The Forgotten Brother: The Innate-like B1 Cell in Multiple Sclerosis
Abstract
1. Introduction
1.1. Multiple Sclerosis and B1 Cells
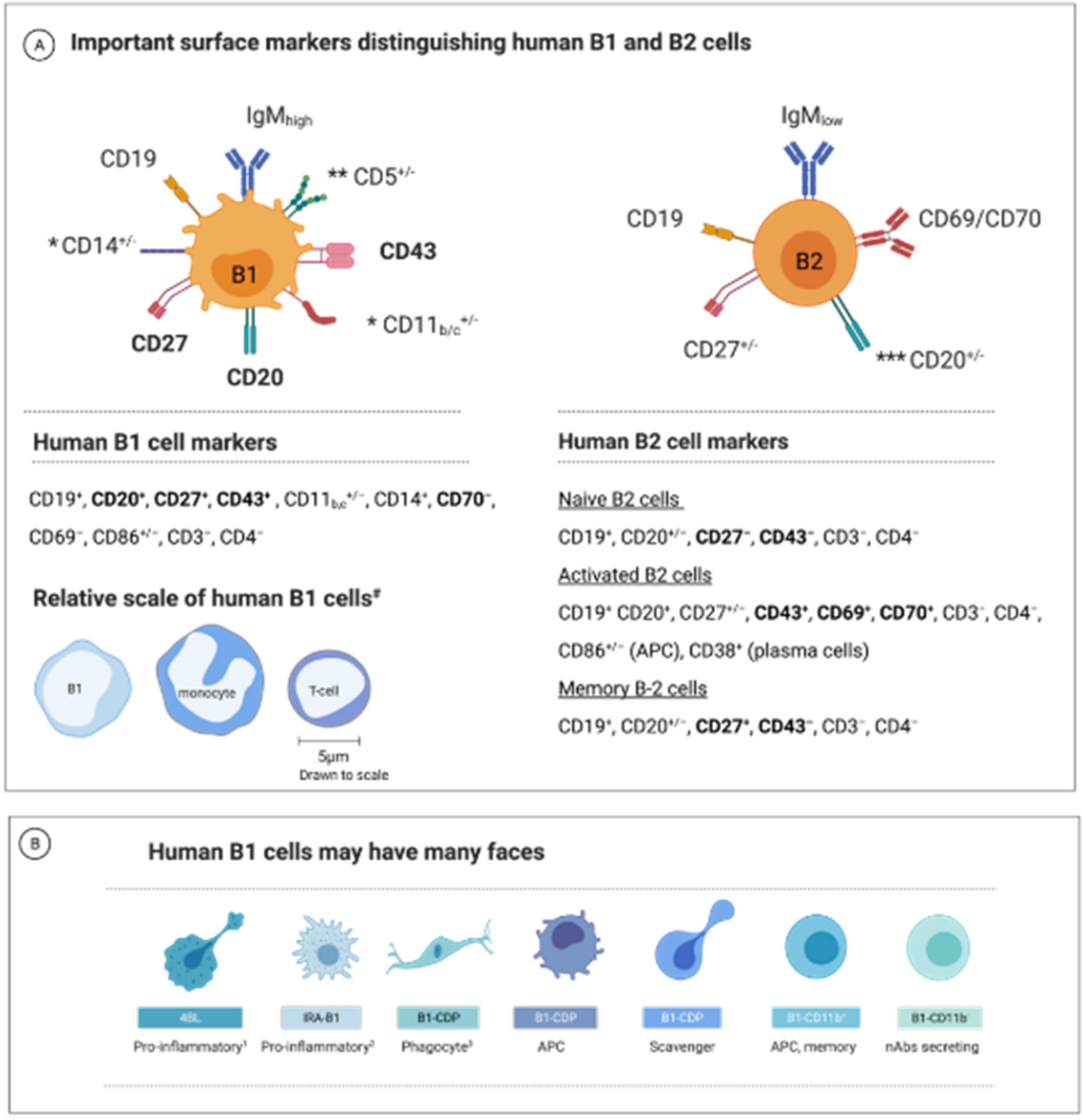
1.2. B1 Cell—A Unique B-Cell Subset
1.3. B1-Cell Subsets Have Distinctive Phenotypes and Functions
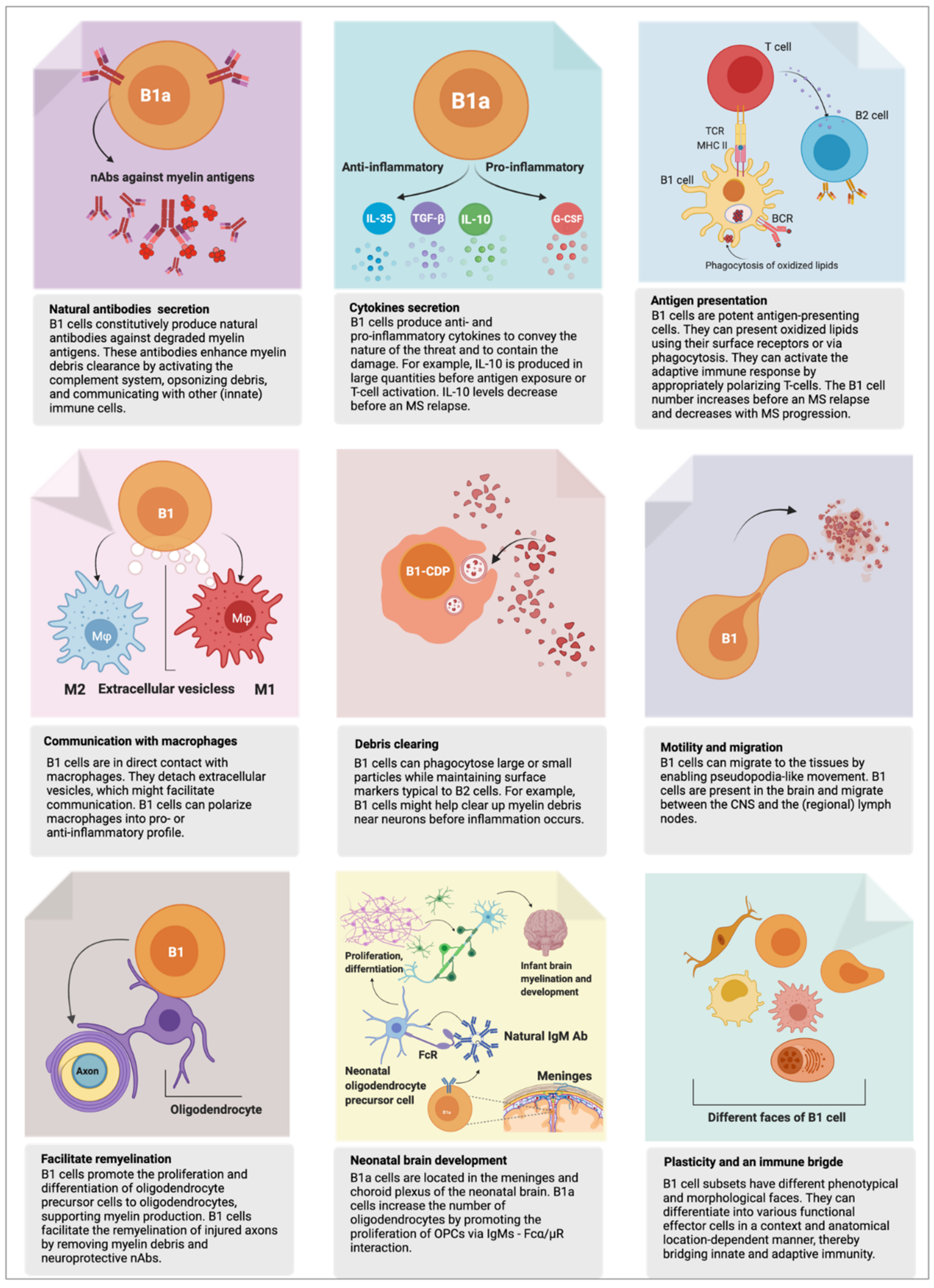
2. B1 Cell’s Functions in Health
2.1. First Responder to Danger
2.2. Phagocytosis—Eating for Elimination
2.3. Antigen Presentation—Eating for Alerting Others
2.4. Humoral Response—Arrows to the Target
2.5. Natural Autoantibodies
2.6. Myelination and Remyelination
3. B1 Cells in MS
3.1. B1 Cells Are Autoreactive by Nature
3.2. B1 Cells Are Linked to Autoimmunity
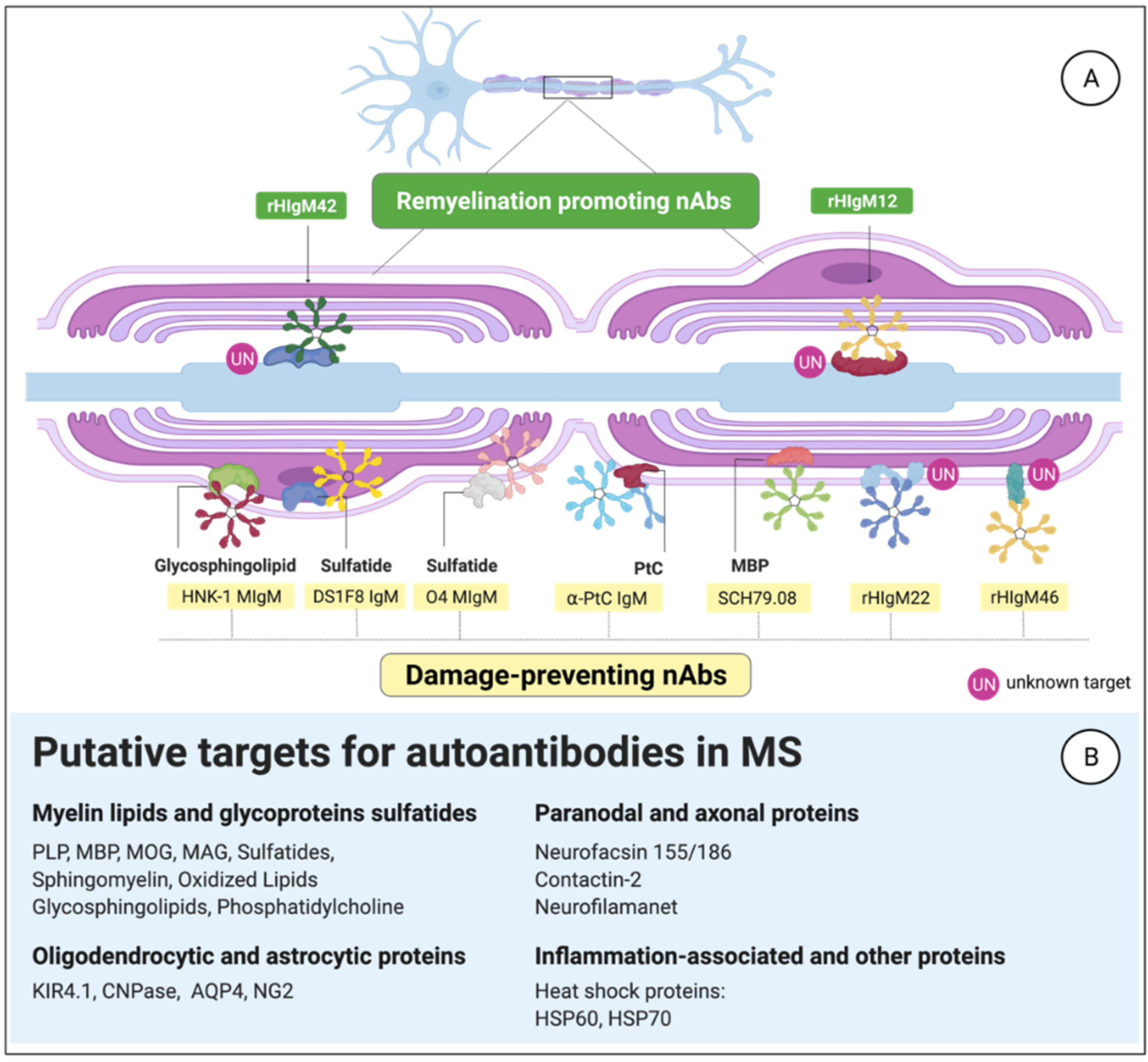
3.3. MS-Related Autoantibody Targets in the CNS
3.3.1. Anti-Phosphatidylcholine aAbs
3.3.2. Citrullinated Myelin-Derived Proteins
3.3.3. B1-Cell-Derived Abs against Myelin
3.4. B1-Cell Frequency Correlates with Relapse and MS Progression
3.5. MS Progression Is Age-Related; So Is B1 Cells’ Quality and Frequency
4. Interpretation
4.1. Emerging Therapeutic Strategies
4.1.1. Cytokine Involvement in MS: A Double-Edged Sword
4.1.2. Manipulating B1-Cell Frequency and nAbs Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stys, P.K.; Tsutsui, S. Recent Advances in Understanding Multiple Sclerosis. F1000Research 2019, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M.; et al. Global, Regional, and National Burden of Multiple Sclerosis 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef]
- Gleicher, N.; Barad, D.H. Gender as Risk Factor for Autoimmune Diseases. J. Autoimmun. 2007, 28, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Pathogenic Mechanisms Associated with Different Clinical Courses of Multiple Sclerosis. Front. Immunol. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Negron, A. The Role of B Cells in Multiple Sclerosis: Current and Future Therapies. Cell Immunol. 2019, 339, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Rahmanzadeh, R.; Weber, M.S.; Brück, W.; Navardi, S.; Sahraian, M.A. B Cells in Multiple Sclerosis Therapy—A Comprehensive Review. Acta Neurol. Scand. 2018, 137, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Simon, M.V.; Zang, Y.C.Q.; Hong, J.; Rivera, V.M.; Zhang, J.Z. Cross-Reactivity with Myelin Basic Protein and Human Herpesvirus-6 in Multiple Sclerosis. Ann. Neurol. 2003, 53, 189–197. [Google Scholar] [CrossRef]
- Belbasis, L.; Bellou, V.; Evangelou, E.; Ioannidis, J.P.A.; Tzoulaki, I. Environmental Risk Factors and Multiple Sclerosis: An Umbrella Review of Systematic Reviews and Meta-Analyses. Lancet Neurol. 2015, 14, 263–273. [Google Scholar] [CrossRef]
- Kolahdouzan, M.; Futhey, N.C.; Kieran, N.W.; Healy, L.M. Novel Molecular Leads for the Prevention of Damage and the Promotion of Repair in Neuroimmunological Disease. Front. Immunol. 2019, 10, 1657. [Google Scholar] [CrossRef]
- Matsiota, P.; Blancher, A.; Doyon, B.; Guilbert, B.; Clanet, M.; Kouvelas, E.D.; Avrameas, S. Comparative Study of Natural Autoantibodies in the Serum and Cerebrospinal Fluid of Normal Individuals and Patients with Multiple Sclerosis and Other Neurological Diseases. Ann. Inst. Pasteur. Immunol. 1988, 139, 99–108. [Google Scholar] [CrossRef]
- Sádaba, M.C.; Rothhammer, V.; Muñoz, Ú.; Sebal, C.; Escudero, E.; Kivisäkk, P.; Garcia Sanchez, M.I.; Izquierdo, G.; Hauser, S.L.; Baranzini, S.E.; et al. Serum Antibodies to Phosphatidylcholine in MS. Neurol. Neuroimmunol. Neuroinflamma. 2020, 7, e765. [Google Scholar] [CrossRef]
- Häusser-Kinzel, S.; Weber, M.S. The Role of B Cells and Antibodies in Multiple Sclerosis, Neuromyelitis Optica, and Related Disorders. Front. Immunol. 2019, 10, 1664–3224. [Google Scholar] [CrossRef]
- Negi, N.; Das, B.K. Decoding Intrathecal Immunoglobulins and B Cells in the CNS: Their Synthesis, Function, and Regulation: Modulation of Immune Responses Mediated by Different B Cells Is a Potential Therapeutic Approach toward Ameliorating Several CNS Disorders. Int. Rev. Immunol. 2020, 39, 67–79. [Google Scholar] [CrossRef]
- Milo, R. Therapies for Multiple Sclerosis Targeting B Cells. Croat. Med. J. 2019, 60, 87–98. [Google Scholar] [CrossRef]
- Tanabe, S.; Yamashita, T. B Lymphocytes: Crucial Contributors to Brain Development and Neurological Diseases. Neurosci. Res. 2019, 139, 37–41. [Google Scholar] [CrossRef]
- Bar-Or, A.; Fawaz, L.; Fan, B.; Darlington, P.J.; Rieger, A.; Ghorayeb, C.; Calabresi, P.A.; Waubant, E.; Hauser, S.L.; Zhang, J.; et al. Abnormal B-Cell Cytokine Responses a Trigger of T-Cell-Mediated Disease in MS? Ann. Neurol. 2010, 67, 452–461. [Google Scholar] [CrossRef]
- Staun-Ram, E.; Miller, A. Effector and Regulatory B Cells in Multiple Sclerosis. Clin. Immunol. 2017, 184, 11–25. [Google Scholar] [CrossRef]
- Hayakawa, K.; Hardy, R.R.; Parks, D.R.; Herzenberg, A.L.A. The “ly-1 b” cell subpopulation in normal, immunodefective, and autoimmune mice. J. Exp. Med. 1983, 157, 202–218. [Google Scholar] [CrossRef]
