Photoactive and Luminescent Transition Metal Complexes as Anticancer Agents: A Guiding Light in the Search for New and Improved Cancer Treatments
Abstract
:1. Introduction
2. Iridium Complexes
2.1. Mitochondrial Targeting
2.2. Lysosomal Targeting
2.3. Other Targets
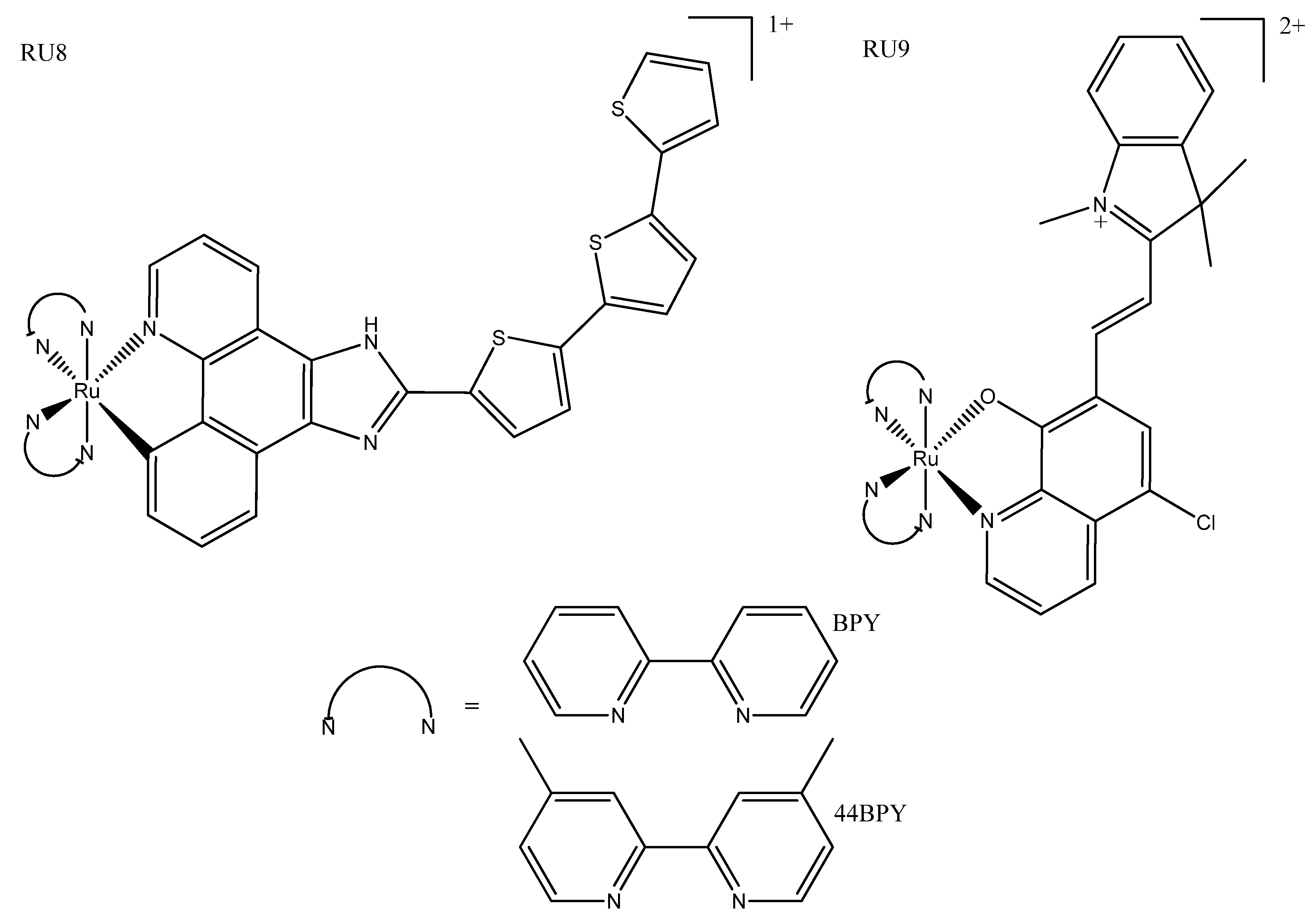
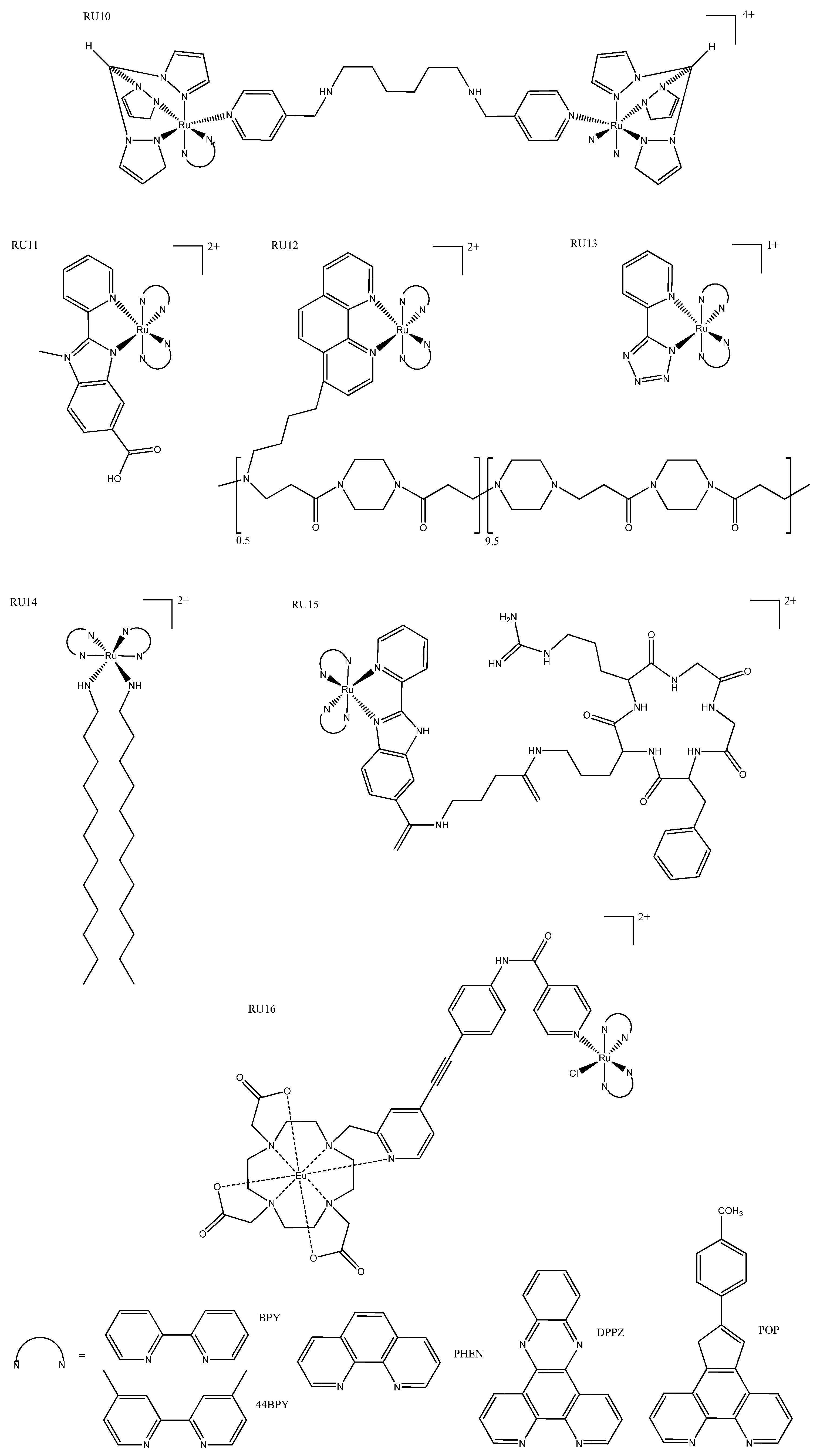
3. Ruthenium Complexes
3.1. Tris(bipyridine)ruthenium(II)-Inspired Complexes
3.2. Piano Stool Complexes
3.3. Cyclometallated/Diverse Coordination Sphere
3.4. Other
4. Platinum Complexes
4.1. Photosensitizers
4.2. Fluorescent Moiety
4.3. Cyclometallated Platinum Complexes
5. Rhenium Complexes
6. Osmium Complexes
7. Other Metal Complexes
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef] [PubMed]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Xu, G.-X.; Mak, E.C.-L.; Lo, K.K.-W. Photofunctional Transition Metal Complexes as Cellular Probes, Bioimaging Reagents and Phototherapeutics. Inorg. Chem. Front. 2021, 8, 4553–4579. [Google Scholar] [CrossRef]
- Ding, S.; Qiao, X.; Suryadi, J.; Marrs, G.S.; Kucera, G.L.; Bierbach, U. Using Fluorescent Post-Labeling to Probe the Subcellular Localization of DNA-Targeted Platinum Anticancer Agents. Angew. Chem. Int. Ed. 2013, 52, 3350–3354. [Google Scholar] [CrossRef] [Green Version]
- Jhaveri, A.; Torchilin, V. Intracellular Delivery of Nanocarriers and Targeting to Subcellular Organelles. Expert Opin. Drug Deliv. 2016, 13, 49–70. [Google Scholar] [CrossRef]
- Qiu, K.; Chen, Y.; Rees, T.W.; Ji, L.; Chao, H. Organelle-Targeting Metal Complexes: From Molecular Design to Bio-Applications. Coord. Chem. Rev. 2019, 378, 66–86. [Google Scholar] [CrossRef]
- Tan, C.-P.; Zhong, Y.-M.; Ji, L.-N.; Mao, Z.-W. Phosphorescent Metal Complexes as Theranostic Anticancer Agents: Combining Imaging and Therapy in a Single Molecule. Chem. Sci. 2021, 12, 2357–2367. [Google Scholar] [CrossRef]
- Zuluaga, M.-F.; Lange, N. Combination of Photodynamic Therapy with Anti-Cancer Agents. Curr. Med. Chem. 2008, 15, 1655–1673. [Google Scholar] [CrossRef] [Green Version]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef]
- Juarranz, A.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic Therapy of Cancer. Basic Principles and Applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Dougherty, T.J. Basic Principles of Photodynamic Therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical Properties of Human Skin, Subcutaneous and Mucous Tissues in the Wavelength Range from 400 to 2000 nm. J. Phys. Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second Window for in vivo Imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, R.R. Photodynamic Therapy: Oncologic Horizons. Available online: https://www.futuremedicine.com/doi/abs/10.2217/fon.13.176 (accessed on 25 January 2022).
