Quantitative Metabolomics of Tissue, Perfusate, and Bile from Rat Livers Subjected to Normothermic Machine Perfusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Surgical Procedure
2.3. Normothermic Machine Perfusion (NMP) Protocol
2.4. Collection, Processing, and Analysis of Perfusate and Bile Samples
2.5. Tissue Sample Collection and Analyses
2.6. Nuclear Magnetic Resonance (NMR) Spectroscopy-Based Metabolomics
2.7. Statistical Analysis
3. Results
3.1. Baseline Perfusate Composition
3.2. Oxygen Delivery/Consumption during NMP
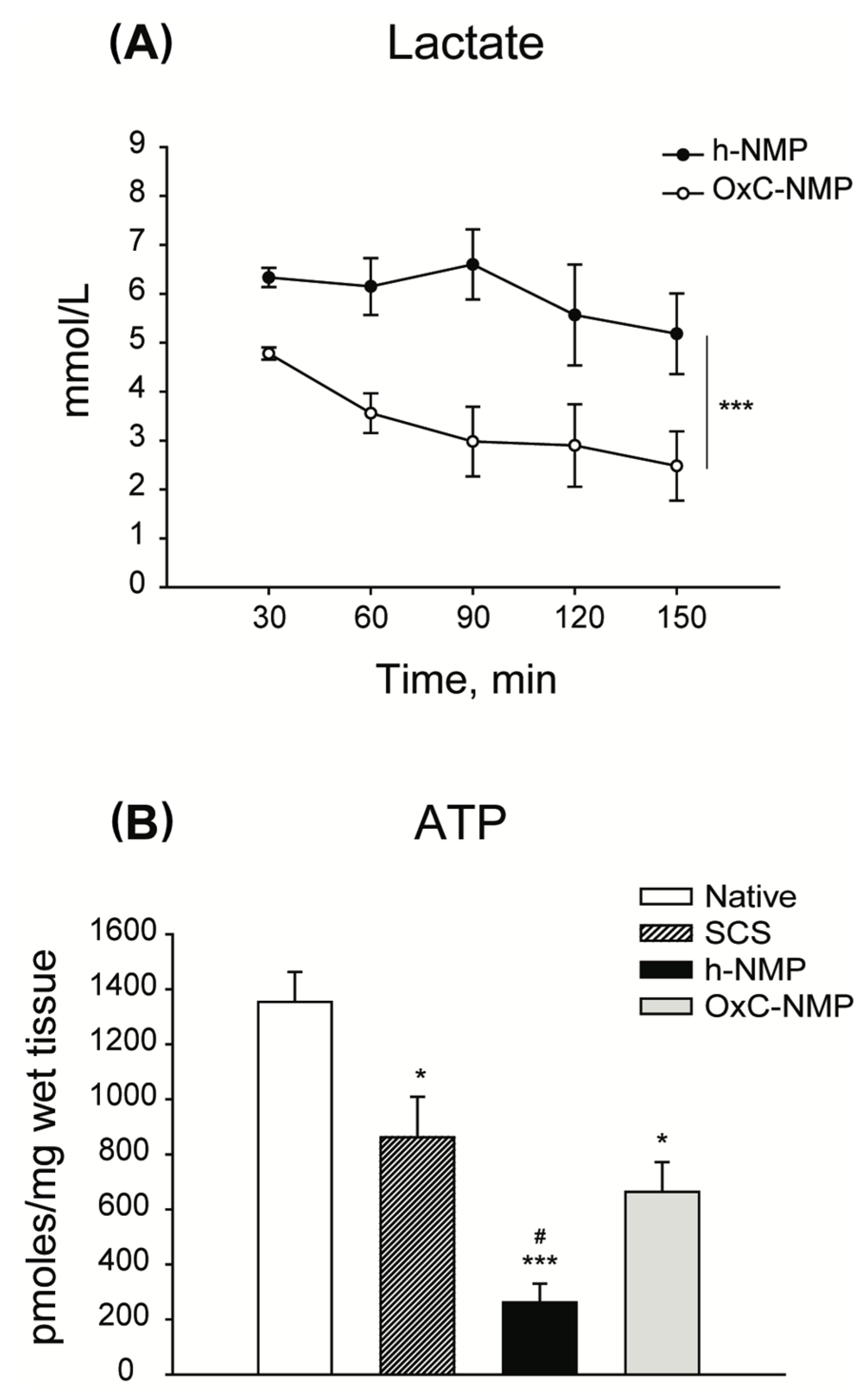
3.3. Liver Graft Viability and Function
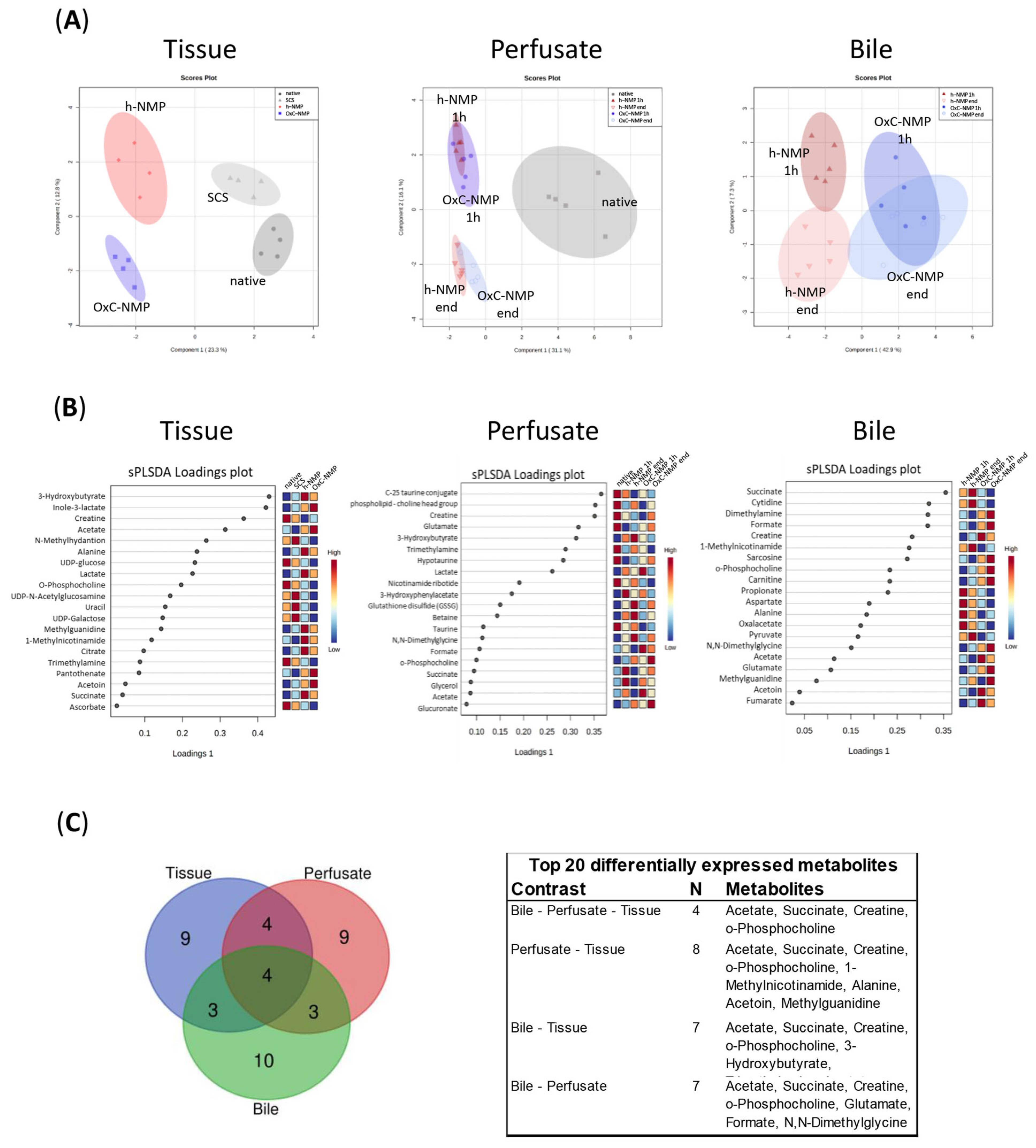
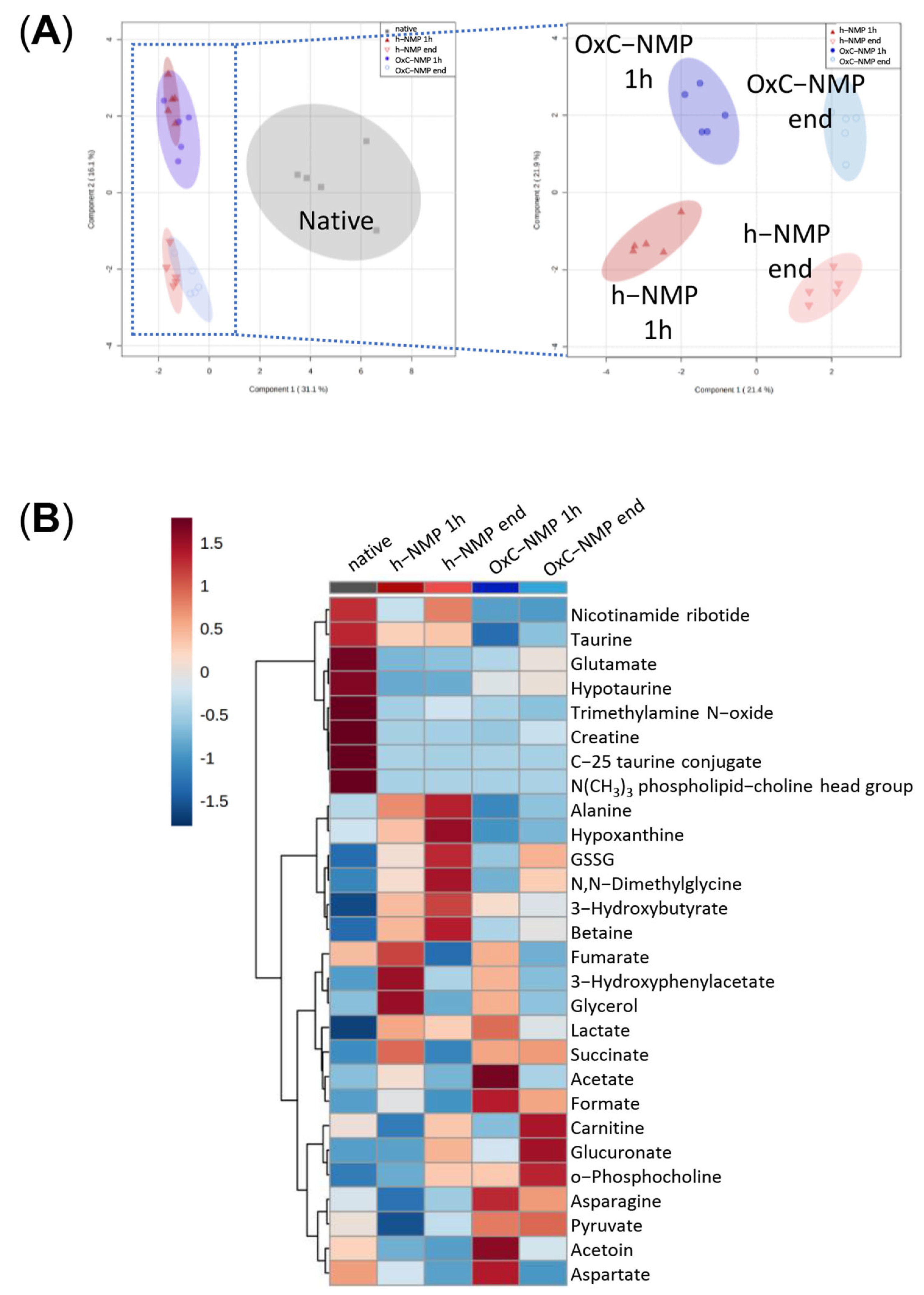
3.4. Unsupervised Analysis of Tissue, Perfusate, and Bile Samples
3.5. Metabolite Changes in Liver Biopsies and Perfusate Samples
3.6. Metabolite Changes in Bile Samples
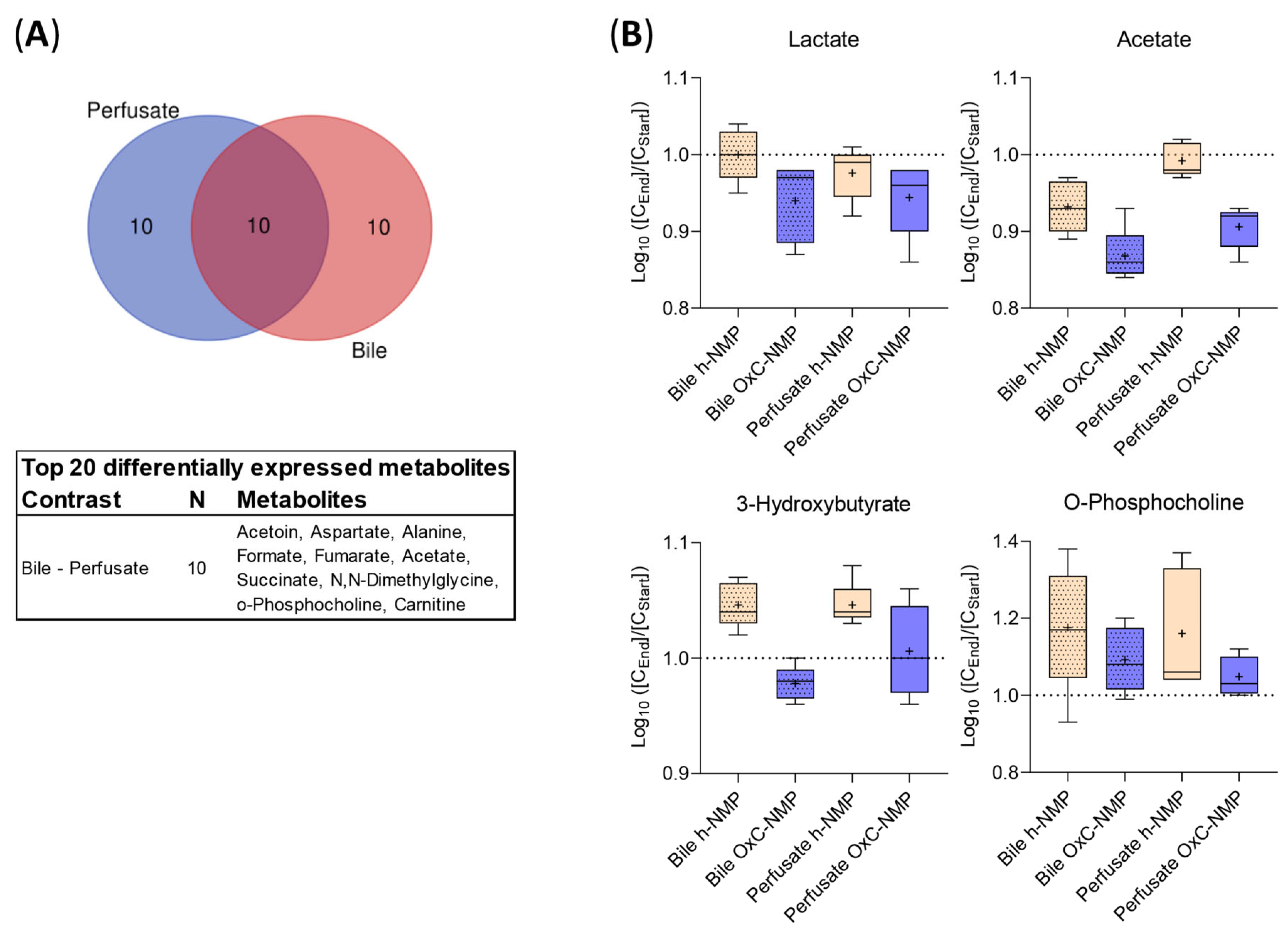
3.7. Comparison between Metabolite Concentration in Bile and Perfusate Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghinolfi, D.; Dondossola, D.; Rreka, E.; Lonati, C.; Pezzati, D.; Cacciatoinsilla, A.; Kersik, A.; Lazzeri, C.; Zanella, A.; Peris, A.; et al. Sequential Use of Normothermic Regional and Ex Situ Machine Perfusion in Donation After Circulatory Death Liver Transplant. Liver Transplant. 2021, 27, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Ghinolfi, D.; Lai, Q.; Dondossola, D.; De Carlis, R.; Zanierato, M.; Patrono, D.; Baroni, S.; Bassi, D.; Ferla, F.; Lauterio, A.; et al. Machine Perfusions in Liver Transplantation: The Evidence-Based Position Paper of the Italian Society of Organ and Tissue Transplantation. Liver Transplant. 2020, 26, 1298–1315. [Google Scholar] [CrossRef] [PubMed]