- Pennell, C.A.; Mercolino, T.J.; Grdina, T.A.; Arnold, L.W.; Haughton, G.; Clarke, S.H. Biased Immunoglobulin Variable Region Gene Expression by Ly-1 B Cells Due to Clonal Selection. Eur. J. Immunol. 1989, 19, 1289–1295. [Google Scholar] [CrossRef]
- Rothstein, T.L.; Quach, T.D. The Human Counterpart of Mouse B-1 Cells. Ann. N. Y. Acad. Sci. 2015, 1362, 143–152. [Google Scholar] [CrossRef]
- Griffin, D.O.; Holodick, N.E.; Rothstein, T.L. Human B1 Cells in Umbilical Cord and Adult Peripheral Blood Express the Novel Phenotype CD20+CD27+CD43+CD70-. J. Exp. Med. 2011, 208, 67–80. [Google Scholar] [CrossRef]
- Tørring, C.; Petersen, C.C.; Bjerg, L.; Kofod-Olsen, E.; Petersen, T.; Höllsberg, P. The B1-Cell Subpopulation Is Diminished in Patients with Relapsing-Remitting Multiple Sclerosis. J. Neuroimmunol. 2013, 262, 92–99. [Google Scholar] [CrossRef]
- Griffin, D.O.; Holodick, N.E.; Rothstein, T.L. Human B1 Cells Are CD3-: A Reply to “A Human Equivalent of Mouse B-1 Cells?” And “The Nature of Circulating CD27+CD43+ B Cells”. J. Exp. Med. 2011, 208, 2566–2569. [Google Scholar] [CrossRef]
- Kageyama, Y.; Katayama, N. Ontogeny of Human B1 Cells. Int. J. Hematol. 2019, 111, 628–633. [Google Scholar] [CrossRef]
- Rothstein, T.L.; Griffin, D.O.; Holodick, N.E.; Quach, T.D.; Kaku, H. Human B-1 Cells Take the Stage. Ann. N. Y. Acad. Sci. 2013, 1285, 97–114. [Google Scholar] [CrossRef]
- Baumgarth, N. A Hard(y) Look at B-1 Cell Development and Function. J. Immunol. 2017, 199, 3387–3394. [Google Scholar] [CrossRef]
- Kyaw, T.; Tay, C.; Krishnamurthi, S.; Kanellakis, P.; Agrotis, A.; Tipping, P.; Bobik, A.; Toh, B.H. B1a B Lymphocytes Are Atheroprotective by Secreting Natural IgM That Increases IgM Deposits and Reduces Necrotic Cores in Atherosclerotic Lesions. Circ. Res. 2011, 109, 830–840. [Google Scholar] [CrossRef]
- Baumgarth, N.; Herman, O.C.; Jager, G.C.; Brown, L.E.; Herzenberg, L.A.; Chen, J. B-1 and b-2 Cell-Derived Immunoglobulin m Antibodies Are Nonredundant Components of the Protective Response to Influenza Virus Infection. J. Exp. Med. 2000, 192, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Zhurbenko, N.; Quach, T.D.; Hopkins, T.J.; Rothstein, T.L.; Hernandez, A.M. Human B-1 Cells and B-1 Cell Antibodies Change with Advancing Age. Front. Immunol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lee, J.G.; Yan, J.J.; Ryu, J.H.; Xu, S.; Yang, J. Human B1 Cells Are the Main Blood Group A-Specific B Cells That Have a Moderate Correlation with Anti-a Antibody Titer. Ann. Lab. Med. 2020, 40, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Baumgarth, N. B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front. Immunol. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Duan, B.; Morel, L. Role of B-1a Cells in Autoimmunity. Autoimmun. Rev. 2006, 5, 403–408. [Google Scholar] [CrossRef]
- Villar, L.M.; Sádaba, M.C.; Roldán, E.; Masjuan, J.; González-Porqué, P.; Villarrubia, N.; Espiño, M.; García-Trujillo, J.A.; Bootello, A.; Álvarez-Cermeño, J.C. Intrathecal Synthesis of Oligoclonal IgM against Myelin Lipids. J. Clin. Investig. 2005, 115, 187–194. [Google Scholar] [CrossRef]
- Popi, A.F.; Longo-Maugéri, I.M.; Mariano, M. An overview of B-1 cells as antigen-presenting cells. Front. Immunol. 2016, 7, 138. [Google Scholar] [CrossRef]
- Tanabe, S.; Yamashita, T. B-1a Lymphocytes Promote Oligodendrogenesis during Brain Development. Nat. Neurosci. 2018, 21, 506–516. [Google Scholar] [CrossRef]
- Brito, N.; Rômulo, R.; Toledo, D.S.; Labussiere, M.; Martins, G.; Dupin, T.V.; Reis, D.C.; Ferraz, N.P.; Cristina, E.; Xander, P. B-1 Cell Response in Immunity against Parasites. Parasitol. Res. 2019, 118, 1343–1352. [Google Scholar] [CrossRef]
- Baumgarth, N. The Double Life of a B-1 Cell: Self-Reactivity Selects for Protective Effector Functions. Nat. Rev. Immunol. 2011, 11, 34–46. [Google Scholar] [CrossRef]
- Gitlin; Nussenzweig. Fifty Years of B Lymphocytes. Nature 2015, 517, 8–10. [Google Scholar] [CrossRef]
- Joseph, S.; Lewis, L. Natural Killer Cells Remember—An Evolutionary Bridge between Innate and Adaptive Immunity? Eur. J. Immunol. 2009, 39, 1–7. [Google Scholar] [CrossRef]
- Waisman, A.; Liblau, R.S.; Becher, B. Innate and Adaptive Immune Responses in the CNS. Lancet Neurol. 2015, 14, 945–955. [Google Scholar] [CrossRef]
- Vale, A.M.; Kearney, J.F.; Nobrega, A.; Schroeder, H.W. Development and Function of B Cell Subsets. In Molecular Biology of B Cells; Academic Press: Cambridge, MA, USA, 2015; pp. 99–119. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Landin, A.M.; Blomberg, B.B. Age Effects on B Cells and Humoral Immunity in Humans. Ageing Res. Rev. 2011, 10, 330–335. [Google Scholar] [CrossRef]
- Wu, L.; Qin, Z.; Liu, H.; Lin, L.; Ye, J.; Li, J. Recent Advances on Phagocytic B Cells in Teleost Fish. Front. Immunol. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, A.; Shao, T.; Nie, L.; Dong, W.; Xiang, L.; Shao, J. B Cells in Teleost Fish Act as Pivotal Initiating APCs in Priming Adaptive Immunity: An Evolutionary Perspective on the Origin of the B-1 Cell Subset and B7 Molecules. J. Immunol. 2014, 192, 2699–2714. [Google Scholar] [CrossRef]