- Welsher, K.; Sherlock, S.P.; Dai, H. Deep-Tissue Anatomical Imaging of Mice Using Carbon Nanotube Fluorophores in the Second near-Infrared Window. Proc. Natl. Acad. Sci. USA 2011, 108, 8943–8948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gkika, K.S.; Byrne, A.; Keyes, T.E. Mitochondrial Targeted Osmium Polypyridyl Probe Shows Concentration Dependent Uptake, Localisation, and Mechanism of Cell Death. Dalton Trans. 2019, 48, 17461–17471. [Google Scholar] [CrossRef]
- Guan, R.; Xie, L.; Ji, L.; Chao, H. Phosphorescent Iridium(III) Complexes for Anticancer Applications. Eur. J. Inorg. Chem. 2020, 2020, 3978–3986. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Xu, C.-X.; He, L.; Wan, Y.-C.; Ji, L.-N.; Mao, Z.-W. Mitochondria-Targeted Phosphorescent Cyclometalated Iridium(III) Complexes: Synthesis, Characterization, and Anticancer Properties. J. Biol. Inorg. Chem. 2020, 25, 597–607. [Google Scholar] [CrossRef]
- Wang, F.-X.; Chen, M.-H.; Hu, X.-Y.; Ye, R.-R.; Tan, C.-P.; Ji, L.-N.; Mao, Z.-W. Ester-Modified Cyclometalated Iridium(III) Complexes as Mitochondria-Targeting Anticancer Agents. Sci. Rep. 2016, 6, 38954. [Google Scholar] [CrossRef]
- Hao, L.; Li, Z.-W.; Zhang, D.-Y.; He, L.; Liu, W.; Yang, J.; Tan, C.-P.; Ji, L.-N.; Mao, Z.-W. Monitoring Mitochondrial Viscosity with Anticancer Phosphorescent Ir(III) Complexes via Two-Photon Lifetime Imaging. Chem. Sci. 2019, 10, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; He, L.; Zhang, D.-Y.; Tan, C.-P.; Ji, L.-N.; Mao, Z.-W. Mixed-Ligand Iridium(III) Complexes as Photodynamic Anticancer Agents. Dalton Trans. 2017, 46, 11395–11407. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Lu, X.-R.; Li, M.-F.; Ji, L.-N.; Mao, Z.-W. Cyclometalated Iridium(III) N-Heterocyclic Carbene Complexes as Potential Mitochondrial Anticancer and Photodynamic Agents. Dalton Trans. 2017, 46, 11363–11371. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.-N.; He, L.; Ji, L.-N.; Mao, Z.-W. Synthesis, Photophysical and Anticancer Properties of Mitochondria-Targeted Phosphorescent Cyclometalated Iridium(III) N-Heterocyclic Carbene Complexes. J. Inorg. Biochem. 2020, 205, 110976. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, S.; Tian, Z.; Xu, Z.; Zhang, Y.; Xia, X.; Chen, X.; Liu, Z. Potential Anticancer Agent for Selective Damage to Mitochondria or Lysosomes: Naphthalimide-Modified Fluorescent Biomarker Half-Sandwich Iridium (III) and Ruthenium (II) Complexes. Eur. J. Med. Chem. 2019, 181, 111599. [Google Scholar] [CrossRef]
- Wu, X.-W.; Zheng, Y.; Wang, F.-X.; Cao, J.-J.; Zhang, H.; Zhang, D.-Y.; Tan, C.-P.; Ji, L.-N.; Mao, Z.-W. Anticancer IrIII–Aspirin Conjugates for Enhanced Metabolic Immuno-Modulation and Mitochondrial Lifetime Imaging. Chem. Weinh. Bergstr. Ger. 2019, 25, 7012–7022. [Google Scholar] [CrossRef]
- Ye, R.-R.; Cao, J.-J.; Tan, C.-P.; Ji, L.-N.; Mao, Z.-W. Valproic Acid-Functionalized Cyclometalated Iridium(III) Complexes as Mitochondria-Targeting Anticancer Agents. Chem. Eur. J. 2017, 23, 15166–15176. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, Q.; Hao, L.; Yang, G.-G.; Hu, W.-L.; Ji, L.-N.; Mao, Z.-W. A Self-Assessed Photosensitizer: Inducing and Dual-Modal Phosphorescence Imaging of Mitochondria Oxidative Stress. Chem. Commun. 2018, 54, 271–274. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Zhao, Z.; Lin, K.; Wang, J. A Phosphorescent Cyclometalated Iridium(III) Complex as Mitochondria-Targeted Theranostic Anticancer Agent. Inorg. Chem. Commun. 2018, 94, 75–79. [Google Scholar] [CrossRef]
- Ye, R.-R.; Tan, C.-P.; Ji, L.-N.; Mao, Z.-W. Coumarin-Appended Phosphorescent Cyclometalated Iridium(III) Complexes as Mitochondria-Targeted Theranostic Anticancer Agents. Dalton Trans. 2016, 45, 13042–13051. [Google Scholar] [CrossRef]
- Li, J.; Tian, Z.; Ge, X.; Xu, Z.; Feng, Y.; Liu, Z. Design, Synthesis, and Evaluation of Fluorine and Naphthyridine-Based Half-Sandwich Organoiridium/Ruthenium Complexes with Bioimaging and Anticancer Activity. Eur. J. Med. Chem. 2019, 163, 830–839. [Google Scholar] [CrossRef]
- Li, J.; Tian, Z.; Xu, Z.; Zhang, S.; Feng, Y.; Zhang, L.; Liu, Z. Highly Potent Half-Sandwich Iridium and Ruthenium Complexes as Lysosome-Targeted Imaging and Anticancer Agents. Dalton Trans. 2018, 47, 15772–15782. [Google Scholar] [CrossRef]
- Du, Q.; Yang, Y.; Guo, L.; Tian, M.; Ge, X.; Tian, Z.; Zhao, L.; Xu, Z.; Li, J.; Liu, Z. Fluorescent Half-Sandwich Phosphine-Sulfonate Iridium(III) and Ruthenium(II) Complexes as Potential Lysosome-Targeted Anticancer Agents. Dyes Pigments 2019, 162, 821–830. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Tian, Z.; Gong, Y.; Zheng, H.; Zhang, S.; Xu, Z.; Ge, X.; Liu, Z. Novel and Versatile Imine-N-Heterocyclic Carbene Half-Sandwich Iridium(III) Complexes as Lysosome-Targeted Anticancer Agents. Inorg. Chem. 2018, 57, 11087–11098. [Google Scholar] [CrossRef]
- Ma, W.; Guo, L.; Tian, Z.; Zhang, S.; He, X.; Li, J.; Yang, Y.; Liu, Z. Rhodamine-Modified Fluorescent Half-Sandwich Iridium and Ruthenium Complexes: Potential Application as Bioimaging and Anticancer Agents. Dalton Trans. 2019, 48, 4788–4793. [Google Scholar] [CrossRef]