- Bral, M.; Gala-Lopez, B.; Bigam, D.L.; Freed, D.H.; Shapiro, A.M.J. Ex situ liver perfusion: Organ preservation into the future. Transplant. Rev. 2018, 32, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Michelotto, J.; Gassner, J.M.G.V.; Moosburner, S.; Muth, V.; Patel, M.S.; Selzner, M.; Pratschke, J.; Sauer, I.M.; Raschzok, N. Ex vivo machine perfusion: Current applications and future directions in liver transplantation. Langenbeck’s Arch. Surg. 2021, 406, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Vogel, T.; Brockmann, J.G.; Coussios, C.; Friend, P.J. The role of normothermic extracorporeal perfusion in minimizing ischemia reperfusion injury. Transplant. Rev. 2012, 26, 156–162. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.L.; Gruszczyk, A.V.; Beach, T.E.; Murphy, M.P.; Saeb-Parsy, K. Mitochondrial mechanisms and therapeutics in ischaemia reperfusion injury. Pediatr. Nephrol. 2019, 34, 1167–1174. [Google Scholar] [CrossRef] [Green Version]
- Saeb-Parsy, K.; Martin, J.L.; Summers, D.M.; Watson, C.J.E.; Krieg, T.; Murphy, M.P. Mitochondria as Therapeutic Targets in Transplantation. Trends Mol. Med. 2021, 27, 185–198. [Google Scholar] [CrossRef]
- Peralta, C.; Bartrons, R.; Riera, L.; Manzano, A.; Xaus, C.; Gelpí, E.; Roselló-Catafau, J. Hepatic preconditioning preserves energy metabolism during sustained ischemia. Am. J. Physiol.-Gastrointest. Liver Physiol. 2000, 279, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S. Metabolomics: The principles and potential applications to transplantation. Am. J. Transplant. 2005, 5, 2814–2820. [Google Scholar] [CrossRef]
- Panconesi, R.; Flores Carvalho, M.; Mueller, M.; Dutkowski, P.; Muiesan, P.; Schlegel, A. Mitochondrial Reprogramming—What Is the Benefit of Hypothermic Oxygenated Perfusion in Liver Transplantation? Transplantology 2021, 2, 15. [Google Scholar] [CrossRef]
