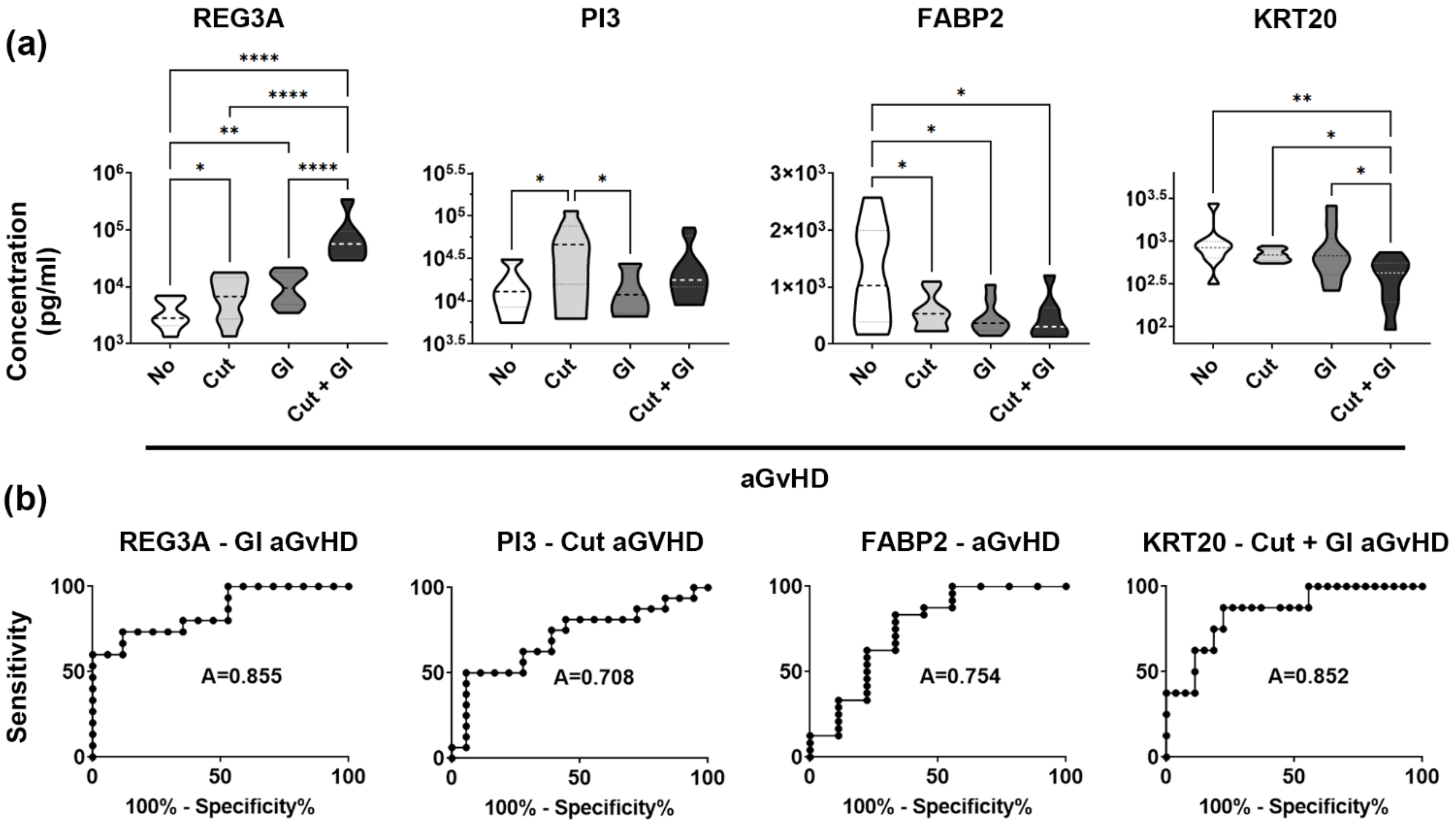
3.2. REG3A and PI3 Validate Patient Selection and Study Design
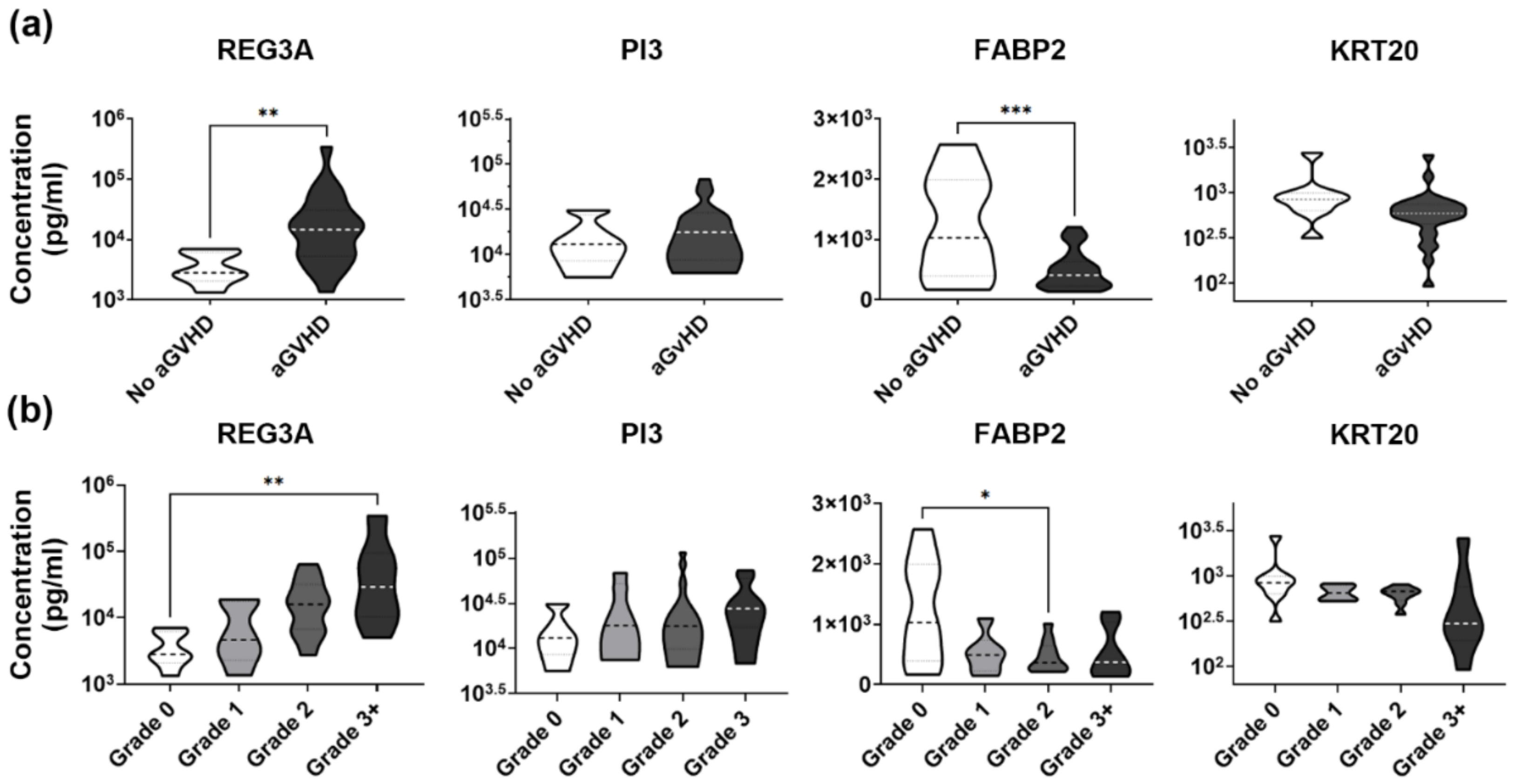
3.3. Low Plasma KRT20 Levels in aGvHD with Skin and Gut Involvement
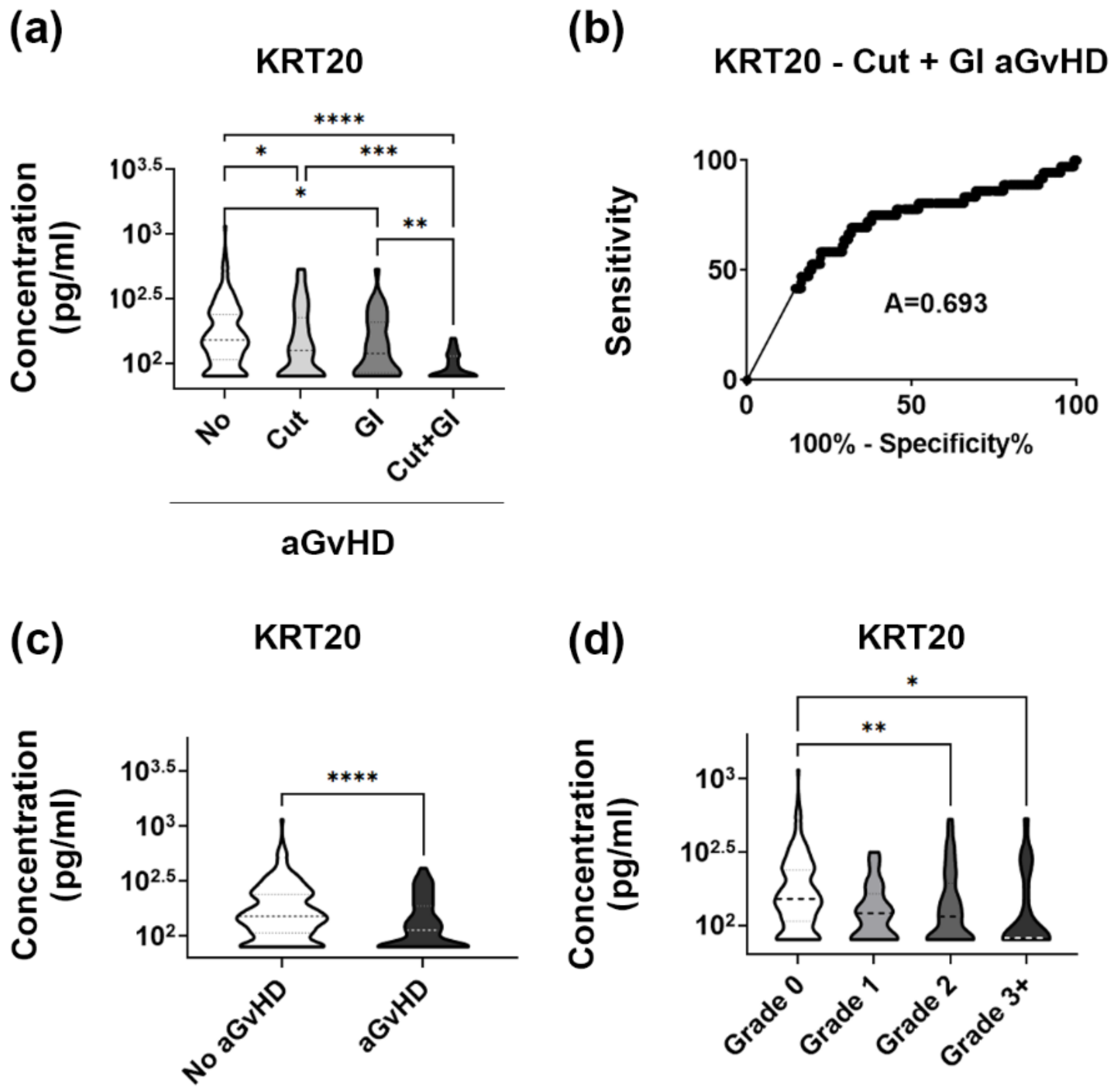
3.4. KRT20 Is a Sensitive and Specific Marker of aGvHD Affecting Multiple Tissues
3.5. Validation Set Confirms Robust Association between Low Plasma KRT20 and Widespread Organ Damage in aGvHD
3.6. Validation Set Discloses Lowered Plasma Levels of KRT20 in Medium to High Grade aGvHD
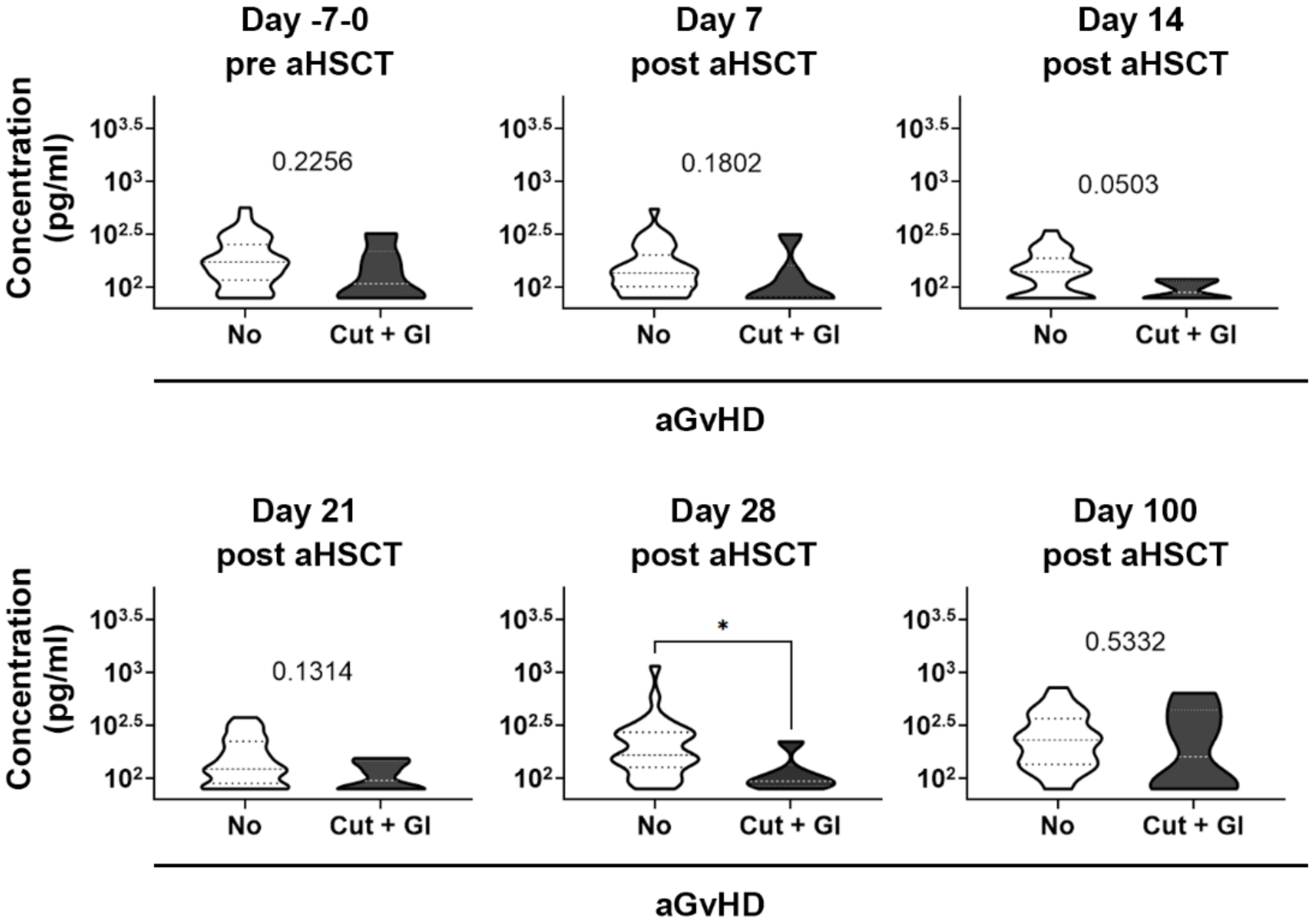
3.7. Analysis of the Kinetics of KRT20 Dysregulation in Severe aGvHD: Possible Prognostic Value?
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Ruutu, T.; van Biezen, A.; Hertenstein, B.; Henseler, A.; Garderet, L.; Passweg, J.; Mohty, M.; Sureda, A.; Niederwieser, D.; Gratwohl, A.; et al. Prophylaxis and treatment of GVHD after allogeneic haematopoietic SCT: A survey of centre strategies by the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012, 47, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Dan, K.; Han, D.; Kim, J.W.; Kim, K.K.; Koh, Y.; Shin, D.Y.; Hong, J.; Yoon, S.S.; Park, S.; et al. Plasma-based protein biomarkers can predict the risk of acute graft-versus-host disease and non-relapse mortality in patients undergoing allogeneic hematopoietic stem cell transplantation. Blood Cells Mol. Dis. 2019, 74, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Kaviany, S.; Kitko, C.L. The role of biomarkers in risk stratification, treatment and outcome in acute GVHD. Curr. Opin. Hematol. 2021, 28, 401–407. [Google Scholar] [CrossRef]
- Chien, J.W.; Zhang, X.C.; Fan, W.; Wang, H.; Zhao, L.P.; Martin, P.J.; Storer, B.E.; Boeckh, M.; Warren, E.H.; Hansen, J.A. Evaluation of published single nucleotide polymorphisms associated with acute GVHD. Blood 2012, 119, 5311–5319. [Google Scholar] [CrossRef] [Green Version]
- Alam, N.; Xu, W.; Atenafu, E.G.; Uhm, J.; Seftel, M.; Gupta, V.; Kuruvilla, J.; Lipton, J.H.; Messner, H.A.; Kim, D.D. Risk model incorporating donor IL6 and IFNG genotype and gastrointestinal GVHD can discriminate patients at high risk of steroid refractory acute GVHD. Bone Marrow Transplant. 2015, 50, 734–742. [Google Scholar] [CrossRef]