- Liu, Z.; Sadler, P.J. Organoiridium Complexes: Anticancer Agents and Catalysts. Acc. Chem. Res. 2014, 47, 1174–1185. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Ge, X.; Zhang, S.; Xu, Z.; Gao, W. Design, Synthesis, and Evaluation of Phosphorescent Ir(III) Complexes with Anticancer Activity. J. Inorg. Biochem. 2019, 197, 110703. [Google Scholar] [CrossRef]
- Wang, F.-X.; Chen, M.-H.; Lin, Y.-N.; Zhang, H.; Tan, C.-P.; Ji, L.-N.; Mao, Z.-W. Dual Functions of Cyclometalated Iridium(III) Complexes: Anti-Metastasis and Lysosome-Damaged Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 42471–42481. [Google Scholar] [CrossRef]
- Liu, X.; Hao, H.; Ge, X.; He, X.; Liu, Y.; Wang, Y.; Wang, H.; Shao, M.; Jing, Z.; Tian, L.; et al. Triphenylamine-Appended Cyclometallated Iridium(III) Complexes: Preparation, Photophysical Properties and Application in Biology/Luminescence Imaging. J. Inorg. Biochem. 2019, 199, 110757. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, H.; Banerjee, S.; Clarkson, G.J.; Ge, C.; Imberti, C.; Sadler, P.J. Nucleus-Targeted Organoiridium-Albumin Conjugate for Photodynamic Cancer Therapy. Angew. Chem. 2019, 58, 2350–2354. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Alonso, M.; Busto, N.; Aguirre, L.D.; Berlanga, L.; Carrión, M.C.; Cuevas, J.V.; Rodríguez, A.M.; Carbayo, A.; Manzano, B.R.; Ortí, E.; et al. Strong Influence of the Ancillary Ligand over the Photodynamic Anticancer Properties of Neutral Biscyclometalated IrIII Complexes Bearing 2-Benzoazole-Phenolates. Chem. Eur. J. 2018, 24, 17523–17537. [Google Scholar] [CrossRef] [Green Version]
- Paitandi, R.P.; Mukhopadhyay, S.; Singh, R.S.; Sharma, V.; Mobin, S.M.; Pandey, D.S. Anticancer Activity of Iridium(III) Complexes Based on a Pyrazole-Appended Quinoline-Based BODIPY. Inorg. Chem. 2017, 56, 12232–12247. [Google Scholar] [CrossRef]
- Chen, F.; Tu, J.; Liang, C.; Yang, B.; Chen, C.; Chen, X.; Cai, C. Fluorescent Drug Screening Based on Aggregation of DNA-Templated Silver Nanoclusters, and Its Application to Iridium (III) Derived Anticancer Drugs. Microchim. Acta 2016, 183, 1571–1577. [Google Scholar] [CrossRef]
- Yellol, J.; Pérez, S.A.; Yellol, G.; Zajac, J.; Donaire, A.; Vigueras, G.; Novohradsky, V.; Janiak, C.; Brabec, V.; Ruiz, J. Highly Potent Extranuclear-Targeted Luminescent Iridium(III) Antitumor Agents Containing Benzimidazole-Based Ligands with a Handle for Functionalization. Chem. Commun. 2016, 52, 14165–14168. [Google Scholar] [CrossRef]
- Thomas, S.J.; Balónová, B.; Cinatl, J.J.; Wass, M.N.; Serpell, C.J.; Blight, B.A.; Michaelis, M. Thiourea and Guanidine Compounds and Their Iridium Complexes in Drug-Resistant Cancer Cell Lines: Structure-Activity Relationships and Direct Luminescent Imaging. ChemMedChem 2020, 15, 349–353. [Google Scholar] [CrossRef]
- Hisamatsu, Y.; Suzuki, N.; Masum, A.-A.; Shibuya, A.; Abe, R.; Sato, A.; Tanuma, S.-I.; Aoki, S. Cationic Amphiphilic Tris-Cyclometalated Iridium(III) Complexes Induce Cancer Cell Death via Interaction with Ca2+-Calmodulin Complex. Bioconjug. Chem. 2017, 28, 507–523. [Google Scholar] [CrossRef]
- Pracharova, J.; Vigueras, G.; Novohradsky, V.; Cutillas, N.; Janiak, C.; Kostrhunova, H.; Kasparkova, J.; Ruiz, J.; Brabec, V. Exploring the Effect of Polypyridyl Ligands on the Anticancer Activity of Phosphorescent Iridium(III) Complexes: From Proteosynthesis Inhibitors to Photodynamic Therapy Agents. Chem. Eur. J. 2018, 24, 4607–4619. [Google Scholar] [CrossRef]
- Liu, B.; Monro, S.; Lystrom, L.; Cameron, C.G.; Colón, K.; Yin, H.; Kilina, S.; McFarland, S.A.; Sun, W. Photophysical and Photobiological Properties of Dinuclear Iridium(III) Bis-Tridentate Complexes. Inorg. Chem. 2018, 57, 9859–9872. [Google Scholar] [CrossRef]
- Masum, A.-A.; Yokoi, K.; Hisamatsu, Y.; Naito, K.; Shashni, B.; Aoki, S. Design and Synthesis of a Luminescent Iridium Complex-Peptide Hybrid (IPH) That Detects Cancer Cells and Induces Their Apoptosis. Bioorg. Med. Chem. 2018, 26, 4804–4816. [Google Scholar] [CrossRef]
- Xiang, H.; Chen, H.; Tham, H.P.; Phua, S.Z.F.; Liu, J.-G.; Zhao, Y. Cyclometalated Iridium(III)-Complex-Based Micelles for Glutathione-Responsive Targeted Chemotherapy and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 27553–27562. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- McFarland, S.A.; Mandel, A.; Dumoulin-White, R.; Gasser, G. Metal-Based Photosensitizers for Photodynamic Therapy: The Future of Multimodal Oncology? Curr. Opin. Chem. Biol. 2020, 56, 23–27. [Google Scholar] [CrossRef]
- Hess, J.; Huang, H.; Kaiser, A.; Pierroz, V.; Blacque, O.; Chao, H.; Gasser, G. Evaluation of the Medicinal Potential of Two Ruthenium(II) Polypyridine Complexes as One- and Two-Photon Photodynamic Therapy Photosensitizers. Chem. Eur. J. 2017, 23, 9888–9896. [Google Scholar] [CrossRef]
- Qu, F.; Lamb, R.W.; Cameron, C.G.; Park, S.; Oladipupo, O.; Gray, J.L.; Xu, Y.; Cole, H.D.; Bonizzoni, M.; Kim, Y.; et al. Singlet Oxygen Formation vs. Photodissociation for Light-Responsive Protic Ruthenium Anticancer Compounds: The Oxygenated Substituent Determines Which Pathway Dominates. Inorg. Chem. 2021, 60, 2138–2148. [Google Scholar] [CrossRef]