- Go, K.L.; Lee, S.; Zendejas, I.; Behrns, K.E.; Kim, J.S. Mitochondrial Dysfunction and Autophagy in Hepatic Ischemia/Reperfusion Injury. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.A.; Richards, D.A.; Bramhall, S.R.; Adams, D.H.; Mirza, D.F. A Study of the Metabolites of Ischemia-Reperfusion Injury and Selected Amino Acids in the Liver Using Microdialysis during Transplantation. Transplantation 2005, 79, 828–835. [Google Scholar] [CrossRef]
- Hrydziuszko, O.; Silva, M.A.; PR Perera, M.T.; Richards, D.A.; Murphy, N.; Mirza, D.; Viant, M.R. Application of metabolomics to investigate the process of human orthotopic liver transplantation: A proof-of-principle study. OMICS 2010, 14, 143–150. [Google Scholar] [CrossRef]
- Singh, H.K.; Yachha, S.K.; Saxena, R.; Gupta, A.; Nagana Gowda, G.A.; Bhandari, M.; Khetrapal, C.L. A new dimension of 1H-NMR spectroscopy in assessment of liver graft dysfunction. NMR Biomed. 2003, 16, 185–188. [Google Scholar] [CrossRef]
- Cortes, M.; Pareja, E.; García-Cañaveras, J.C.; Donato, M.T.; Montero, S.; Mir, J.; Castell, J.V.; Lahoz, A. Metabolomics discloses donor liver biomarkers associated with early allograft dysfunction. J. Hepatol. 2014, 61, 564–574. [Google Scholar] [CrossRef]
- Faitot, F.; Besch, C.; Battini, S.; Ruhland, E.; Onea, M.; Addeo, P.; Woehl-Jaeglé, M.L.; Ellero, B.; Bachellier, P.; Namer, I.J. Impact of real-time metabolomics in liver transplantation: Graft evaluation and donor-recipient matching. J. Hepatol. 2018, 68, 699–706. [Google Scholar] [CrossRef]
- Dondossola, D.; Santini, A.; Lonati, C.; Zanella, A.; Merighi, R.; Vivona, L.; Battistin, M.; Galli, A.; Biancolilli, O.; Maggioni, M.; et al. Human Red Blood Cells as Oxygen Carriers to Improve Ex-Situ Liver Perfusion in a Rat Model. J. Clin. Med. 2019, 8, 1918. [Google Scholar] [CrossRef] [Green Version]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Roffia, V.; De Palma, A.; Lonati, C.; Silvestre, D.D.; Rossi, R.; Mantero, M.; Gatti, S.; Dondossola, D.; Valenza, F.; Mauri, P.; et