- Hartwell, M.J.; Ozbek, U.; Holler, E.; Renteria, A.S.; Major-Monfried, H.; Reddy, P.; Aziz, M.; Hogan, W.J.; Ayuk, F.; Efebera, Y.A.; et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight 2017, 2, e89798. [Google Scholar] [CrossRef]
- Levine, J.E.; Braun, T.M.; Harris, A.C.; Holler, E.; Taylor, A.; Miller, H.; Magenau, J.; Weisdorf, D.J.; Ho, V.T.; Bolanos-Meade, J.; et al. A prognostic score for acute graft-versus-host disease based on biomarkers: A multicentre study. Lancet Haematol. 2015, 2, e21–e29. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.; Signorino, E.; Muraro, M.; Quarello, P.; Biasin, E.; Nesi, F.; Vassallo, E.; Fagioli, F. Monitoring of TNFR1, IL-2Ralpha, HGF, CCL8, IL-8 and IL-12p70 following HSCT and their role as GVHD biomarkers in paediatric patients. Bone Marrow Transplant. 2013, 48, 1230–1236. [Google Scholar] [CrossRef]
- Ranganathan, P.; Heaphy, C.E.; Costinean, S.; Stauffer, N.; Na, C.; Hamadani, M.; Santhanam, R.; Mao, C.; Taylor, P.A.; Sandhu, S.; et al. Regulation of acute graft-versus-host disease by microRNA-155. Blood 2012, 119, 4786–4797. [Google Scholar] [CrossRef]
- Lia, G.; Brunello, L.; Bruno, S.; Carpanetto, A.; Omede, P.; Festuccia, M.; Tosti, L.; Maffini, E.; Giaccone, L.; Arpinati, M.; et al. Extracellular vesicles as potential biomarkers of acute graft-vs-host disease. Leukemia 2018, 32, 765–773. [Google Scholar] [CrossRef]
- Holler, E.; Butzhammer, P.; Schmid, K.; Hundsrucker, C.; Koestler, J.; Peter, K.; Zhu, W.; Sporrer, D.; Hehlgans, T.; Kreutz, M.; et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: Loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transplant. 2014, 20, 640–645. [Google Scholar] [CrossRef] [Green Version]
- Jenq, R.R.; Ubeda, C.; Taur, Y.; Menezes, C.C.; Khanin, R.; Dudakov, J.A.; Liu, C.; West, M.L.; Singer, N.V.; Equinda, M.J.; et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med. 2012, 209, 903–911. [Google Scholar] [CrossRef]
- Zhao, X.S.; Huang, X.J. Seeking biomarkers for acute graft-versus-host disease: Where we are and where we are heading? Biomark. Res. 2019, 7, 17. [Google Scholar] [CrossRef]