- Wang, L.; Yin, H.; Jabed, M.A.; Hetu, M.; Wang, C.; Monro, S.; Zhu, X.; Kilina, S.; McFarland, S.A.; Sun, W. π-Expansive Heteroleptic Ruthenium(II) Complexes as Reverse Saturable Absorbers and Photosensitizers for Photodynamic Therapy. Inorg. Chem. 2017, 56, 3245–3259. [Google Scholar] [CrossRef]
- Estalayo-Adrián, S.; Blasco, S.; Bright, S.A.; McManus, G.J.; Orellana, G.; Williams, D.C.; Kelly, J.M.; Gunnlaugsson, T. Effect of Alkyl Chain Length on the Photophysical, Photochemical, and Photobiological Properties of Ruthenium(II) Polypyridyl Complexes for Their Application as DNA-Targeting, Cellular-Imaging, and Light-Activated Therapeutic Agents. ACS Appl. Bio Mater. 2021, 4, 6664–6681. [Google Scholar] [CrossRef]
- Lari, M.; Martínez-Alonso, M.; Busto, N.; Manzano, B.R.; Rodríguez, A.M.; Acuña, M.I.; Domínguez, F.; Albasanz, J.L.; Leal, J.M.; Espino, G.; et al. Strong Influence of Ancillary Ligands Containing Benzothiazole or Benzimidazole Rings on Cytotoxicity and Photoactivation of Ru(II) Arene Complexes. Inorg. Chem. 2018, 57, 14322–14336. [Google Scholar] [CrossRef]
- Srivastava, P.; Verma, M.; Kumar, A.; Srivastava, P.; Mishra, R.; Sivakumar, S.; Patra, A.K. Luminescent Naphthalimide-Tagged Ruthenium(II)-Arene Complexes: Cellular Imaging, Photocytotoxicity and Transferrin Binding. Dalton Trans. 2021, 50, 3629–3640. [Google Scholar] [CrossRef]
- Miachin, K.; Del Solar, V.; El Khoury, E.; Nayeem, N.; Khrystenko, A.; Appelt, P.; Neary, M.C.; Buccella, D.; Contel, M. Intracellular Localization Studies of the Luminescent Analogue of an Anticancer Ruthenium Iminophosphorane with High Efficacy in a Triple-Negative Breast Cancer Mouse Model. Inorg. Chem. 2021, 60, 19152–19164. [Google Scholar] [CrossRef]
- Ghosh, G.; Colón, K.L.; Fuller, A.; Sainuddin, T.; Bradner, E.; McCain, J.; Monro, S.M.A.; Yin, H.; Hetu, M.W.; Cameron, C.G.; et al. Cyclometalated Ruthenium(II) Complexes Derived from α-Oligothiophenes as Highly Selective Cytotoxic or Photocytotoxic Agents. Inorg. Chem. 2018, 57, 7694–7712. [Google Scholar] [CrossRef]
- McCain, J.; Colón, K.L.; Barrett, P.C.; Monro, S.M.A.; Sainuddin, T.; Roque Iii, J.; Pinto, M.; Yin, H.; Cameron, C.G.; McFarland, S.A. Photophysical Properties and Photobiological Activities of Ruthenium(II) Complexes Bearing π-Expansive Cyclometalating Ligands with Thienyl Groups. Inorg. Chem. 2019, 58, 10778–10790. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Tian, N.; Li, C.; Wang, X. Ru(II)-Complex-Based DNA Photocleaver Having Intense Absorption in the Phototherapeutic Window. Inorg. Chem. 2017, 56, 1865–1873. [Google Scholar] [CrossRef]
- Saeed, H.K.; Jarman, P.J.; Archer, S.; Sreedharan, S.; Saeed, I.Q.; Mckenzie, L.K.; Weinstein, J.A.; Buurma, N.J.; Smythe, C.G.W.; Thomas, J.A. Homo- and Heteroleptic Phototoxic Dinuclear Metallo-Intercalators Based on RuII(dppn) Intercalating Moieties: Synthesis, Optical, and Biological Studies. Angew. Chem. 2017, 56, 12628–12633. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, R.; Shi, H.; Wang, Y.; Ji, L.; Zhang, P.; Zhang, Q. Design of Ruthenium-Albumin Hydrogel for Cancer Therapeutics and Luminescent Imaging. J. Inorg. Biochem. 2019, 194, 19–25. [Google Scholar] [CrossRef]
- Mascheroni, L.; Dozzi, M.V.; Ranucci, E.; Ferruti, P.; Francia, V.; Salvati, A.; Maggioni, D. Tuning Polyamidoamine Design to Increase Uptake and Efficacy of Ruthenium Complexes for Photodynamic Therapy. Inorg. Chem. 2019, 58, 14586–14599. [Google Scholar] [CrossRef]
- Yang, J.; He, X.; Ke, Z.; Chen, J.; Zou, Z.; Wei, B.; Zou, D.; Zou, J. Two Photoactive Ru (II) Compounds Based on Tetrazole Ligands for Photodynamic Therapy. J. Inorg. Biochem. 2020, 210, 111127. [Google Scholar] [CrossRef]
- Nehru, S.; Veeralakshmi, S.; Kalaiselvam, S.; Subin David, S.P.; Sandhya, J.; Arunachalam, S. DNA Binding, Antibacterial, Hemolytic and Anticancer Studies of Some Fluorescent Emissive Surfactant-Ruthenium(II) Complexes. J. Biomol. Struct. Dyn. 2021, 39, 2242–2256. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Li, C.-E.; Chen, T. Designing Luminescent Ruthenium Prodrug for Precise Cancer Therapy and Rapid Clinical Diagnosis. Biomaterials 2019, 192, 579–589. [Google Scholar] [CrossRef]
- Li, H.; Xie, C.; Lan, R.; Zha, S.; Chan, C.-F.; Wong, W.-Y.; Ho, K.-L.; Chan, B.D.; Luo, Y.; Zhang, J.-X.; et al. A Smart Europium-Ruthenium Complex as Anticancer Prodrug: Controllable Drug Release and Real-Time Monitoring under Different Light Excitations. J. Med. Chem. 2017, 60, 8923–8932. [Google Scholar] [CrossRef]
- Dos Santos, J.S.; Ramos, L.C.; Ferreira, L.P.; Campo, V.L.; de Rezende, L.C.D.; da Silva Emery, F.; Santana da Silva, R. Cytotoxicity, Cellular Uptake, and Subcellular Localization of a Nitrogen Oxide and Aminopropyl-β-Lactose Derivative Ruthenium Complex Used as Nitric Oxide Delivery Agent. Nitric Oxide Biol. Chem. 2019, 86, 38–47. [Google Scholar] [CrossRef]