al. Proteome investigation of rat lungs subjected to Ex vivo perfusion (EVLP). Molecules 2018, 23, 3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonati, C.; Battistin, M.; Dondossola, D.E.; Bassani, G.A.; Brambilla, D.; Merighi, R.; Leonardi, P.; Carlin, A.; Meroni, M.; Zanella, A.; et al. NDP-MSH treatment recovers marginal lungs during ex vivo lung perfusion (EVLP). Peptides 2021, 141, 170552. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; De Rougemont, O.; Graf, R.; Clavien, P.A.; Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 2013, 58, 278–286. [Google Scholar] [CrossRef]
- Bruinsma, B.G.; Sridharan, G.V.; Weeder, P.D.; Avruch, J.H.; Saeidi, N.; Özer, S.; Geerts, S.; Porte, R.J.; Heger, M.; Van Gulik, T.M.; et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hrydziuszko, O.; Thamara, M.T.P.R.; Laing, R.; Kirwan, J.; Silva, M.A.; Richards, D.A.; Murphy, N.; Mirza, D.F.; Viant, M.R. Mass spectrometry based metabolomics comparison of liver grafts from donors after circulatory death (DCD) and Donors after Brain Death (DBD) Used in human orthotopic liver transplantation. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef]
- Martin, J.L.; Costa, A.S.H.; Gruszczyk, A.V.; Beach, T.E.; Allen, F.M.; Prag, H.A.; Hinchy, E.C.; Mahbubani, K.; Hamed, M.; Tronci, L.; et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 2019, 1, 966–974. [Google Scholar] [CrossRef] [Green Version]
- Tretter, L.; Patocs, A.; Chinopoulos, C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 1086–1101. [Google Scholar] [CrossRef]
- Mierziak, J.; Burgberger, M.; Wojtasik, W. 3-Hydroxybutyrate As a Metabolite and a Signal Molecule Regulating Processes of Living Organisms. Biomolecules 2021, 11, 402. [Google Scholar] [CrossRef]