- Srinagesh, H.K.; Levine, J.E.; Ferrara, J.L.M. Biomarkers in acute graft-versus-host disease: New insights. Ther. Adv. Hematol. 2019, 10, 2040620719891358. [Google Scholar] [CrossRef] [Green Version]
- Srinagesh, H.K.; Ozbek, U.; Kapoor, U.; Ayuk, F.; Aziz, M.; Ben-David, K.; Choe, H.K.; DeFilipp, Z.; Etra, A.; Grupp, S.A.; et al. The MAGIC algorithm probability is a validated response biomarker of treatment of acute graft-versus-host disease. Blood Adv. 2019, 3, 4034–4042. [Google Scholar] [CrossRef]
- Ferrara, J.L.; Harris, A.C.; Greenson, J.K.; Braun, T.M.; Holler, E.; Teshima, T.; Levine, J.E.; Choi, S.W.; Huber, E.; Landfried, K.; et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood 2011, 118, 6702–6708. [Google Scholar] [CrossRef] [Green Version]
- Solan, L.; Kwon, M.; Carbonell, D.; Dorado, N.; Balsalobre, P.; Serrano, D.; Chicano-Lavilla, M.; Anguita, J.; Gayoso, J.; Diez-Martin, J.L.; et al. ST2 and REG3alpha as Predictive Biomarkers after Haploidentical Stem Cell Transplantation Using Post-transplantation High-Dose Cyclophosphamide. Front. Immunol. 2019, 10, 2338. [Google Scholar] [CrossRef]
- Paczesny, S.; Braun, T.M.; Levine, J.E.; Hogan, J.; Crawford, J.; Coffing, B.; Olsen, S.; Choi, S.W.; Wang, H.; Faca, V.; et al. Elafin is a biomarker of graft-versus-host disease of the skin. Sci. Transl. Med. 2010, 2, 13ra12. [Google Scholar] [CrossRef] [Green Version]
- Bruggen, M.C.; Petzelbauer, P.; Greinix, H.; Contassot, E.; Jankovic, D.; French, L.; Socie, G.; Rabitsch, W.; Kuzmina, Z.; Kalhs, P.; et al. Epidermal elafin expression is an indicator of poor prognosis in cutaneous graft-versus-host disease. J. Invest. Dermatol. 2015, 135, 999–1006. [Google Scholar] [CrossRef] [Green Version]
- Zewde, M.G.; Morales, G.; Gandhi, I.; Ozbek, U.; Aguayo-Hiraldo, P.; Ayuk, F.; Baez, J.; Chanswangphuwana, C.; Choe, H.; DeFilipp, Z.; et al. Evaluation of Elafin as a Prognostic Biomarker in Acute Graft-versus-Host Disease. Transplant. Cell. Ther. 2021, 27, 988-e1. [Google Scholar] [CrossRef]
- George, L.; Mahabal, G.; Mohanan, E.; Balasubramanian, P.; Peter, D.; Pulimood, S.; Lakshmi, K.; Jeyaseelan, L.; Abraham, A.; Srivastava, A.; et al. Limited utility of plasma elafin as a biomarker for skin graft-versus-host disease following allogeneic stem cell transplantation. Clin. Exp. Dermatol. 2021, 46, 1482–1487. [Google Scholar] [CrossRef]