- Weynand, J.; Diman, A.; Abraham, M.; Marcélis, L.; Jamet, H.; Decottignies, A.; Dejeu, J.; Defrancq, E.; Elias, B. Towards the Development of Photo-Reactive Ruthenium(II) Complexes Targeting Telomeric G-Quadruplex DNA. Chemistry 2018, 24, 19216–19227. [Google Scholar] [CrossRef]
- Zhong, Y.-F.; Zhang, H.; Liu, W.-T.; Zheng, X.-H.; Zhou, Y.-W.; Cao, Q.; Shen, Y.; Zhao, Y.; Qin, P.Z.; Ji, L.-N.; et al. A Platinum(II)-Based Photosensitive Tripod as an Effective Photodynamic Anticancer Agent through DNA Damage. Chem. Eur. J. 2017, 23, 16442–16446. [Google Scholar] [CrossRef]
- Xue, X.; Zhu, C.; Chen, H.; Bai, Y.; Shi, X.; Jiao, Y.; Chen, Z.; Miao, Y.; He, W.; Guo, Z. A New Approach to Sensitize Antitumor Monofunctional Platinum(II) Complexes via Short Time Photo-Irradiation. Inorg. Chem. 2017, 56, 3754–3762. [Google Scholar] [CrossRef]
- Shi, H.; Clarkson, G.J.; Sadler, P.J. Dual Action Photosensitive Platinum(II) Anticancer Prodrugs with Photoreleasable Azide Ligands. Inorganica Chim. Acta 2019, 489, 230–235. [Google Scholar] [CrossRef]
- Thiabaud, G.; Arambula, J.F.; Siddik, Z.H.; Sessler, J.L. Photoinduced Reduction of PtIV within an Anti-Proliferative PtIV-Texaphyrin Conjugate. Chemistry 2014, 20, 8942–8947. [Google Scholar] [CrossRef] [Green Version]
- Lv, S.; Miao, Y.; Zheng, D.; Li, X.; Liu, D.; Song, F. Self-Assembled Platinum Supramolecular Metallacycles Based on a Novel TADF Photosensitizer for Efficient Cancer Photochemotherapy. Mol. Pharm. 2021, 18, 1229–1237. [Google Scholar] [CrossRef]
- Presa, A.; Vázquez, G.; Barrios, L.A.; Roubeau, O.; Korrodi-Gregório, L.; Pérez-Tomás, R.; Gamez, P. Photoactivation of the Cytotoxic Properties of Platinum(II) Complexes through Ligand Photoswitching. Inorg. Chem. 2018, 57, 4009–4022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laranjo, M.; Aguiar, M.C.; Pereira, N.A.M.; Brites, G.; Nascimento, B.F.O.; Brito, A.F.; Casalta-Lopes, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Pineiro, M.; et al. Platinum(II) Ring-Fused Chlorins as Efficient Theranostic Agents: Dyes for Tumor-Imaging and Photodynamic Therapy of Cancer. Eur. J. Med. Chem. 2020, 200, 112468. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Xie, B.-R.; Cheng, H.; Li, C.-X.; Zhang, M.-K.; Qiu, W.-X.; Liu, W.-L.; Wang, X.-S.; Zhang, X.-Z. A Biomimetic Theranostic O2-Meter for Cancer Targeted Photodynamic Therapy and Phosphorescence Imaging. Biomaterials 2018, 151, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kuang, Y.; Lv, R.; Yang, P.; Li, C.; Bi, H.; Liu, B.; Yang, D.; Dai, Y.; Gai, S.; et al. Charge Convertibility and near Infrared Photon Co-Enhanced Cisplatin Chemotherapy Based on Upconversion Nanoplatform. Biomaterials 2017, 130, 42–55. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Zhu, Y.; Zhang, C.; Cao, H.; Ma, W.; Tian, X.; Wu, J.; Zhou, H.; Tian, Y. Functional Platinum(II) Complexes with Four-Photon Absorption Activity, Lysosome Specificity, and Precise Cancer Therapy. Inorg. Chem. 2021, 60, 2362–2371. [Google Scholar] [CrossRef]
- Kuang, G.; Lu, H.; He, S.; Xiong, H.; Yu, J.; Zhang, Q.; Huang, Y. Near-Infrared Light-Triggered Polyprodrug/SiRNA Loaded Upconversion Nanoparticles for Multi-Modality Imaging and Synergistic Cancer Therapy. Adv. Healthc. Mater. 2021, 10. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, F.; Chen, Z.; Zhang, R.; Li, C.; Xu, Y.; Zhang, Y.; Ni, R.; Li, X.; Yang, G.; et al. Melanin-Dot-Mediated Delivery of Metallacycle for NIR-II/Photoacoustic Dual-Modal Imaging-Guided Chemo-Photothermal Synergistic Therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 16729–16735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Lv, B.; Tang, Z.; Zhang, M.; Ge, W.; Liu, Y.; He, X.; Zhao, K.; Zheng, X.; He, M.; et al. Scintillator-Based Nanohybrids with Sacrificial Electron Prodrug for Enhanced X-Ray-Induced Photodynamic Therapy. Nano Lett. 2018, 18, 5768–5774. [Google Scholar] [CrossRef] [PubMed]
- Şahin, Ö.; Özdemir, Ü.Ö.; Seferoğlu, N.; Genc, Z.K.; Kaya, K.; Aydıner, B.; Tekin, S.; Seferoğlu, Z. New Platinum (II) and Palladium (II) Complexes of Coumarin-Thiazole Schiff Base with a Fluorescent Chemosensor Properties: Synthesis, Spectroscopic Characterization, X-ray Structure Determination, in vitro Anticancer Activity on Various Human Carcinoma Cell Lines and Computational Studies. J. Photochem. Photobiol. B 2018, 178, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Schlagintweit, J.F.; Jakob, C.H.G.; Meighen-Berger, K.; Gronauer, T.F.; Weigert Muñoz, A.; Weiß, V.; Feige, M.J.; Sieber, S.A.; Correia, J.D.G.; Kühn, F.E. Fluorescent Palladium(II) and Platinum(II) NHC/1,2,3-Triazole Complexes: Antiproliferative Activity and Selectivity against Cancer Cells. Dalton Trans. 2021, 50, 2158–2166. [Google Scholar] [CrossRef]
- Hadian Rasanani, S.; Eslami Moghadam, M.; Soleimani, E.; Divsalar, A.; Ajloo, D.; Tarlani, A.; Amiri, M. Anticancer Activity of New Imidazole Derivative of 1R,2R-Diaminocyclohexane Palladium, and Platinum Complexes as DNA Fluorescent Probes. J. Biomol. Struct. Dyn. 2018, 36, 3058–3076. [Google Scholar] [CrossRef]