- Rojas-morales, P.; Tapia, E.; Pedraza-chaverri, J. β-Hydroxybutyrate: A signaling metabolite in starvation response ? Cell. Signal. 2016, 28, 917–923. [Google Scholar] [CrossRef]
- Rojas-Morales, P.; Pedraza-Chaverri, J.; Tapia, E. Ketone bodies, stress response, and redox homeostasis. Redox Biol. 2020, 29, 101395. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cui, J.; Ma, H.; Lu, W.; Huang, J. Targeting Pyrimidine Metabolism in the Era of Precision Cancer Medicine. Front. Oncol. 2021, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Duarte, I.F.; Stanley, E.G.; Holmes, E.; Lindon, J.C.; Gil, A.M.; Tang, H.; Ferdinand, R.; McKee, C.G.; Nicholson, J.K.; Vilca-Melendez, H.; et al. Metabolic assessment of human liver transplants from biopsy samples at donor and recipient stages using high-resolution magic angle spinning 1H NMR spectroscopy. Anal. Chem. 2005, 77, 5570–5578. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; da Silva, R.P.; Brosnan, M.E. The metabolic burden of creatine synthesis. Amino Acids 2011, 40, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Zervou, S.; Lygate, C.A. The creatine kinase system as a therapeutic target for myocardial ischaemia-reperfusion injury. Biochem. Soc. Trans. 2018, 46, 1119–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legido-Quigley, C.; Mcdermott, L.; Vilca-Melendez, H.; Murphy, G.M.; Heaton, N.; Lindon, J.C.; Nicholson, J.K.; Holmes, E. Bile UPLC-MS fingerprinting and bile acid fluxes during human liver transplantation. Electrophoresis 2011, 32, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridonos, K.; Garcia-Perez, I.; Angulo, S.; Domann, P.J.; Vilca-Melendez, H.; Heaton, N.; Murphy, G.M.; Holmes, E.; Barbas, C.; Legido-Quigley, C. Fingerprinting of human bile during liver transplantation by capillary electrophoresis. J. Sep. Sci. 2008, 31, 3058–3064. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transplant. 