- Suchanek, O.; Sadler, R.; Bateman, E.A.; Patel, S.Y.; Ferry, B.L. Immunophenotyping of Putative Human B1 B Cells in Healthy Controls and Common Variable Immunodeficiency (CVID) Patients. Clin. Exp. Immunol. 2012, 170, 333–341. [Google Scholar] [CrossRef]
- Plytycz, B.; Seljelid, R. B-1/Macrophages as “Living Fossils”. Immunol. Today 1997, 18, 505. [Google Scholar] [CrossRef]
- Quách, T.D.; Hopkins, T.J.; Holodick, N.E.; Vuyyuru, R.; Manser, T.; Bayer, R.-L.; Rothstein, T.L. Human B-1 and B-2 B Cells Develop from Lin—CD34 + CD38 Lo Stem Cells. J. Immunol. 2016, 197, 3950–3958. [Google Scholar] [CrossRef] [PubMed]
- Sauerborn, M.; Schellekens, H. B-1 Cells and Naturally Occurring Antibodies: Influencing the Immunogenicity of Recombinant Human Therapeutic Proteins? Curr. Opin. Biotechnol. 2009, 20, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.M.B.; Felippe, M.J.B. Development, Phenotype, and Function of Non-Conventional B Cells. Comp. Immunol. Microbiol. Infect. Dis. 2017, 54, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Elsner, R.A.; Baumgarth, N. Natural IgM Prevents Autoimmunity by Enforcing B Cell Central Tolerance Induction Trang. J Immunol. 2015, 194, 1489. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Puri, B.K.; Olive, L.; Carvalho, A.F.; Berk, M.; Maes, M. Emerging Role of Innate B1 Cells in the Pathophysiology of Autoimmune and Neuroimmune Diseases: Association with Inflammation, Oxidative and Nitrosative Stress and Autoimmune Responses. Pharmacol. Res. 2019, 148, 104408. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, C.A.; Weill, J.C. Gene profiling of CD11b+ and CD11b− B1 cell subsets reveals potential cell sorting artifacts. J. Exp. Med. 2012, 209, 433. [Google Scholar] [CrossRef]
- Popi, A.F. B-1 Phagocytes: The Myeloid Face of B-1 Cells. Ann. N. Y. Acad. Sci. 2015, 1362, 86–97. [Google Scholar] [CrossRef]
- Aziz, M.; Holodick, N.E.; Rothstein, T.L.; Wang, P. The Role of B-1 Cells in Inflammation. Immunol. Res. 2015, 63, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Quách, T.D.; Rodríguez-Zhurbenko, N.; Hopkins, T.J.; Guo, X.; Hernández, A.M.; Li, W.; Rothstein, T.L. Distinctions among Circulating Antibody-Secreting Cell Populations, Including B-1 Cells, in Human Adult Peripheral Blood. J. Immunol. 2016, 196, 1060–1069. [Google Scholar] [CrossRef]
- Verbinnen, B.; Covens, K.; Moens, L.; Meyts, I.; Bossuyt, X. Human CD20+ CD43+ CD27+ CD5− B cells generate antibodies to capsular polysaccharides of Streptococcus pneumoniae. J. Allergy Clin. Immunol. 2012, 130, 272–275. [Google Scholar] [CrossRef]
- Covens, K.; Verbinnen, B.; Geukens, N.; Meyts, I.; Schuit, F.; Lommel, L.V.; Jacquemin, M.; Bossuyt, X. Characterization of Proposed Human B-1 Cells Reveals Pre-Plasmablast Phenotype. Blood 2013, 121, 5176–5183. [Google Scholar] [CrossRef]
- Descatoire, M.; Weill, J.C.; Reynaud, C.A.; Weller, S. A Human Equivalent of Mouse B-1 Cells? J. Exp. Med. 2011, 208, 2563–2564. [Google Scholar] [CrossRef]
- Romero-Ramírez, S.; Navarro-Hernandez, I.C.; Cervantes-Díaz, R.; Sosa-Hernández, V.A.; Acevedo-Ochoa, E.; Kleinberg-Bild, A.; Valle-Rios, R.; Meza-Sánchez, D.E.; Hernández-Hernández, J.M.; Maravillas-Montero, J.L. Innate-like B Cell Subsets during Immune Responses: Beyond Antibody Production. J. Leukoc. Biol. 2019, 105, 843–856. [Google Scholar] [CrossRef]
- Gambero, M.; Teixeira, D.; Butin, L.; Ishimura, M.E.; Mariano, M.; Popi, A.F.; Longo-Maugéri, I.M. Propionibacterium Acnes Induces an Adjuvant Effect in B-1 Cells and Affects Their Phagocyte Differentiation via a TLR2-Mediated Mechanism. Immunobiology 2016, 221, 1001–1011. [Google Scholar] [CrossRef]
- Vo, H.; Chiu, J.; Allaimo, D.; Mao, C.; Wang, Y.; Gong, Y.; Ow, H.; Porter, T.; Zhong, X. High Fat Diet Deviates Ptc-Specific B1 b Cell Phagocytosis in Obese Mice. Immun. Inflamm. Dis. 2014, 2, 254–261. [Google Scholar] [CrossRef]
- Parra, D.; Rieger, A.M.; Li, J.; Zhang, Y.-A.; Randall, L.M.; Hunter, C.A.; Barreda, D.R.; Sunyer, J.O. Pivotal Advance: Peritoneal Cavity B-1 B Cells Have Phagocytic and Microbicidal Capacities and Present Phagocytosed Antigen to CD4+ T Cells. J. Leukoc. Biol. 2012, 91, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ma, X.; Gu, W.; Fu, M.; An, J.; Xing, Y.; Gao, T.; Li, W.; Liu, Y. Novel Functions of Murine B1 Cells: Active Phagocytic and Microbicidal Abilities. Eur. J. Immunol. 2012, 42, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, T.L. Natural Antibodies as Rheostats for Susceptibility to Chronic Diseases in the Aged. Front. Immunol. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee-Chang, C.; Bodogai, M.; Moritoh, K.; Chen, X.; Wersto, R.; Sen, R.; Young, H.A.; Croft, M.; Ferrucci, L.; Biragyn, A. Aging Converts Innate B1a Cells into Potent CD8 + T Cell Inducers. J. Immunol. 2016, 196, 3385–3397. [Google Scholar] [CrossRef]
- Wu, Y.-Y. Concordance of Increased B1 Cell Subset and Lupus Phenotypes in Mouse and Human Dependent on BLK Expression Levels. J. Immunol. 2015, 194, 5692–5703. [Google Scholar] [CrossRef]