- Lo, A.T.S.; Bryce, N.S.; Klein, A.V.; Todd, M.H.; Hambley, T.W. Novel Polyamide Amidine Anthraquinone Platinum(II) Complexes: Cytotoxicity, Cellular Accumulation, and Fluorescence Distributions in 2D and 3D Cell Culture Models. J. Biol. Inorg. Chem. 2021, 26, 217–233. [Google Scholar] [CrossRef]
- Millán, G.; Giménez, N.; Lara, R.; Berenguer, J.R.; Moreno, M.T.; Lalinde, E.; Alfaro-Arnedo, E.; López, I.P.; Piñeiro-Hermida, S.; Pichel, J.G. Luminescent Cycloplatinated Complexes with Biologically Relevant Phosphine Ligands: Optical and Cytotoxic Properties. Inorg. Chem. 2019, 58, 1657–1673. [Google Scholar] [CrossRef]
- Lázaro, A.; Balcells, C.; Quirante, J.; Badia, J.; Baldomà, L.; Ward, J.S.; Rissanen, K.; Font-Bardia, M.; Rodríguez, L.; Crespo, M.; et al. Luminescent PtII and PtIV Platinacycles with Anticancer Activity Against Multiplatinum-Resistant Metastatic CRC and CRPC Cell Models. Chemistry 2020, 26, 1947–1952. [Google Scholar] [CrossRef]
- Lo, K.K.-W. Luminescent Rhenium(I) and Iridium(III) Polypyridine Complexes as Biological Probes, Imaging Reagents, and Photocytotoxic Agents. Available online: http://pubs.acs.org/doi/abs/10.1021/acs.accounts.5b00211 (accessed on 24 January 2022).
- Ramos, L.D.; de Macedo, L.H.; Gobo, N.R.S.; de Oliveira, K.T.; Cerchiaro, G.; Morelli Frin, K.P. Understanding the Photophysical Properties of Rhenium(I) Compounds Coordinated to 4,7-Diamine-1,10-Phenanthroline: Synthetic, Luminescence and Biological Studies. Dalton Trans. 2020, 49, 16154–16165. [Google Scholar] [CrossRef]
- Murphy, B.L.; Marker, S.C.; Lambert, V.J.; Woods, J.J.; MacMillan, S.N.; Wilson, J.J. Synthesis, Characterization, and Biological Properties of Rhenium(I) Tricarbonyl Complexes Bearing Nitrogen-Donor Ligands. J. Organomet. Chem. 2020, 907. [Google Scholar] [CrossRef]
- Marker, S.C.; MacMillan, S.N.; Zipfel, W.R.; Li, Z.; Ford, P.C.; Wilson, J.J. Photoactivated in Vitro Anticancer Activity of Rhenium(I) Tricarbonyl Complexes Bearing Water-Soluble Phosphines. Inorg. Chem. 2018, 57, 1311–1331. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-X.; Lee, L.C.-C.; Kwok, C.W.-C.; Leung, P.K.-K.; Zhu, J.-H.; Lo, K.K.-W. Utilization of Rhenium(I) Polypyridine Complexes Featuring a Dinitrophenylsulfonamide Moiety as Biothiol-Selective Phosphorogenic Bioimaging Reagents and Photocytotoxic Agents. Eur. J. Inorg. Chem. 2021, 2021, 3432–3442. [Google Scholar] [CrossRef]
- Yang, J.; Cao, Q.; Zhang, H.; Hao, L.; Zhou, D.; Gan, Z.; Li, Z.; Tong, Y.-X.; Ji, L.-N.; Mao, Z.-W. Targeted Reversal and Phosphorescence Lifetime Imaging of Cancer Cell Metabolism via a Theranostic Rhenium(I)-DCA Conjugate. Biomaterials 2018, 176, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, J.-X.; Cao, Q.; Hao, L.; Zhou, D.; Gan, Z.; Ji, L.-N.; Mao, Z.-W. Simultaneously Inducing and Tracking Cancer Cell Metabolism Repression by Mitochondria-Immobilized Rhenium(I) Complex. ACS Appl. Mater. Interfaces 2017, 9, 13900–13912. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Pan, Z.-Y.; Qin, W.-W.; Li, Y.; Tan, C.-P.; Mao, Z.-W. Impairment of the Autophagy-Related Lysosomal Degradation Pathway by an Anticancer Rhenium(I) Complex. Dalton Trans. 2019, 48, 4398–4404. [Google Scholar] [CrossRef]
- Knopf, K.M.; Murphy, B.L.; MacMillan, S.N.; Baskin, J.M.; Barr, M.P.; Boros, E.; Wilson, J.J. In Vitro Anticancer Activity and in vivo Biodistribution of Rhenium(I) Tricarbonyl Aqua Complexes. J. Am. Chem. Soc. 2017, 139, 14302–14314. [Google Scholar] [CrossRef]
- Ye, R.-R.; Chen, B.-C.; Lu, J.-J.; Ma, X.-R.; Li, R.-T. Phosphorescent Rhenium(I) Complexes Conjugated with Artesunate: Mitochondrial Targeting and Apoptosis-Ferroptosis Dual Induction. J. Inorg. Biochem. 2021, 223. [Google Scholar] [CrossRef]
- Pan, Z.-Y.; Cai, D.-H.; He, L. Dinuclear Phosphorescent Rhenium(I) Complexes as Potential Anticancer and Photodynamic Therapy Agents. Dalton Trans. 2020, 49, 11583–11590. [Google Scholar] [CrossRef]
- Luengo, A.; Fernández-Moreira, V.; Marzo, I.; Gimeno, M.C. Trackable Metallodrugs Combining Luminescent Re(I) and Bioactive Au(I) Fragments. Inorg. Chem. 2017, 56, 15159–15170. [Google Scholar] [CrossRef]
- Luengo, A.; Fernandez-Moreira, V.; Marzo, I.; Concepcion Gimeno, M. Bioactive Heterobimetallic Re(I)/Au(I) Complexes Containing Bidentate N-Heterocyclic Carbenes. Organometallics 2018, 37, 3993–4001. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Huang, H. Future Potential of Osmium Complexes as Anticancer Drug Candidates, Photosensitizers and Organelle-Targeted Probes. Dalton Trans. 2018, 47, 14841–14854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, Y.; Qiu, K.; Zhao, Z.; Hu, R.; He, C.; Zhang, Q.; Chao, H. A NIR Phosphorescent Osmium(II) Complex as a Lysosome Tracking Reagent and Photodynamic Therapeutic Agent. Chem. Commun. 2017, 53, 12341–12344. [Google Scholar] [CrossRef]
- Ge, C.; Zhu, J.; Ouyang, A.; Lu, N.; Wang, Y.; Zhang, Q.; Zhang, P. Near-Infrared Phosphorescent Terpyridine Osmium(Ii) Photosensitizer Complexes for Photodynamic and Photooxidation Therapy. Inorg. Chem. Front. 2020, 7, 4020–4027. [Google Scholar] [CrossRef]