2018, 18, 2005–2020. [Google Scholar] [CrossRef] [Green Version]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.T.P.R.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Perera, M.T.P.R.; Richards, D.A.; Silva, M.A.; Ahmed, N.; Neil, D.A.; Murphy, N.; Mirza, D.F. Comparison of energy metabolism in liver grafts from donors after circulatory death and donors after brain death during cold storage and reperfusion. Br. J. Surg. 2014, 101, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Pan, Q.; Cheng, Y.; Shao, Z.G.; Wang, A.Y.; Liu, Y.F. Prediction of rat liver transplantation outcomes using energy metabolites measured by microdialysis. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 392–401. [Google Scholar] [CrossRef]
- Patrono, D.; Roggio, D.; Mazzeo, A.T.; Catalano, G.; Mazza, E.; Rizza, G.; Gambella, A.; Rigo, F.; Leone, N.; Elia, V.; et al. Clinical assessment of liver metabolism during hypothermic oxygenated machine perfusion using microdialysis. Artif. Organs 2022, 46, 281–295. [Google Scholar] [CrossRef]
- Von Platen, A.; Souza, M.A.D.; Rooyackers, O.; Nowak, G. Intrahepatic Microdialysis for Monitoring of Metabolic Markers to Detect Rejection Early After Liver Transplantation. Transplant. Proc. 2021, 53, 130–135. [Google Scholar] [CrossRef]
- Lonati, C.; Schlegel, A.; Battistin, M.; Merighi, R.; Carbonaro, M.; Dongiovanni, P.; Leonardi, P.; Zanella, A.; Dondossola, D. Effluent Molecular Analysis Guides Liver Graft Allocation to Clinical Hypothermic Oxygenated Machine Perfusion. Biomedicines 2021, 9, 1444. [Google Scholar] [CrossRef]
- Serkova, N.J.; Zhang, Y.; Coatney, J.L.; Hunter, L.; Wachs, M.E.; Niemann, C.U.; Mandell, M.S. Early detection of graft failure using the blood metabolic profile of a liver recipient. Transplantation 2007, 83, 517–521. [Google Scholar] [CrossRef]