- Lundy, S.K.; Wu, Q.; Wang, Q.; Dowling, C.A.; Taitano, S.H.; Mao, G.; Mao-Draayer, Y. Dimethyl Fumarate Treatment of Relapsing-Remitting Multiple Sclerosis Influences B-Cell Subsets. Neurol. Neuroimmunol. NeuroInflammation 2016, 3, e211. [Google Scholar] [CrossRef]
- Upadhye, A.; Srikakulapu, P.; Gonen, A.; Hendrikx, S.; Perry, H.M.; Nguyen, A.; McSkimming, C.; Marshall, M.A.; Garmey, J.C.; Taylor, A.M.; et al. Diversification and CXCR4-Dependent Establishment of the Bone Marrow B-1a Cell Pool Governs Atheroprotective IgM Production Linked to Human Coronary Atherosclerosis. Circ. Res. 2019, 125, e55–e70. [Google Scholar] [CrossRef]
- Passos, L.S.A.; Magalhães, L.M.D.; Soares, R.P.; Marques, A.F.; Alves, M.L.R.; Giunchetti, R.C.; do Carmo Pereira Nunes, M.; Gollob, K.J.; Dutra, W.O. Activation of Human CD11b+ B1 B-Cells by Trypanosoma Cruzi-Derived Proteins Is Associated with Protective Immune Response in Human Chagas Disease. Front. Immunol. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Demoersman, J.; Pochard, P.; Framery, C.; Simon, Q.; Boisramé, S.; Soueidan, A.; Pers, J.O. B Cell Subset Distribution Is Altered in Patients with Severe Periodontitis. PLoS ONE 2018, 13, e0192986. [Google Scholar] [CrossRef]
- Lee, J.G.; Jang, J.Y.; Fang, T.; Xu, Y.; Yan, J.J.; Ryu, J.H.; Jeon, H.J.; Koo, T.Y.; Kim, D.K.; Oh, K.H.; et al. Identification of Human B-1 Helper T Cells with a Th1-like Memory Phenotype and High Integrin CD49d Expression. Front. Immunol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Lee-Chang, C.; Bodogai, M.; Moritoh, K.; Olkhanud, P.B.; Chan, A.C.; Croft, M.; Mattison, J.A.; Holst, P.J.; Gress, R.E.; Ferrucci, L.; et al. Accumulation of 4-1BBL+ B Cells in the Elderly Induces the Generation of Granzyme-B+ CD8+ T Cells with Potential Antitumor Activity. Blood 2014, 124, 1450–1459. [Google Scholar] [CrossRef]
- Hilgendorf, I.; Theurl, I.; Gerhardt, L.M.S.; Robbins, C.S.; Weber, G.F.; Gonen, A.; Iwamoto, Y.; Degousee, N.; Holderried, T.A.W.; Winter, C.; et al. Innate Response Activator b Cells Aggravate Atherosclerosis by Stimulating t Helper-1 Adaptive Immunity. Circulation 2014, 129, 1677–1687. [Google Scholar] [CrossRef]
- Griffin, D.O.; Rothstein, T.L. Human B1 Cell Frequency: Isolation and Analysis of Human B1 Cells. Front. Immunol. 2012, 3, 1–10. [Google Scholar] [CrossRef]
- Maddur, M.S.; Lacroix-Desmazes, S.; Dimitrov, J.D.; Kazatchkine, M.D.; Bayry, J.; Kaveri, S.V. Natural Antibodies: From First-Line Defense Against Pathogens to Perpetual Immune Homeostasis. Clin. Rev. Allergy Immunol. 2020, 58, 213–228. [Google Scholar] [CrossRef]
- Care, C.; Us, A. CD11b: Marker for a New Type of B Cell that Participates in Cell-Mediated Immunity. Available online: https://www.novusbio.com/antibody-news/antibodies/cd11b-marking-a-new-b-cell-type-responsible-for-cell-mediated-immunity (accessed on 26 January 2022).
- Griffin, D.O.; Rothstein, T.L. A Small Cd11b + Human B1 Cell Subpopulation Stimulates T Cells and Is Expanded in Lupus. J. Exp. Med. 2011, 208, 2591–2598. [Google Scholar] [CrossRef]
- Upadhye, A.; Sturek, J.M.; McNamara, C.A. B Lymphocyte–Mediated Protective Immunity in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 309–322. [Google Scholar] [CrossRef]
- Griffin, D.O.; Rothstein, T.L. Human “Orchestrator” CD11b(+) B1 Cells Spontaneously Secrete Interleukin-10 and Regulate T-Cell Activity. Mol. Med. 2012, 18, 1003–1008. [Google Scholar] [CrossRef]
- Miles, K.; Simpson, J.; Brown, S.; Cowan, G.; Gray, D.; Gray, M. Immune Tolerance to Apoptotic Self Is Mediated Primarily by Regulatory B1a Cells. Front. Immunol. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Yamamoto, W.; Toyoda, H.; Xu, D.Q.; Hanaki, R.; Morimoto, M.; Nakato, D.; Ito, T.; Iwamoto, S.; Bonno, M.; Tanaka, S.; et al. CD3+ B-1a Cells as a Mediator of Disease Progression in Autoimmune-Prone Mice. Mediators Inflamm. 2018, 2018, 9289417. [Google Scholar] [CrossRef]
- Hsu, L.H.; Li, K.P.; Chu, K.H.; Chiang, B.L. A B-1a Cell Subset Induces Foxp3-T Cells with Regulatory Activity through an IL-10-Independent Pathway. Cell. Mol. Immunol. 2015, 12, 354–365. [Google Scholar] [CrossRef]
- Rauch, P.J.; Chudnovskiy, A.; Robbins, C.S.; Weber, G.F.; Etzrodt, M.; Hilgendorf, I.; Tiglao, E.; Figueiredo, J.L.; Iwamoto, Y.; Theurl, I.; et al. Innate Response Activator B Cells Protect against Microbial Sepsis. Science 2012, 335, 597–601. [Google Scholar] [CrossRef]
- Hastings, W.D.; Gurdak, S.M.; Tumang, J.R.; Rothstein, T.L. CD5+/Mac-1- Peritoneal B Cells: A Novel B Cell Subset That Exhibits Characteristics of B-1 Cells. Immunol. Lett. 2006, 105, 90–96. [Google Scholar] [CrossRef]
- Minton, K. B1 B Cells Link Gut Dysbiosis and Insulin Resistance. Nat. Rev. Immunol. 2019, 19, 4271. [Google Scholar] [CrossRef]
- Berland, R.; Wortis, H.H. Origins and Functions of B-1 Cells with Notes on the Role of CD5. Annu. Rev. Immunol. 2002, 20, 253–300. [Google Scholar] [CrossRef]
- Chin, S.S.; Chorro, L.; Chan, J.; Lauvau, G. Splenic Innate B1 B Cell Plasmablasts Produce Sustained Granulocyte-Macrophage Colony-Stimulating Factor and Interleukin-3 Cytokines during Murine Malaria Infections. Infect. Immun. 2019, 87, 1–14. [Google Scholar] [CrossRef]