- Coverdale, J.P.C.; Bridgewater, H.E.; Song, J.-I.; Smith, N.A.; Barry, N.P.E.; Bagley, I.; Sadler, P.J.; Romero-Canelon, I. In Vivo Selectivity and Localization of Reactive Oxygen Species (ROS) Induction by Osmium Anticancer Complexes That Circumvent Platinum Resistance. J. Med. Chem. 2018, 61, 9246–9255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazic, S.; Kaspler, P.; Shi, G.; Monro, S.; Sainuddin, T.; Forward, S.; Kasimova, K.; Hennigar, R.; Mandel, A.; McFarland, S.; et al. Novel Osmium-Based Coordination Complexes as Photosensitizers for Panchromatic Photodynamic Therapy. Photochem. Photobiol. 2017, 93, 1248–1258. [Google Scholar] [CrossRef]
- Azzouzi, A.R.; Barret, E.; Bennet, J.; Moore, C.; Taneja, S.; Muir, G.; Villers, A.; Coleman, J.; Allen, C.; Scherz, A.; et al. TOOKAD® Soluble Focal Therapy: Pooled Analysis of Three Phase II Studies Assessing the Minimally Invasive Ablation of Localized Prostate Cancer. World J. Urol. 2015, 33, 945–953. [Google Scholar] [CrossRef] [Green Version]
- Bernd, M.A.; Bauer, E.B.; Oberkofler, J.; Bauer, A.; Reich, R.M.; Kühn, F.E. Macrocyclic NHC Complexes of Group 10 Elements with Enlarged Aromaticity for Biological Studies. Dalton Trans. 2020, 49, 14106–14114. [Google Scholar] [CrossRef]
- Tripathi, M.; Syed, R.; Stalin, A.; Malik, A.; Pande, R.; Asatkar, A.K. In Vitro Investigation of Biophysical Interactions between Ag(I) Complexes of Bis(Methyl)(Thia/Selena)Salen and Ct-DNA via Multi-Spectroscopic, Physicochemical and Molecular Docking Methods along with Cytotoxicity Study. Luminescence 2021, 36, 1277–1284. [Google Scholar] [CrossRef]
- Fedorenko, S.V.; Grechkina, S.L.; Mukhametshina, A.R.; Solovieva, A.O.; Pozmogova, T.N.; Miroshnichenko, S.M.; Alekseev, A.Y.; Shestopalov, M.A.; Kholin, K.V.; Nizameev, I.R.; et al. Silica Nanoparticles with Tb(III)-Centered Luminescence Decorated by Ag0 as Efficient Cellular Contrast Agent with Anticancer Effect. J. Inorg. Biochem. 2018, 182, 170–176. [Google Scholar] [CrossRef]
- Huang, L.; Liu, R.; Li, J.; Liang, X.; Lan, Q.; Shi, X.; Pan, L.; Chen, H.; Ma, Z. Synthesis, Characterization, Anti-Tumor Activity, Photo-Luminescence and BHb/HHb/Hsp90 Molecular Docking of Zinc(II) Hydroxyl-Terpyridine Complexes. J. Inorg. Biochem. 2019, 201, 110790. [Google Scholar] [CrossRef]
- Liang, X.; Jiang, J.; Xue, X.; Huang, L.; Ding, X.; Nong, D.; Chen, H.; Pan, L.; Ma, Z. Synthesis, Characterization, Photoluminescence, Anti-Tumor Activity, DFT Calculations and Molecular Docking with Proteins of Zinc(Ii) Halogen Substituted Terpyridine Compounds. Dalton Trans. 2019, 48, 10488–10504. [Google Scholar] [CrossRef] [PubMed]
- Vellaiswamy, G.; Ramaswamy, S. Co(II) Complexes Of4-((3-Ethoxy-2-Hydroxybenzylidene)Amino)-N-(Thiazol-2-Yl)Benzenesulphonamide and 4-((Pyridin-2-Ylmethylene)Amino)-N-(Thiazol-2-Tl)Benzenesulfonamide: Synthesis, Fluorescence Properties, and Anticancer Activity. J. Fluoresc. 2017, 27, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
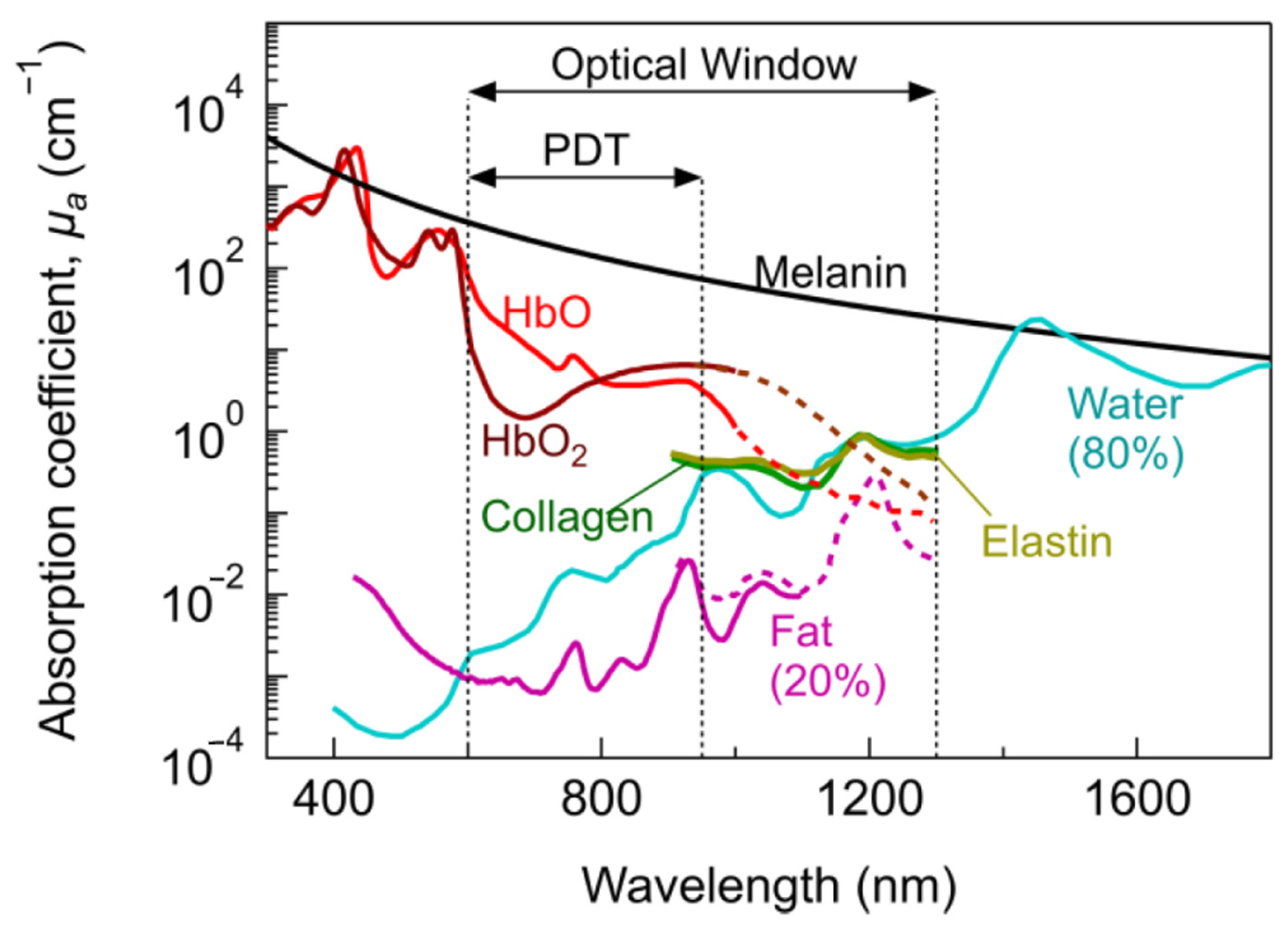
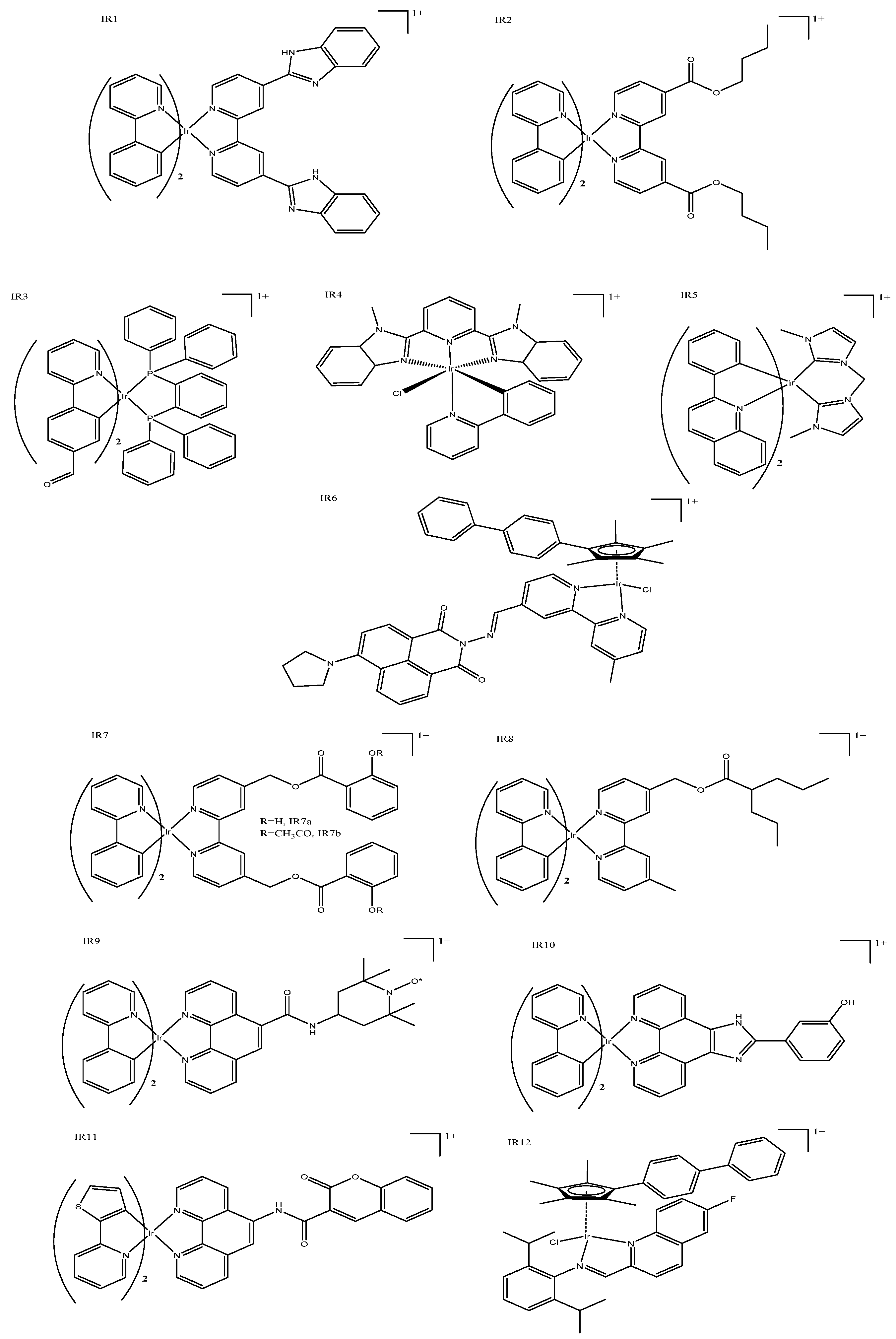
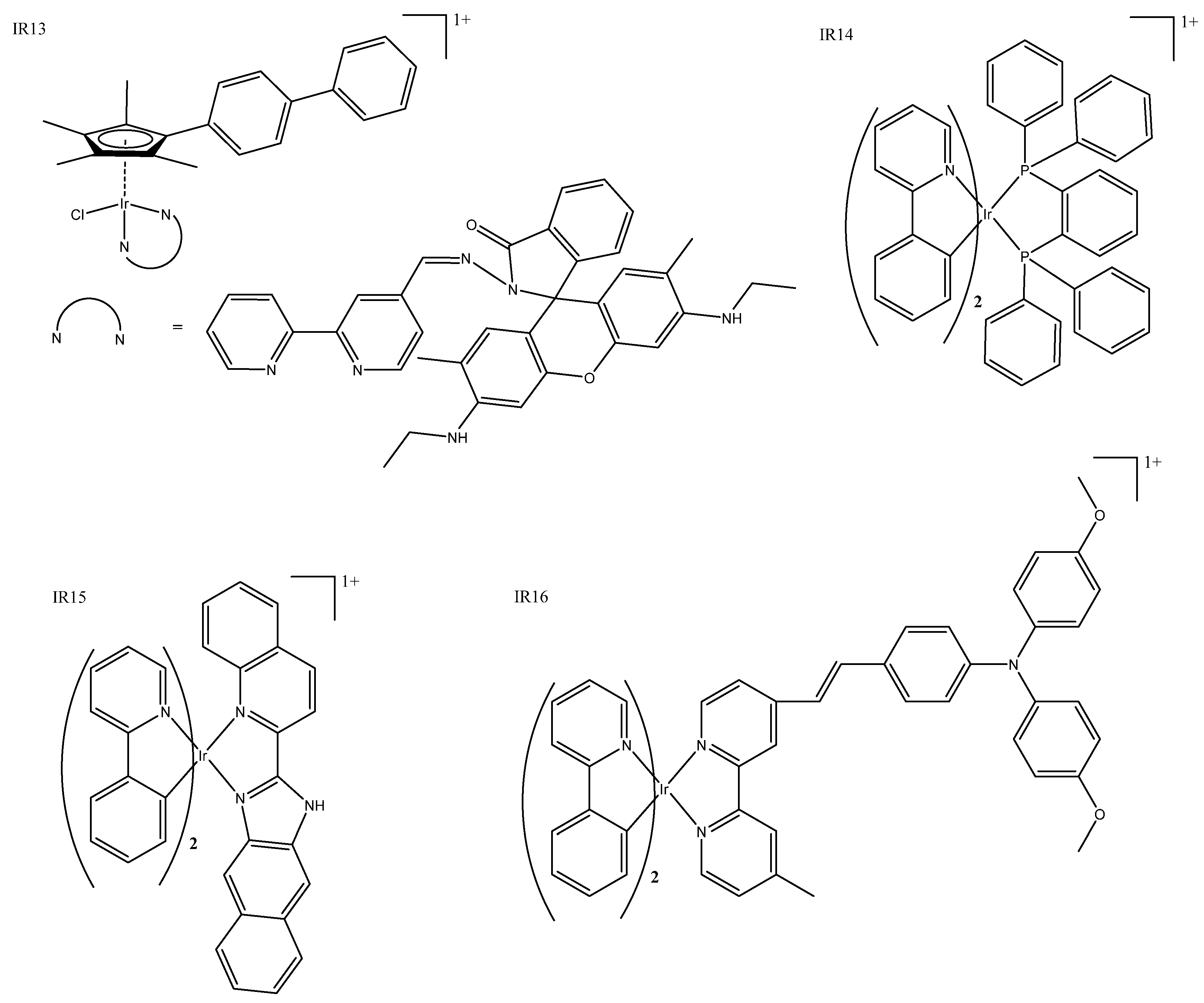
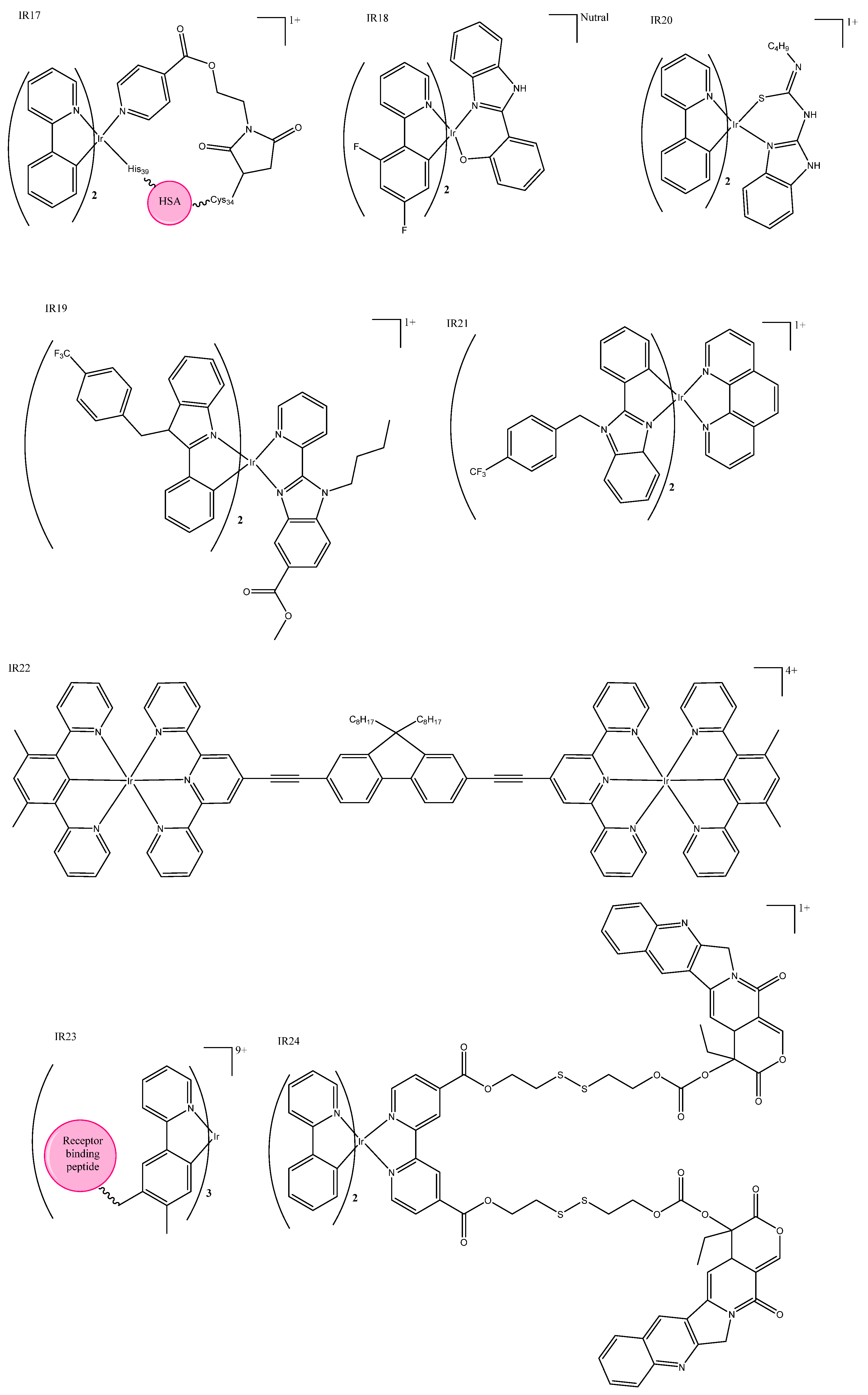
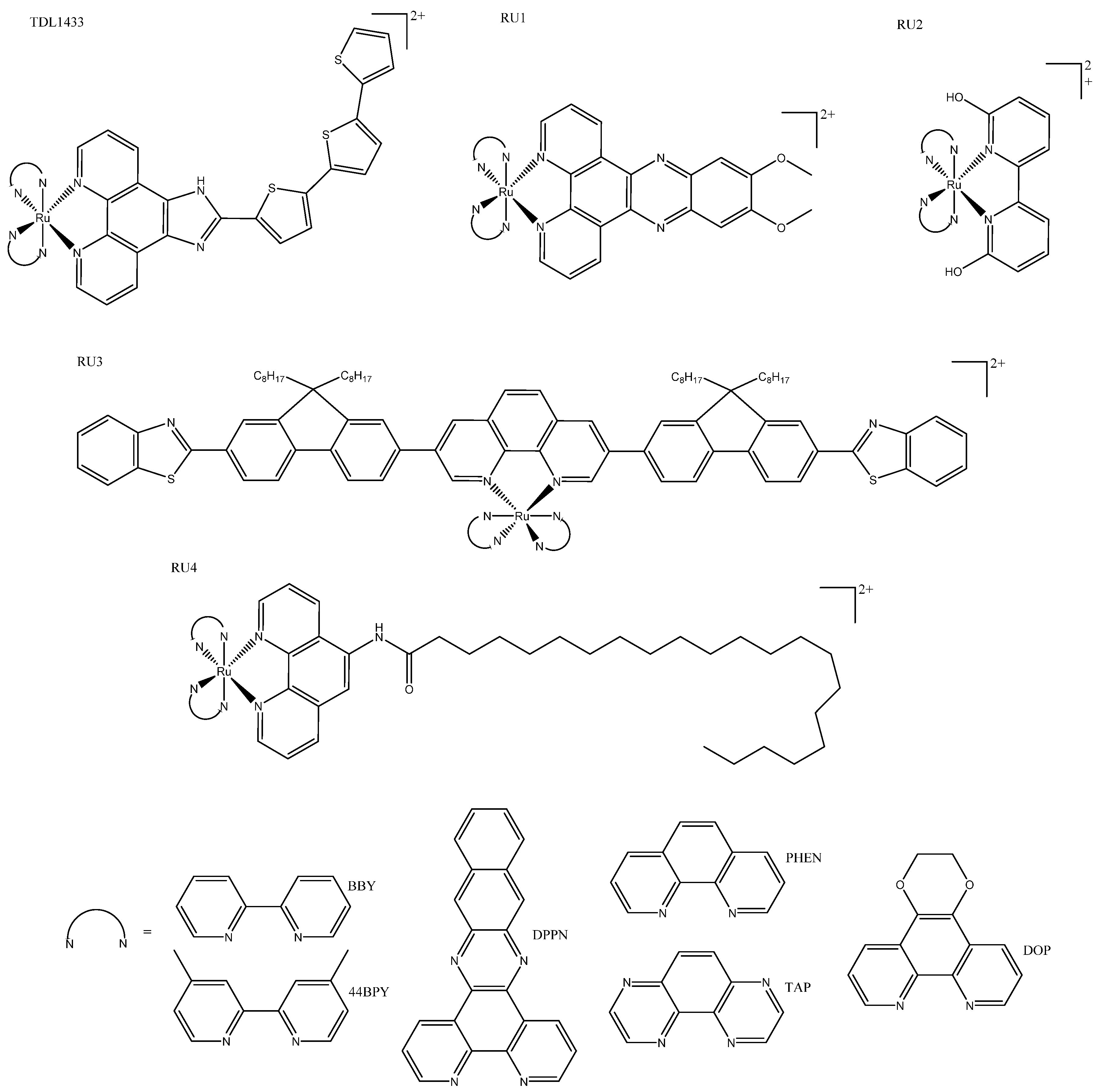
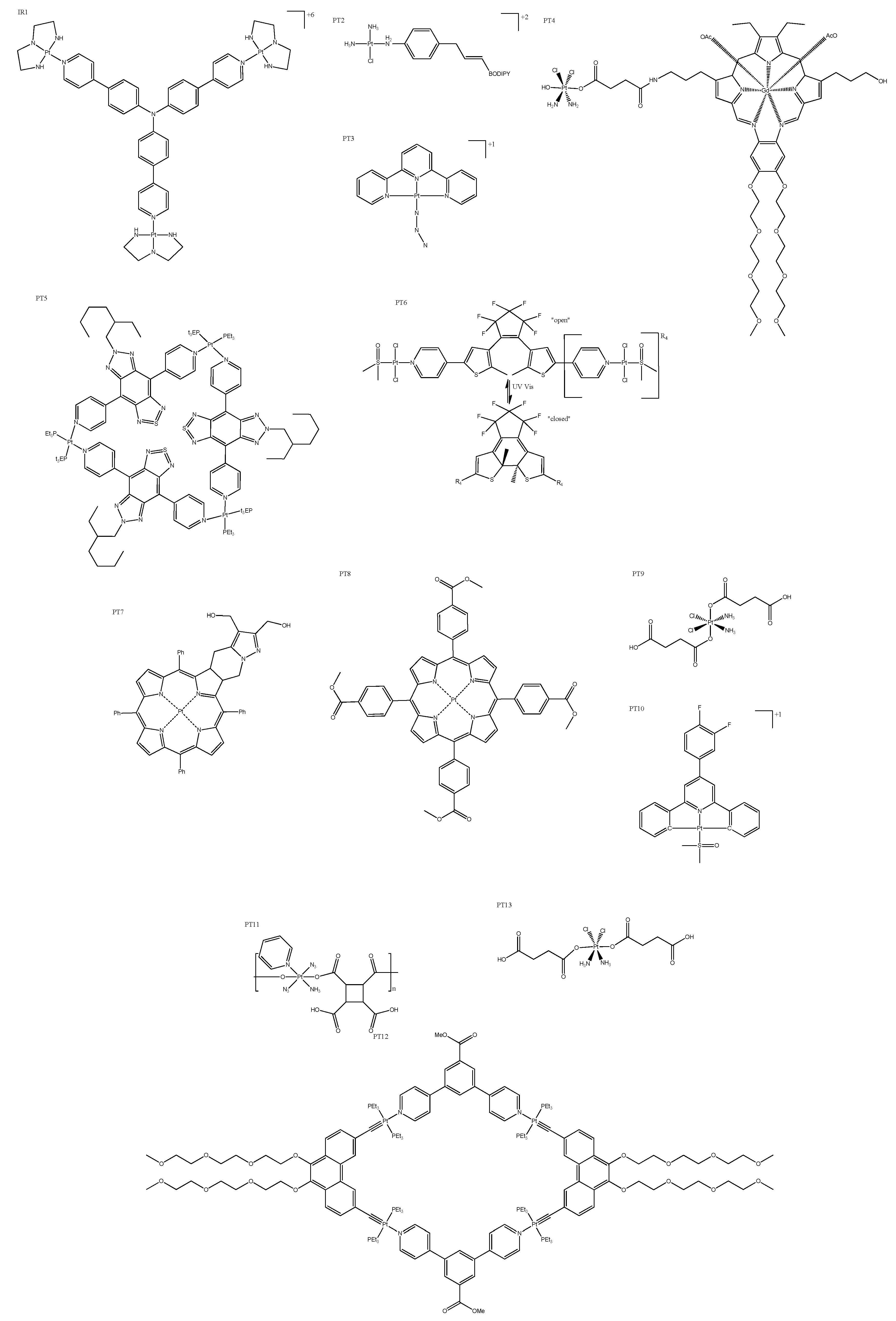


| Ir Complex | HeLa IC50 | A549 IC50 | Luminescence | Special Features | |||
|---|---|---|---|---|---|---|---|
| Dark | Light (Irradiation nm) | Dark | Light (Irradiation nm) | λEm (nm) | λEx (nm) | ||
| IR2 | 2.1 ± 0.2 | 1.7 ± 0.1 | 630–690 | 405 | Could cause concentration-dependent cell cycle arrest, pro-death autophagy, and caspase-dependent apoptosis in A549 cells. | ||
| IR6 | 11.3 ± 0.1 | 15.6 ± 1.2 | Could induce cancer cell death in a variety of ways and had a good antimetastatic ability to cancer cells. | ||||
| IR13 | 3.6 ± 0.8 | 2.6 ± 0.3 | 500–600 | 488 | Fluorescence could be used for cellular imaging under both acidic and neutral conditions. | ||
| IR15 | >100 | 0.21 ± 0.01 (425) | >100 | 0.31 ± 0.02 (425) | 670 | 405 | Had enhanced emission in lysosomes and could inhibit several key cancerous events, including cell migration, invasion, colony formation, and in vivo angiogenesis. |
| IR16 | 4.34 ± 0.01 | 591 | 488 | Potential anticancer agents with dual functions, including metastasis-inhibition and lysosomal damage. | |||
| IR17 | 62.3 ± 2.6 | 1.1 ± 0.3 (465) | 580–630 | 563 | Photo-selective and cancer-selective in cell and spheroids. | ||
| IR21 | 11.5 ± 0.2 | 0.044 ± 0.006 (420) | 557 | 381 | The first iridium complexes to induce cancer cell death by inhibition of translation targeting the endoplasmic reticulum. | ||
| IR22 | 49.9 ± 0.1 * | 0.75 ± 0.01 (visible) | 608 | 405 | Nanomolar photocytotoxicity and visible PI > 280. | ||
| IR23 | 506 | 366 | The first example of an artificial TRAIL mimic that induced apoptosis-like cell death. | ||||
| IR24 | 460–510 | 400 | Folate targeted multi-action micelle, activated by GHS and irradiation at 400nm. | ||||
| Ru Complex | HeLa IC50 | SK-MEL-28 IC50 | Luminescence | Special Features | |||
|---|---|---|---|---|---|---|---|
| Dark | Light (Irradiation nm) | Dark | Light (Irradiation nm) | λEm (nm) | λEx (nm) | ||
| TDL1433 | 137 ± 3 | 1.9 ± 0.1 (400-700) | 525 | Optimized clinical procedure and completion of human clinical trials. | |||