- Lounder, D.T.; Khandelwal, P.; Dandoy, C.E.; Jodele, S.; Grimley, M.S.; Wallace, G.; Lane, A.; Taggart, C.; Teusink-Cross, A.C.; Lake, K.E.; et al. Lower levels of vitamin A are associated with increased gastrointestinal graft-versus-host disease in children. Blood 2017, 129, 2801–2807. [Google Scholar] [CrossRef] [Green Version]
- Rashidi, A.; Shanley, R.; Holtan, S.G.; MacMillan, M.L.; Blazar, B.R.; Khoruts, A.; Weisdorf, D.J. Pretransplant Serum Citrulline Predicts Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2018, 24, 2190–2196. [Google Scholar] [CrossRef] [Green Version]
- Ponten, F.; Gry, M.; Fagerberg, L.; Lundberg, E.; Asplund, A.; Berglund, L.; Oksvold, P.; Bjorling, E.; Hober, S.; Kampf, C.; et al. A global view of protein expression in human cells, tissues, and organs. Mol. Syst. Biol. 2009, 5, 337. [Google Scholar] [CrossRef]
- Zhan, Q.; Signoretti, S.; Whitaker-Menezes, D.; Friedman, T.M.; Korngold, R.; Murphy, G.F. Cytokeratin15-positive basal epithelial cells targeted in graft-versus-host disease express a constitutive antiapoptotic phenotype. J. Investig. Dermatol. 2007, 127, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Noth, R.; Lange-Grumfeld, J.; Stuber, E.; Kruse, M.L.; Ellrichmann, M.; Hasler, R.; Hampe, J.; Bewig, B.; Rosenstiel, P.; Schreiber, S.; et al. Increased intestinal permeability and tight junction disruption by altered expression and localization of occludin in a murine graft versus host disease model. BMC Gastroenterol. 2011, 11, 109. [Google Scholar] [CrossRef] [Green Version]
- Calnek, D.; Quaroni, A. Differential localization by in situ hybridization of distinct keratin mRNA species during intestinal epithelial cell development and differentiation. Differentiation 1993, 53, 95–104. [Google Scholar] [CrossRef]
- Zhou, Q.; Toivola, D.M.; Feng, N.; Greenberg, H.B.; Franke, W.W.; Omary, M.B. Keratin 20 helps maintain intermediate filament organization in intestinal epithelia. Mol. Biol. Cell 2003, 14, 2959–2971. [Google Scholar] [CrossRef] [Green Version]