- Baumgarth, N. The Shaping of a B Cell Pool Maximally Responsive to Infections. Annu. Rev. Immunol. 2021, 39, 103–129. [Google Scholar] [CrossRef]
- Cunningham, A.F.; Flores-Langarica, A.; Bobat, S.; Medina, C.C.D.; Cook, C.N.L.; Ross, E.A.; Lopez-Macias, C.; Henderson, I.R. B1b Cells Recognize Protective Antigens after Natural Infection and Vaccination. Front. Immunol. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Zhang, X. Regulatory Functions of Innate-like B Cells. Cell. Mol. Immunol. 2013, 10, 113–121. [Google Scholar] [CrossRef]
- Alugupalli, K.R.; Leong, J.M.; Woodland, R.T.; Muramatsu, M.; Honjo, T.; Gerstein, R.M. B1b Lymphocytes Confer T Cell-Independent Long-Lasting Immunity. Immunity 2004, 21, 379–390. [Google Scholar] [CrossRef]
- Kreuk, L.S.M.; Koch, M.A.; Slayden, L.C.; Lind, N.A.; Chu, S.; Savage, H.P.; Kantor, A.B.; Baumgarth, N.; Barton, G.M. B Cell Receptor and Toll-like Receptor Signaling Coordinate to Control Distinct B-1 Responses to Both Self and the Microbiota. eLife 2019, 8, 1–25. [Google Scholar] [CrossRef]
- Oliveira, V.C.D.; Sodré, A.C.P.; Gomes, C.P.; Moretti, N.S.; Pesquero, J.B.; Popi, A.F. Alteration in Ikaros Expression Promotes B-1 Cell Differentiation into Phagocytes. Immunobiology 2018, 223, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.R.; Aroeira, L.S.; Frymuller, E.; Dias, M.Â.A.; Bogsan, C.S.B.; Lopes, J.D.; Mariano, M. Mouse B-1 Cell-Derived Mononuclear Phagocyte, a Novel Cellular Component of Acute Non-Specific Inflammatory Exudate. Int. Immunol. 2001, 13, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Popi, A.F.; Osugui, L.; Perez, K.R.; Longo-Maugéri, I.M.; Mariano, M. Could a B-1 Cell Derived Phagocyte “Be One” of the Peritoneal Macrophages during LPS-Driven Inflammation? PLoS ONE 2012, 7, e34570. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, M.M.; Costa, C.R.; Barbosa, F.M.C.; Vivanco, B.C.; Gonzaga, W.F.K.M.; Novaes e Brito, R.R.; Popi, A.F.; Lopes, J.D.; Xander, P. In Vivo and in Vitro Phagocytosis of Leishmania (Leishmania) Amazonensis Promastigotes by B-1 Cells. Parasite Immunol. 2016, 38, 365–376. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Popi, A.F.; Bachi, A.L.L.; Nonogaki, S.; Lopes, J.D.; Mariano, M. B-1 Cells Modulate the Kinetics of Wound-Healing Process in Mice. Immunobiology 2010, 215, 215–222. [Google Scholar] [CrossRef]
- Popi, A.F.; Motta, F.L.T.; Mortara, R.A.; Schenkman, S.; Lopes, J.D.; Mariano, M. Co-Ordinated Expression of Lymphoid and Myeloid Specific Transcription Factors during B-1b Cell Differentiation into Mononuclear Phagocytes in Vitro. Immunology 2009, 126, 114–122. [Google Scholar] [CrossRef]
- Weber, G.F.; Chousterman, B.G.; He, S.; Fenn, A.M.; Nairz, M.; Anzai, A.; Brenner, T.; Uhle, F.; Iwamoto, Y.; Robbins, C.S.; et al. Interleukin-3 Amplifies Acute Inflammation and Is a Potential Therapeutic Target in Sepsis. Science 2015, 347, 1260–1265. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K. Innate Response Activator B Cells: Origins and Functions. Int. Immunol. 2015, 27, 537–541. [Google Scholar] [CrossRef]
- Weber, G.F.; Chousterman, B.G.; Hilgendorf, I.; Robbins, C.S.; Theurl, I.; Gerhardt, L.M.S.; Iwamoto, Y.; Quach, T.D.; Ali, M.; Chen, J.W.; et al. Pleural Innate Response Activator B Cells Protect against Pneumonia via a GM-CSF-IgM Axis. J. Exp. Med. 2014, 211, 1243–1256. [Google Scholar] [CrossRef]
- Robbins, C.S.; Swirski, F.K. Newly Discovered Innate Response Activator B Cells: Crucial Responders against Microbial Sepsis. Expert Rev. Clin. Immunol. 2012, 8, 405–407. [Google Scholar] [CrossRef]
- Kaku, H.; Holodick, N.E.; Tumang, J.R.; Rothstein, T.L. CD25+ B-1a Cells Express Aicda. Front. Immunol. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Yanaba, K.; Bouaziz, J.D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A Regulatory B Cell Subset with a Unique CD1dhiCD5+ Phenotype Controls T Cell-Dependent Inflammatory Responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef]
- Wu, H.; Su, Z.; Barnie, P.A. The Role of B Regulatory (B10) Cells in Inflammatory Disorders and Their Potential as Therapeutic Targets. Int. Immunopharmacol. 2020, 78, 106111. [Google Scholar] [CrossRef]
- Matsushita, T.; Horikawa, M.; Iwata, Y.; Tedder, T.F. Regulatory B Cells (B10 Cells) and Regulatory T Cells Have Independent Roles in Controlling Experimental Autoimmune Encephalomyelitis Initiation and Late-Phase Immunopathogenesis. J. Immunol. 2010, 185, 2240–2252. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The Regulation of IL-10 Production by Immune Cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Pereira, A.; Alvares-Saraiva, A.M.; De Camargo Konno, F.T.; Spadacci-Morena, D.D.; Perez, E.C.; Mariano, M.; Lallo, M.A. B-1 Cell-Mediated Modulation of M1 Macrophage Profile Ameliorates Microbicidal Functions and Disrupt the Evasion Mechanisms of Encephalitozoon Cuniculi. PLoS Negl. Trop. Dis. 2019, 13, e0007674. [Google Scholar] [CrossRef]