- Land, W.G.; Agostinis, P.; Gasser, S.; Garg, A.D.; Linkermann, A. Transplantation and Damage-Associated Molecular Patterns (DAMPs). Am. J. Transplant. 2016, 16, 3338–3361. [Google Scholar] [CrossRef]
- Peralta, C.; Jiménez-Castro, M.B.; Gracia-Sancho, J. Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J. Hepatol. 2013, 59, 1094–1106. [Google Scholar] [CrossRef] [Green Version]
- Ryan, D.G.; Murphy, M.P.; Frezza, C.; Prag, H.A.; Chouchani, E.T.; O’Neill, L.A.; Mills, E.L. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat. Metab. 2019, 1, 16–33. [Google Scholar] [CrossRef]


| Modulation vs. Native Bile | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| h-NMP | OxC-NMP | ||||||||
| Class | Metabolite | Start | End | Start | End | ||||
| (1) Increased | |||||||||
| 3-HB | ↑ | <0.001 | ↑ | <0.001 | ↑ | <0.001 | ↑ | <0.001 | |
| Lactate | ↑ | <0.010 | ↑ | <0.010 | ↑ | <0.001 | ↑ | <0.050 | |
| O-phosphocholine | = | ↑ | <0.050 | = | ↑ | <0.010 | |||
| GSSG | = | ↑ | <0.010 | = | ↑ | <0.050 | |||
| Glucuronic acid | = | ↑ | <0.010 | = | ↑ | <0.0001 | |||
| (2) Decreased | |||||||||
| TMAO | ↓ | <0.001 | ↓ | <0.010 | ↓ | <0.001 | ↓ | <0.001 | |
| Creatine | ↓ | <0.0001 | ↓ | <0.0001 | ↓ | <0.0001 | ↓ | <0.0001 | |
| C-25 tau conjugate | ↓ | <0.0001 | ↓ | <0.0001 | ↓ | <0.0001 | ↓ | <0.0001 | |
| N(CH3)3 phospholipid-CHO | ↓ | <0.0001 | ↓ | <0.0001 | ↓ | <0.0001 | ↓ | <0.0001 | |
| Glutammate | ↓ | <0.0001 | ↓ | <0.0001 | ↓ | <0.0001 | ↓ | <0.0001 | |
| Hypotaurine | ↓ | <0.0001 | ↓ | <0.0001 | ↓ | <0.010 | ↓ | <0.010 | |
| Fumarate | = | ↓ | <0.010 | = | ↓ | <0.010 | |||
| NMN | ↓ | <0.010 | = | ↓ | <0.0001 | ↓ | <0.0001 | ||
| (3) Increased at the “start” and restored at the “end” | |||||||||
| Acetate | ↑ | <0.050 | = | ↑ | <0.0001 | = | |||
| Glycerol | ↑ | <0.0001 | = | ↑ | <0.050 | = | |||
| 3-HPA | ↑ | <0.0001 | = | ↑ | <0.0001 | = | |||
| (4) Unchanged | |||||||||