- Pastuszak, M.; Groszewski, K.; Pastuszak, M.; Dyrla, P.; Wojtun, S.; Gil, J. Cytokeratins in gastroenterology. Systematic review. Prz. Gastroenterol. 2015, 10, 61–70. [Google Scholar] [CrossRef]
- Moll, R.; Lowe, A.; Laufer, J.; Franke, W.W. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am. J. Pathol. 1992, 140, 427–447. [Google Scholar] [PubMed]
- McGregor, D.K.; Wu, T.T.; Rashid, A.; Luthra, R.; Hamilton, S.R. Reduced expression of cytokeratin 20 in colorectal carcinomas with high levels of microsatellite instability. Am. J. Surg. Pathol. 2004, 28, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Lugli, A.; Tzankov, A.; Zlobec, I.; Terracciano, L.M. Differential diagnostic and functional role of the multi-marker phenotype CDX2/CK20/CK7 in colorectal cancer stratified by mismatch repair status. Mod. Pathol. 2008, 21, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, V.; Sokic-Milutinovic, A.; Drndarevic, N.; Micev, M.; Mitrovic, O.; Nikolic, I.; Wex, T.; Milosavljevic, T.; Malfertheiner, P. Expression of cytokeratins in Helicobacter pylori-associated chronic gastritis of adult patients infected with cagA+ strains: An immunohistochemical study. World J. Gastroenterol. 2006, 12, 1865–1873. [Google Scholar] [CrossRef]
- Liu, G.S.; Gong, J.; Cheng, P.; Zhang, J.; Chang, Y.; Qiang, L. Distinction between short-segment Barrett’s esophageal and cardiac intestinal metaplasia. World J. Gastroenterol. 2005, 11, 6360–6365. [Google Scholar] [CrossRef]
- Helenius, T.O.; Antman, C.A.; Asghar, M.N.; Nystrom, J.H.; Toivola, D.M. Keratins Are Altered in Intestinal Disease-Related Stress Responses. Cells 2016, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Crenn, P.; Vahedi, K.; Lavergne-Slove, A.; Cynober, L.; Matuchansky, C.; Messing, B. Plasma citrulline: A marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology 2003, 124, 1210–1219. [Google Scholar] [CrossRef]
- Blijlevens, N.M.; Lutgens, L.C.; Schattenberg, A.V.; Donnelly, J.P. Citrulline: A potentially simple quantitative marker of intestinal epithelial damage following myeloablative therapy. Bone Marrow Transplant. 2004, 34, 193–196. [Google Scholar] [CrossRef]