- Baumgarth, N.; Waffarn, E.E.; Nguyen, T.T.T. Natural and Induced B-1 Cell Immunity to Infections Raises Questions of Nature versus Nurture. Ann. N. Y. Acad. Sci. 2015, 1362, 188–199. [Google Scholar] [CrossRef]
- Kampen, R. The Role of Innate-like B1 Cells in the Regulation of Sex-Dependent Immune Responses to Chlamydia Infection; Dalhousie University: Halifax, NS, Canada, 2017; Available online: https://dalspace.library.dal.ca//handle/10222/76673 (accessed on 26 January 2022).
- Yong, L.; Tang, Y.; Ren, C.; Liu, M.; Shen, J.; Hou, X. B1 Cells Protect against Schistosoma Japonicum–Induced Liver Inflammation and Fibrosis by Controlling Monocyte Infiltration. PLoS Negl. Trop. Dis. 2019, 13, e0007474. [Google Scholar] [CrossRef]
- Ansel, K.M.; Harris, R.B.S.; Cyster, J.G. CXCL13 Is Required for B1 Cell Homing, Natural Antibody Production, and Body Cavity Immunity. Immunity 2002, 16, 67–76. [Google Scholar] [CrossRef]
- Hauser, A.E.; Höpken, U.E. B Cell Localization and Migration in Health and Disease, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Aramaki, M.; Nagasawa, T.; Koseki, T.; Ishikawa, I. Presence of Activated B-1 Cells in Chronic Inflamed Gingival Tissue. J. Clin. Immunol. 1998, 18, 421–429. [Google Scholar] [CrossRef]
- Bogsan, C.S.B.; Novaes E Brito, R.R.; Da Cruz Palos, M.; Mortara, R.A.; Almeida, S.R.; Lopes, J.D.; Mariano, M. B-1 Cells Are Pivotal for in Vivo Inflammatory Giant Cell Formation. Int. J. Exp. Pathol. 2005, 86, 257–265. [Google Scholar] [CrossRef]
- Ha, S.A.; Tsuji, M.; Suzuki, K.; Meek, B.; Yasuda, N.; Kaisho, T.; Fagarasan, S. Regulation of B1 Cell Migration by Signals through Toll-like Receptors. J. Exp. Med. 2006, 203, 2541–2550. [Google Scholar] [CrossRef]
- Jackson-Jones, L.H.; Bénézech, C. Control of Innate-like B Cell Location for Compartmentalised IgM Production. Curr. Opin. Immunol. 2018, 50, 9–13. [Google Scholar] [CrossRef]
- Smith, F.L.; Baumgarth, N. B-1 Cell Responses to Infections. Curr. Opin. Immunol. 2019, 57, 23–31. [Google Scholar] [CrossRef]
- Waffarn, E.E. Infection-Induced Type I Interferons Activate CD11b on B-1 Cells for Subsequent Lymph Node Accumulation. Nat. Commun. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Tauber, A.I. Metchnikoff and the Phagocytosis Theory. Nat. Rev. Mol. Cell Biol. 2003, 4, 897–901. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Jaumouillé, V.; Grinstein, S. The Cell Biology of Phagocytosis. Annu. Rev. Pathol. Mech. Dis. 2012, 7, 61–98. [Google Scholar] [CrossRef]
- Vidard, L.; Kovacsovics-Bankowski, M.; Kraeft, S.K.; Chen, L.B.; Benacerraf, B.; Rock, K.L. Analysis of MHC Class II Presentation of Particulate Antigens of B Lymphocytes. J. Immunol. 1996, 156, 2809–2818. [Google Scholar]
- Li, J.; Barreda, D.R.; Zhang, Y.A.; Boshra, H.; Gelman, A.E.; LaPatra, S.; Tort, L.; Sunyer, J.O. B Lymphocytes from Early Vertebrates Have Potent Phagocytic and Microbicidal Abilities. Nat. Immunol. 2006, 7, 1116–1124. [Google Scholar] [CrossRef]
- Ghosn, E.E.B.; Russo, M.; Almeida, S.R. Nitric Oxide-Dependent Killing of Cryptococcus Neoformans by B-1-Derived Mononuclear Phagocyte. J. Leukoc. Biol. 2006, 80, 36–44. [Google Scholar] [CrossRef]
- Pérez, E.C.; Machado, J.; Aliperti, F.; Freymüller, E.; Mariano, M.; Lopes, J.D. B-1 Lymphocytes Increase Metastatic Behavior of Melanoma Cells through the Extracellular Signal-Regulated Kinase Pathway. Cancer Sci. 2008, 99, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Balsamo Abrahão, T.; Freymüller, E.; Arruda Mortara, R.; Lopes, J.D.; Mariano, M. Morphological Characterization of Mouse B-1 Cells. Immunobiology 2003, 208, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Firmino-Cruz, L.; Decote-Ricardo, D.; Gomes, D.C.D.O.; Morrot, A.; Freire-de-Lima, C.G.; De Matos Guedes, H.L. How to B(e)-1 Important Cell During Leishmania Infection. Front. Cell. Infect. Microbiol. 2020, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.; Morel, L.; Yang, P.; Wakeland, E.K. Accumulation of Splenic B1a Cells with Potent Antigen-Presenting Capability in NZM2410 Lupus-Prone Mice. Arthritis Rheum. 1998, 41, 1652–1662. [Google Scholar] [CrossRef]
- Margry, B.; Wieland, W.H.; Van Kooten, P.J.; Van Eden, W.; Broere, F. Peritoneal Cavity B-1a Cells Promote Peripheral CD4+ T-Cell Activation. Eur. J. Immunol. 2013, 43, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Gao, W.; Degauque, N.; Bai, C.; Lu, Y.; Kenny, J.; Oukka, M.; Strom, T.B.; Rothstein, T.L. Reciprocal Generation of Th1/Th17 and Treg Cells by B1 and B2 B Cells. Eur. J. Immunol. 2007, 37, 2400–2404. [Google Scholar] [CrossRef]
- Choi, Y.S.; Baumgarth, N. Dual Role for B-1a Cells in Immunity to Influenza Virus Infection. J. Exp. Med. 2008, 205, 3053–3064. [Google Scholar] [CrossRef]
- Noal, V.; Santos, S.; Ferreira, K.S.; Almeida, S.R. Infection with Paracoccidioides Brasiliensis Induces B-1 Cell Migration and Activation of Regulatory T Cells. Microbes Infect. 2016, 18, 798–803. [Google Scholar] [CrossRef]