| Alanine | = | = | = | = | |||||
| Hypoxantine | = | = | = | = | |||||
| Carnitine | = | = | = | = | |||||
| (5) Differentially modulated in the h-NMP and OxC-NMP groups | |||||||||
| DMG | ↑ | ↑ | <0.010 | = | = | ||||
| Betaine | = | ↑ | <0.010 | = | = | ||||
| Formate | = | = | ↑ | <0.001 | ↑ | <0.050 | |||
| Taurine | = | = | ↓ | <0.050 | ↓ | <0.050 | |||
| Asparagine | = | = | ↑ | <0.050 | = | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lonati, C.; Dondossola, D.; Zizmare, L.; Battistin, M.; Wüst, L.; Vivona, L.; Carbonaro, M.; Zanella, A.; Gatti, S.; Schlegel, A.; et al. Quantitative Metabolomics of Tissue, Perfusate, and Bile from Rat Livers Subjected to Normothermic Machine Perfusion. Biomedicines 2022, 10, 538. https://doi.org/10.3390/biomedicines10030538
Lonati C, Dondossola D, Zizmare L, Battistin M, Wüst L, Vivona L, Carbonaro M, Zanella A, Gatti S, Schlegel A, et al. Quantitative Metabolomics of Tissue, Perfusate, and Bile from Rat Livers Subjected to Normothermic Machine Perfusion. Biomedicines. 2022; 10(3):538. https://doi.org/10.3390/biomedicines10030538
Chicago/Turabian StyleLonati, Caterina, Daniele Dondossola, Laimdota Zizmare, Michele Battistin, Leonie Wüst, Luigi Vivona, Margherita Carbonaro, Alberto Zanella, Stefano Gatti, Andrea Schlegel, and et al. 2022. "Quantitative Metabolomics of Tissue, Perfusate, and Bile from Rat Livers Subjected to Normothermic Machine Perfusion" Biomedicines 10, no. 3: 538. https://doi.org/10.3390/biomedicines10030538
APA StyleLonati, C., Dondossola, D., Zizmare, L., Battistin, M., Wüst, L., Vivona, L., Carbonaro, M., Zanella, A., Gatti, S., Schlegel, A., & Trautwein, C. (2022). Quantitative Metabolomics of Tissue, Perfusate, and Bile from Rat Livers Subjected to Normothermic Machine Perfusion. Biomedicines, 10(3), 538. https://doi.org/10.3390/biomedicines10030538