- Majhail, N.S.; Mau, L.W.; Denzen, E.M.; Arneson, T.J. Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: A study using a large national private claims database. Bone Marrow Transplant. 2013, 48, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Parasuraman, S.; Shah, A.; Weisdorf, D. Mortality, length of stay and costs associated with acute graft-versus-host disease during hospitalization for allogeneic hematopoietic stem cell transplantation. Curr. Med. Res. Opin. 2019, 35, 983–988. [Google Scholar] [CrossRef]
- Diep, P.P.; Melberg, H.O.; Brinch, L.; Buechner, J.; Fløisand, Y.; Gedde-Dahl, T.; Loge, J.H.; Tjønnfjord, G.E.; Ruud, E. Cost-utility of allogeneic hematopoietic stem cell transplantation in Norway. Bone Marrow Transplant. 2018, 53, 657–660. [Google Scholar] [CrossRef] [Green Version]
- Matthes-Martin, S.; Potschger, U.; Barr, R.; Martin, M.; Boztug, H.; Klingebiel, T.; Attarbaschi, A.; Eibler, W.; Mann, G. Costs and cost-effectiveness of allogeneic stem cell transplantation in children are predictable. Biol. Blood Marrow Transplant. 2012, 18, 1533–1539. [Google Scholar] [CrossRef] [Green Version]
- Weissinger, E.M.; Metzger, J.; Schleuning, M.; Schmid, C.; Messinger, D.; Beutel, G.; Wagner-Drouet, E.M.; Schetelig, J.; Baurmann, H.; Rank, A.; et al. A multicenter prospective, randomized, placebo-controlled phase II/III trial for preemptive acute graft-versus-host disease therapy. Leukemia 2021, 35, 1763–1772. [Google Scholar] [CrossRef]
- Weissinger, E.M.; Schiffer, E.; Hertenstein, B.; Ferrara, J.L.; Holler, E.; Stadler, M.; Kolb, H.J.; Zander, A.; Zurbig, P.; Kellmann, M.; et al. Proteomic patterns predict acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood 2007, 109, 5511–5519. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, T.; Kamal, H.; Rank, A.; Kolb, H.J.; Holler, E.; Ganser, A.; Hertenstein, B.; Mischak, H.; Weissinger, E.M. Proteomics applied to the clinical follow-up of patients after allogeneic hematopoietic stem cell transplantation. Blood 2004, 104, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, R.; Daniels, J.; Fusaro, V.; Lundqvist, A.; Killian, J.K.; Geho, D.; Quezado, M.; Kleiner, D.; Rucker, S.; Espina, V.; et al. Accurate diagnosis of acute graft-versus-host disease using serum proteomic pattern analysis. Exp. Hematol. 2006, 34, 796–801. [Google Scholar] [CrossRef]