- Sato, T.; Ishikawa, S.; Akadegawa, K.; Ito, T.; Yurino, H.; Kitabatake, M.; Yoneyama, H.; Matsushima, K. Aberrant B1 Cell Migration into the Thymus Results in Activation of CD4 T Cells through Its Potent Antigen-Presenting Activity in the Development of Murine Lupus. Eur. J. Immunol. 2004, 34, 3346–3358. [Google Scholar] [CrossRef]
- Youinou, P.; Lydyard, P.M. CD5+ B Cells in Nonorgan-Specific Autoimmune Diseases: A Fresh Look. Lupus 2001, 10, 523–525. [Google Scholar] [CrossRef]
- Tumang, J.R.; Hastings, W.D.; Bai, C.; Rothstein, T.L. Peritoneal and Splenic B-1 Cells Are Separable by Phenotypic, Functional, and Transcriptomic Characteristics. Eur. J. Immunol. 2004, 34, 2158–2167. [Google Scholar] [CrossRef]
- Dempsey, P.W.; Vaidya, S.A.; Cheng, G. The Art of War: Innate and Adaptive Immune Responses. Cell. Mol. Life Sci. 2003, 60, 2604–2621. [Google Scholar] [CrossRef]
- Sospedra, M.; Martin, R. Immunology of Multiple Sclerosis. Annu. Rev. Immunol. 2005, 23, 683–747. [Google Scholar] [CrossRef]
- Göbel, K.; Ruck, T.; Meuth, S.G. Cytokine Signaling in Multiple Sclerosis: Lost in Translation. Mult. Scler. J. 2018, 24, 432–439. [Google Scholar] [CrossRef]
- Codarri, L.; Fontana, A.; Becher, B. Cytokine Networks in Multiple Sclerosis: Lost in Translation. Curr. Opin. Neurol. 2010, 23, 205–211. [Google Scholar] [CrossRef]
- Berger, T. Immunological Processes Related to Cognitive Impairment in MS. Acta Neurol. Scand. 2016, 134, 34–38. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 Microglia: The Good, the Bad, and the Inflamed. J. Neuroinflammation 2014, 11, 1–15. [Google Scholar] [CrossRef]
- Correa, S.G.; Sotomayor, C.E.; Rodrĺguez-Galán, M.C. Cytokines and the Immune–Neuroendocrine Network. NeuroImmune Biol. 2010, 9, 79–90. [Google Scholar] [CrossRef]
- Mosser, D.M.; Zhang, X. Interleukin-10: New Perspectives on an Old Cytokine. Immunol. Rev. 2008, 226, 205–218. [Google Scholar] [CrossRef]
- Gonzaga, W.F.K.M.; Xavier, V.; Vivanco, B.C.; Lopes, J.D.; Xander, P. B-1 Cells Contribute to Susceptibility in Experimental Infection with Leishmania (Leishmania) Chagasi. Parasitology 2015, 142, 1506–1515. [Google Scholar] [CrossRef]
- O’garra, A.; Howard, M. IL-10 Production by CD5 B Cells. Ann. N. Y. Acad. Sci. 1992, 651, 182–199. [Google Scholar] [CrossRef]
- Aziz, M.; Ode, Y.; Zhou, M.; Ochani, M.; Holodick, N.E.; Rothstein, T.L.; Wang, P. B-1a Cells Protect Mice from Sepsis-Induced Acute Lung Injury. Mol. Med. 2018, 24, 1–12. [Google Scholar] [CrossRef]
- Popi, A.F.; Lopes, J.D.; Mariano, M. Interleukin-10 Secreted by B-1 Cells Modulates the Phagocytic Activity of Murine Macrophages in Vitro. Immunology 2004, 113, 348–354. [Google Scholar] [CrossRef]
- Ahmed, A.; Koma, M.K. Interleukin-33 Triggers B1 Cell Expansion and Its Release of Monocyte/Macrophage Chemoattractants and Growth Factors. Scand. J. Immunol. 2015, 82, 118–124. [Google Scholar] [CrossRef]
- Royster, W.; Jin, H.; Wang, P.; Aziz, M. Extracellular CIRP Decreases Siglec-G Expression on B-1a Cells Skewing Them towards a pro-Inflammatory Phenotype in Sepsis. Mol. Med. 2021, 27, 1–12. [Google Scholar] [CrossRef]
- Da Costa, L.F.V.; Alvares-Saraiva, A.M.; Dell’Armelina Rocha, P.R.; Spadacci-Morena, D.D.; Perez, E.C.; Mariano, M.; Lallo, M.A. B-1 Cell Decreases Susceptibility to Encephalitozoonosis in Mice. Immunobiology 2017, 222, 218–227. [Google Scholar] [CrossRef]
- Murakami, M.; Yoshioka, H.; Shirai, T.; Tsubata, T.; Honjo, T. Prevention of Autoimmune Symptoms in Autoimmune-Prone Mice by Elimination of B-1 Cells. Int. Immunol. 1995, 7, 877–882. [Google Scholar] [CrossRef]
- Zhong, X.; Lau, S.; Bai, C.; Degauque, N.; Holodick, N.E.; Steven, S.J.; Tumang, J.; Gao, W.; Rothstein, T.L. A Novel Subpopulation of B-1 Cells Is Enriched with Autoreactivity in Normal and Lupus-Prone Mice. Arthritis Rheum 2009, 60, 3734–3743. [Google Scholar] [CrossRef]
- Rasheed, M.A.U.; Latner, D.R.; Aubert, R.D.; Gourley, T.; Spolski, R.; Davis, C.W.; Langley, W.A.; Ha, S.-J.; Ye, L.; Sarkar, S.; et al. Interleukin-21 Is a Critical Cytokine for the Generation of Virus-Specific Long-Lived Plasma Cells. J. Virol. 2013, 87, 7737–7746. [Google Scholar] [CrossRef]
- Mühl, H. Pro-Inflammatory Signaling by IL-10 and IL-22: Bad Habit Stirred up by Interferons? Front. Immunol. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Tilg, H.; Van Montfrans, C.; Van den Ende, A.; Kaser, A.; Van Deventer, S.J.H.; Schreiber, S.; Gregor, M.; Ludwiczek, O.; Rutgeerts, P.; Gasche, C.; et al. Treatment of Crohn’s Disease with Recombinant Human Interleukin 10 Induces the Proinflammatory Cytokine Interferon γ. Gut 2002, 50, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Lauw, F.N.; Pajkrt, D.; Hack, C.E.; Kurimoto, M.; Van Deventer, S.J.H.; Van der Poll, T. Proinflammatory Effects of IL-10 During Human Endotoxemia. J. Immunol. 2000, 165, 2783–2789. [Google Scholar] [CrossRef] [PubMed]
- Getahun, A.; Cambier, J.C. Non-Antibody-Secreting Functions of B Cells and Their Contribution to Autoimmune Disease. Annu. Rev. Cell Dev. Biol. 2019, 35, 337–356. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Yanaba, K.; Bouaziz, J.D.; Fujimoto, M.; Tedder, T.F. Regulatory B Cells Inhibit EAE Initiation in Mice While Other B Cells Promote Disease Progression. J. Clin. Investig. 2008, 118, 3420–3430. [Google Scholar] [CrossRef]