| RU1 | 36.5 ± 3.0 | 3.1 ± 0.6 (420) | 620 | 420 | Photosensitizers for one- and two-photon PDT. | ||
| RU3 | 123 ± 3.62 | 3.77 ± 0.18 | 557/640 | 413 | Activated at multiple wavelengths; tracking possible both before and after photo treatment. | ||
| RU4a | 13 ± 2 | 0.47 ± 0.01 ** | 614 | 440 | Shows how modification of ancillary ligand and lipophilicity enhances therapeutic effect. | ||
| RU4b | 11 ± 3 | 2 ± 1 ** | 643 | 418 | |||
| RU6 | 83.1 ± 6.2 * | 34.1 ± 2.4 (460) | 500 | 355 | Slight variations in structure led to phototoxic or other photoactivated complexes. | ||
| RU6 | 31.3 ± 4.5 | 11.5 ± 2.5 (488) | 534 | 440 | Tagged with napthalamide derivative to target DNA. | ||
| RU8a | >300 | 12.0 ± 0.4 (633) | 805 | 540 | More lipophilic and absorption; more red-shifted than their non-cyclometallated counterparts. | ||
| RU8b | >300 | 16.6 ± 1.53 (625) | 728 | 550 | |||
| RU16 | 277.0 ± 7.1 | 32.5 ± 8.2 (488) | 570-750 | 350 | The linker could be irradiated at different wavelengths for different functionalization (prodrug activation or fluorescence). | ||
| Pt Complex | IC50 (μM) | Luminescence | Special Features | ||
|---|---|---|---|---|---|
| Dark (Cell Line) | Light (Irradiation nm) | λEm(max) (nm) | λEx(max) (nm) | ||
| PT2 | >50 (HeLa) | 7.4 ± 0.3(635) | 550–650 | 566 | Positively charged Pt(II) center not only provided the cell membrane with an anchoring ability, but also made the complex a mild photosensitizer. |
| PT6 | >75 (A375) | 3.1 ± 0.2(365) | 470 | 300 | Light-mediated conversion from open to closed. |
| PT9 | None (HeLa) | “Increased” (NIR) * | Negatively charged in normal physiological conditions, and converted to positive charge in acidic tumor extracellular microenvironments. | ||
| PT15 | 410, 470 | 300 | DNA groove via hydrogenic or hydrophobic interaction. | ||
| PT16 | 16.2 ± 0.3 (MDA-MB-231) | 615 | 525 | Spheroid penetration. | |
| PT17a | 20.29 ± 2.10 (HeLa) | 400 | 475 | Shows potential as an anti-tubulin agent, and also provides useful information for our understanding of the mechanism responsible for the cytotoxic activity caused by these cyclometallated complexes. | |
| PT17b | 12.45 ± 2.50 (HeLa) | 388 | 458 | Selective generation of oxidative stress in cancer cells over noncancerous cells. | |
| Re Complex | HeLa IC50 (μM) | A549 IC50 (μM) | Luminescence | Special Features | |||
|---|---|---|---|---|---|---|---|
| Dark | Light (Irradiation nm) | Dark | Light (Irradiation nm) | λEm (nm) | λEx (nm) | ||
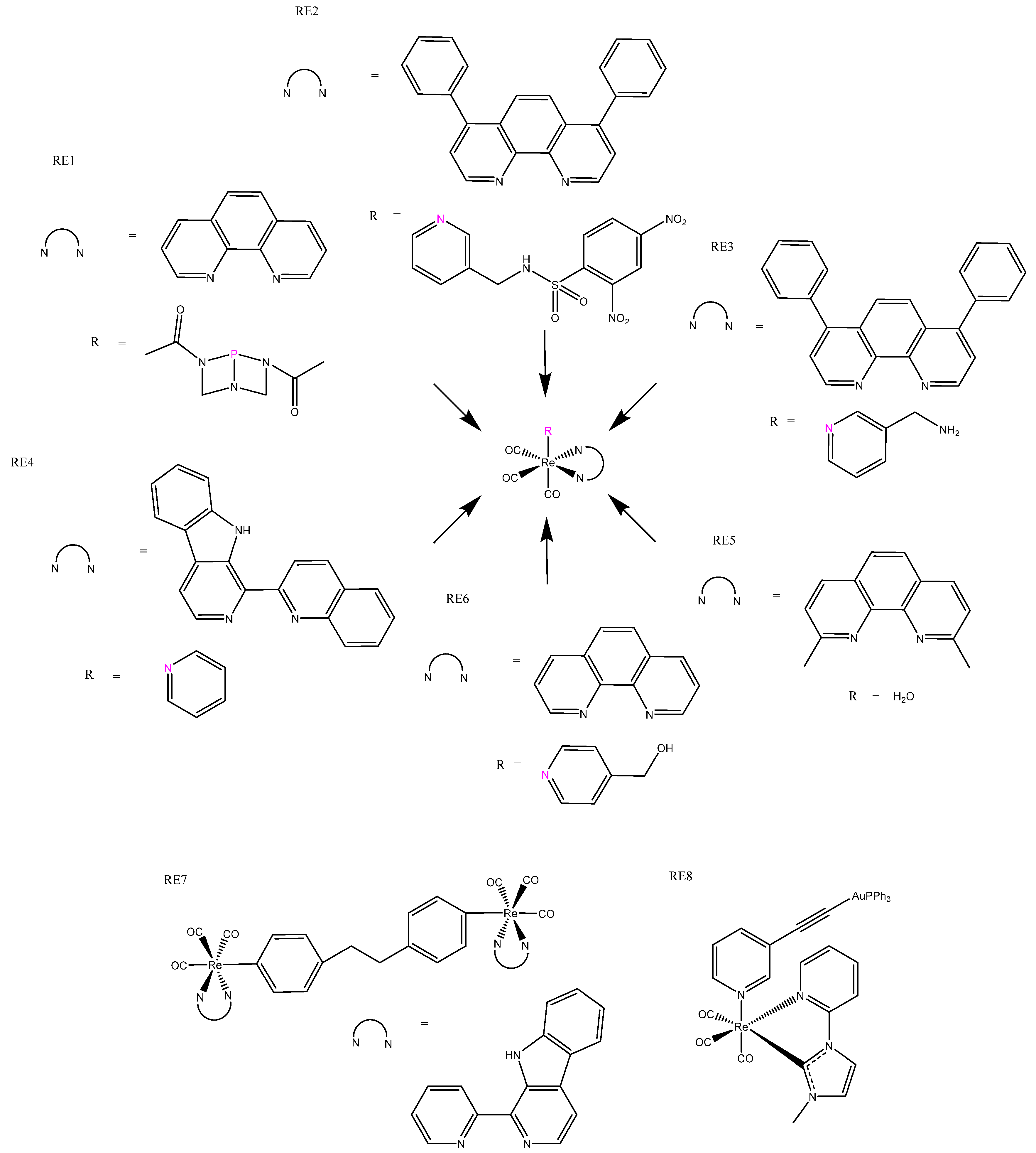
| RE1 | >200 | 5.9 ± 1.4 (365) | 516 | ~360 | The phosphine ligand regulated luminescence, which strongly correlated to cytotoxicity. | ||
| RE2 | 6.50 ± 2.0 | 541 | 405 | Showed significant emission enhancement when in the presence of GHS. | |||
| RE3 | 0.52 ± 0.07 | 3.4 ± 0.6 | 540 | 405 | Effective repression of mitochondrial metabolism; had O2-sensitive phosphorescent lifetimes | ||
| RE5 | 6.7 ± 4.9 | 560–590 | Induced caspase-independent cell death accompanied by cytoplasmic vacuolization. | ||||
| RE7 | 4.0 ± 0.6 | 15.8 ± 10 | 0.26 ± 0.04 (425) | ~550 | 405 | Homodinuclear Re(I) for chemo-photodynamic therapy. | |
| RE8 | 12.18 ± 1.19 | 4.48 ± 0.71 (405) | 460-514 | 414 | Heterodinuclear Au(II)–Re(I) complex; gold brought additional bioactivity. | ||
| Os Complex | IC50 (μM) | Luminescence | Special Features | ||
|---|---|---|---|---|---|
| Dark (Cell Line) | Light (Irradiation nm) | λEm(max) (nm) | λEx(max) (nm) | ||
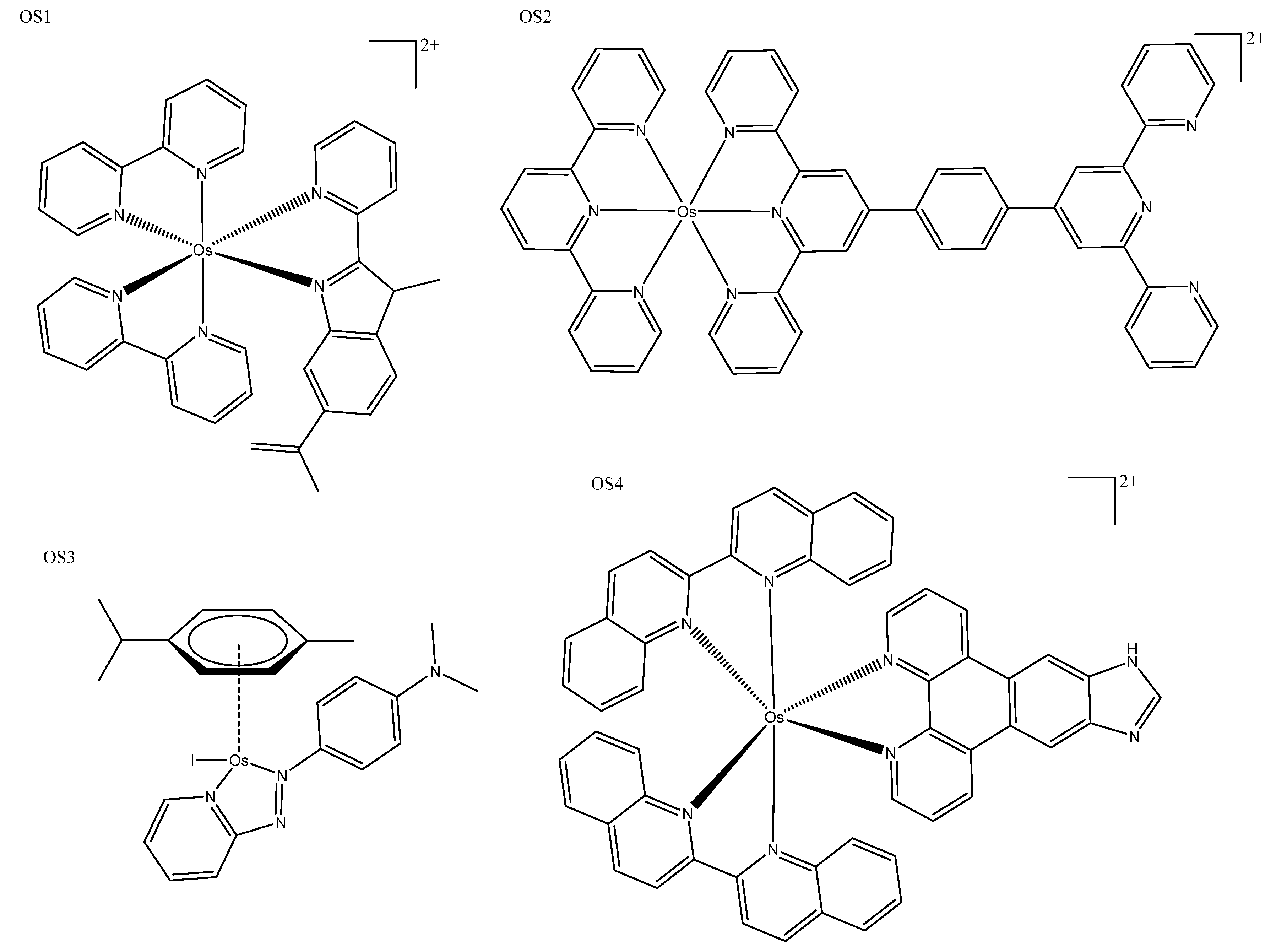
| OS2 | >100 (Hep-G2) | 1.23 ± 0.12 (465) 4.05 ± 0.05 (633) | ~570–770 | 488 | Exhibited high phototoxicities against cancer cells upon both 465 and 633 nm light irradiation. |
| OS3 | 1.1 ± 0.2(A549) | Selective generation of oxidative stress in cancer cells over noncancerous cells. | |||
| OS4 | 550 ± 49 (U87) | 45 ± 5 (NIR) | 940 | 200–900 | These photosensitizers were panchromatic (i.e., black absorbers), activatable from 200 to 900 nm. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGhie, B.S.; Aldrich-Wright, J.R. Photoactive and Luminescent Transition Metal Complexes as Anticancer Agents: A Guiding Light in the Search for New and Improved Cancer Treatments. Biomedicines 2022, 10, 578. https://doi.org/10.3390/biomedicines10030578
McGhie BS, Aldrich-Wright JR. Photoactive and Luminescent Transition Metal Complexes as Anticancer Agents: A Guiding Light in the Search for New and Improved Cancer Treatments. Biomedicines. 2022; 10(3):578. https://doi.org/10.3390/biomedicines10030578
Chicago/Turabian StyleMcGhie, Brondwyn S., and Janice R. Aldrich-Wright. 2022. "Photoactive and Luminescent Transition Metal Complexes as Anticancer Agents: A Guiding Light in the Search for New and Improved Cancer Treatments" Biomedicines 10, no. 3: 578. https://doi.org/10.3390/biomedicines10030578
APA StyleMcGhie, B. S., & Aldrich-Wright, J. R. (2022). Photoactive and Luminescent Transition Metal Complexes as Anticancer Agents: A Guiding Light in the Search for New and Improved Cancer Treatments. Biomedicines, 10(3), 578. https://doi.org/10.3390/biomedicines10030578