- Geyer, P.E.; Holdt, L.M.; Teupser, D.; Mann, M. Revisiting biomarker discovery by plasma proteomics. Mol. Syst. Biol. 2017, 13, 942. [Google Scholar] [CrossRef]
- Burger, B.; Lereim, R.R.; Berven, F.S.; Barsnes, H. Detecting single amino acids and small peptides by combining isobaric tags and peptidomics. Eur. J. Mass Spectrom. 2019, 25, 451–456. [Google Scholar] [CrossRef]

| No aGvHD | Cutaneous aGvHD | Gastrointestinal aGvHD | Cutaneous and Gastrointestinal aGvHD | ||
|---|---|---|---|---|---|
| Group size (n) | 10 | 10 | 10 | 9 | |
| Age; median (range) | 44 (22–58) | 41 (20–68) | 33.5 (19–57) | 58 (21–66) | |
| Gender | Female | 3 | 3 | 1 | 4 |
| Male | 7 | 7 | 9 | 5 | |
| Conditioning regimen | Myeloablative | 6 | 6 | 7 | 2 |
| Reduced intensity | 4 | 4 | 3 | 7 | |
| Indication of aHSCT | 1ALL | 2 | 2 | 4 | 1 |
| 2AML | 4 | 3 | 4 | 2 | |
| 3CML | 0 | 1 | 0 | 0 | |
| 5HD | 1 | 0 | 0 | 0 | |
| 9MPN | 1 | 1 | 1 | 1 | |
| 6MDS | 0 | 1 | 1 | 5 | |
| 8MM | 1 | 1 | 0 | 0 | |
| 10NHL | 1 | 1 | 0 | 0 | |
| Donor type | Sibling | 3 | 6 | 4 | 2 |
| 4Haplo | 0 | 1 | 1 | 2 | |
| 7MUD | 7 | 3 | 5 | 5 | |
| Status (aHSCT + day 100) | Dead | 3 | 2 | 2 | 7 |
| Alive | 7 | 8 | 7 | 2 | |
| Unknown | 0 | 0 | 1 | 0 | |
| aGvHD grade | 0 | 10 | 0 | 0 | 0 |
| I | 0 | 4 | 2 | 0 | |
| II | 0 | 4 | 4 | 4 | |
| III | 0 | 0 | 1 | 1 | |
| III-IV | 0 | 0 | 2 | 3 | |
| IV | 0 | 2 | 1 | 1 | |
| No aGvHD | Cutaneous aGvHD | Gastrointestinal aGvHD | Cutaneous and Gastrointestinal aGvHD | ||
|---|---|---|---|---|---|
| Group size (n) | 40 | 14 | 7 | 6 | |
| Age; median (range) | 43 (19–66) | 43 (21–63) | 39 (20–46) | 36 (19–66) | |
| Gender | Female | 19 | 8 | 4 | 5 |
| Male | 21 | 6 | 3 | 1 | |
| Conditioning regimen | Myeloablative | 28 | 10 | 5 | 4 |
| Reduced intensity | 12 | 4 | 2 | 2 | |
| Indication of aHSCT | 1ALL | 0 | 1 | 0 | 0 |
| 2AML | 17 | 2 | 1 | 1 | |
| 2AML/6MDS | 3 | 0 | 0 | 0 | |
| 3B-ALL | 3 | 1 | 3 | 2 | |
| 4B-TH | 1 | 0 | 0 | 0 | |
| 7HLH | 0 | 0 | 0 | 1 | |
| 8MDS | 4 | 2 | 0 | 1 | |
| 9MDS RAEB-II | 0 | 1 | 0 | 0 | |
| 10MF | 4 | 1 | 0 | 0 | |
| 11MM | 0 | 0 | 1 | 1 | |
| 12MPN | 1 | 0 | 0 | 0 | |
| 14NHL | 0 | 2 | 0 | 0 | |
| 16PreB-ALL | 0 | 1 | 0 | 0 | |
| 17PNH | 1 | 1 | 0 | 0 | |
| kidney amyloidosis | 1 | 0 | 0 | 0 | |
| Sézary disease | 1 | 0 | 0 | 0 | |
| 18SAA | 2 | 1 | 1 | 0 | |
| 19T-ALL | 2 | 1 | 1 | 0 | |
| Donor type | Sibling | 14 | 4 | 3 | 2 |
| 6Haplo | 7 | 3 | 0 | 1 | |
| 13MUD | 19 | 7 | 4 | 3 | |
| Status (aHSCT + day 100) | Dead | 10 | 3 | 3 | 2 |
| Alive | 30 | 11 | 4 | 4 | |
| Unknown | 0 | 0 | 0 | 0 | |
| aGvHD grade | 0 | 40 | 0 | 0 | 0 |
| I | 0 | 5 | 0 | 0 | |
| II | 0 | 9 | 4 | 5 | |
| II-III | 0 | 0 | 1 | 1 | |
| III | 0 | 0 | 2 | 0 | |
| IV | 0 | 0 | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupsa, N.; Szegedi, Á.; Gézsi, A.; Vuncs, Z.; Masszi, T.; Mikala, G.; Reményi, P.; Deola, S.; Lakshmanan, A.P.; Terranegra, A.; et al. Decreased Plasma Level of Cytokeratin 20 (KRT20) Is Indicative of the Emergence and Severity of Acute GvHD Irrespective to the Type of Organ Involvement. Biomedicines 2022, 10, 519. https://doi.org/10.3390/biomedicines10030519
Lupsa N, Szegedi Á, Gézsi A, Vuncs Z, Masszi T, Mikala G, Reményi P, Deola S, Lakshmanan AP, Terranegra A, et al. Decreased Plasma Level of Cytokeratin 20 (KRT20) Is Indicative of the Emergence and Severity of Acute GvHD Irrespective to the Type of Organ Involvement. Biomedicines. 2022; 10(3):519. https://doi.org/10.3390/biomedicines10030519
Chicago/Turabian StyleLupsa, Nikolett, Ákos Szegedi, András Gézsi, Zoltán Vuncs, Tamás Masszi, Gábor Mikala, Péter Reményi, Sara Deola, Arun Prasath Lakshmanan, Annalisa Terranegra, and et al. 2022. "Decreased Plasma Level of Cytokeratin 20 (KRT20) Is Indicative of the Emergence and Severity of Acute GvHD Irrespective to the Type of Organ Involvement" Biomedicines 10, no. 3: 519. https://doi.org/10.3390/biomedicines10030519
APA StyleLupsa, N., Szegedi, Á., Gézsi, A., Vuncs, Z., Masszi, T., Mikala, G., Reményi, P., Deola, S., Lakshmanan, A. P., Terranegra, A., Buzás, E. I., & Pós, Z. (2022). Decreased Plasma Level of Cytokeratin 20 (KRT20) Is Indicative of the Emergence and Severity of Acute GvHD Irrespective to the Type of Organ Involvement. Biomedicines, 10(3), 519. https://doi.org/10.3390/biomedicines10030519