- O’garra, A.; Chang, R.; Go, N.; Hastings, R.; Haughton, G.; Howard, M. Ly-1 B (B-1) Cells Are the Main Source of B Cell-derived Interleukin 10. Eur. J. Immunol. 1992, 22, 711–717. [Google Scholar] [CrossRef]
- Firmino-Cruz, L.; Ramos, T.D.; Da Fonseca-Martins, A.M.; Oliveira-Maciel, D.; Oliveira-Silva, G.; Dos Santos, J.S.; Cavazzoni, C.; Morrot, A.; Gomes, D.C.O.; Vale, A.M.; et al. B-1 Lymphocytes Are Able to Produce IL-10, but Is Not Pathogenic during Leishmania (Leishmania) Amazonensis Infection. Immunobiology 2020, 225, 151857. [Google Scholar] [CrossRef]
- Palma, J.; Tokarz-Deptuła, B.; Deptuła, J.; Deptuła, W. Natural Antibodies—Facts Known and Unknown. Cent. Eur. J. Immunol. 2018, 43, 466–475. [Google Scholar] [CrossRef]
- Narang, A.; Qiao, F.; Atkinson, C.; Zhu, H.; Yang, X.; Kulik, L.; Holers, V.M.; Tomlinson, S. Natural IgM Antibodies That Bind Neoepitopes Exposed as a Result of Spinal Cord Injury, Drive Secondary Injury by Activating Complement. J. Neuroinflamm. 2017, 14, 1–12. [Google Scholar] [CrossRef]
- Lobo, P.I. Role of Natural Autoantibodies and Natural IgM Anti-Leucocyte Autoantibodies in Health and Disease. Front. Immunol. 2016, 7, 198. [Google Scholar] [CrossRef]
- Holodick, N.E.; Rodríguez-Zhurbenko, N.; Hernández, A.M. Defining Natural Antibodies. Front. Immunol. 2017, 8, 2–9. [Google Scholar] [CrossRef]
- Panda, S.; Ding, J.L. Natural Antibodies Bridge Innate and Adaptive Immunity. J. Immunol. 2015, 194, 13–20. [Google Scholar] [CrossRef]
- Kumar, D.; Romero, Y.; Schuck, K.N.; Smalley, H.; Subedi, B.; Fleming, S.D. Drivers and Regulators of Humoral Innate Immune Responses to Infection and Cancer. Mol. Immunol. 2020, 121, 99–110. [Google Scholar] [CrossRef]
- Nagele, E.P.; Han, M.; Acharya, N.K.; DeMarshall, C.; Kosciuk, M.C.; Nagele, R.G. Natural IgG Autoantibodies Are Abundant and Ubiquitous in Human Sera, and Their Number Is Influenced by Age, Gender, and Disease. PLoS ONE 2013, 8, e60726. [Google Scholar] [CrossRef]
- Grönwall, C.; Silverman, G.J. Natural IgM: Beneficial Autoantibodies for the Control of Inflammatory and Autoimmune Disease? J. Clin. Immunol. 2014, 34 (Suppl. 1), 12–21. [Google Scholar] [CrossRef]
- Okech, B.A.; Nalunkuma, A.; Okello, D.; Pang, X.L.; Suzue, K.; Li, J.; Horii, T.; Egwang, T.G. Natural Human Immunoglobulin g Subclass Responses to Plasmodium Falciparum Serine Repeat Antigen in Uganda. Am. J. Trop. Med. Hyg. 2001, 65, 912–917. [Google Scholar] [CrossRef]
- Ohdan, H.; Swenson, K.G.; Kruger Gray, H.S.; Yang, Y.-G.; Xu, Y.; Thall, A.D.; Sykes, M. Mac-1-Negative B-1b Phenotype of Natural Antibody-Producing Cells, Including Those Responding to Galα1,3Gal Epitopes in A1,3-Galactosyltransferase-Deficient Mice. J. Immunol. 2000, 165, 5518–5529. [Google Scholar] [CrossRef]
- Vas, J.; Grönwal, C.; Silverman, G.J. Fundamental Roles of the Innate-like Repertoire of Natural Antibodies in Immune Homeostasis. Front. Immunol. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Wen, W.; Su, W.; Tang, H.; Le, W.; Zhang, X.; Zheng, Y.; Liu, X.; Xie, L.; Li, J.; Ye, J.; et al. Immune Cell Profiling of COVID-19 Patients in the Recovery Stage by Single-Cell Sequencing. Cell Discov. 2020, 6, 1–18. [Google Scholar] [CrossRef]
- Aziz, M.; Brenner, M.; Wang, P. Therapeutic Potential of B-1a Cells in COVID-19. Shock 2020, 54, 586. [Google Scholar] [CrossRef]
- Herzenberg, L.A. B-1 Cells: The Lineage Question Revisited. Immunol. Rev. 2000, 175, 9–22. [Google Scholar] [CrossRef]
- Tanabe, S.; Yamashita, T. Function of Lymphocytes in Oligodendrocyte Development. Neuroscientist 2020, 26, 74–86. [Google Scholar] [CrossRef]
- Schutter, D.J.; Van Honk, J.; D’Alfonso, A.A.; Peper, J.S.; Panksepp, J. Expression of Fc Receptor for Immunoglobulin M in Oligodendrocytes and Myelin of Mouse Central Nervous System. Neurosci. Lett. 2003, 337, 73–76. [Google Scholar] [CrossRef]
- Tan, C.; Noviski, M.; Huizar, J.; Zikherman, J. Self-Reactivity on a Spectrum: A Sliding Scale of Peripheral B Cell Tolerance. Immunol. Rev. 2019, 292, 37–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ko, Y.; Kim, T.J. Homeostasis and Regulation of Autoreactive B Cells. Cell. Mol. Immunol. 2020, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Viau, M.; Zouali, M. B-Lymphocytes, Innate Immunity, and Autoimmunity. Clin. Immunol. 2005, 114, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ng, S.M.; Hassouna, E.; Warrington, A.; Oh, S.H.; Rodriguez, M. Human-Derived Natural Antibodies: Biomarkers and Potential Therapeutics. Future Neurol. 2015, 10, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Diamond, B.; Honig, G.; Mader, S.; Brimberg, L.; Volpe, B.T. Brain-Reactive Antibodies and Disease. Annu. Rev. Immunol. 2013, 31, 345–385. [Google Scholar] [CrossRef]
- Rodriguez, M.; Warrington, A.E.; Pease, L.R. Invited Article: Human Natural Autoantibodies in the Treatment of Neurologic Disease. Neurology 2009, 72, 1269–1276. [Google Scholar] [CrossRef]
- Micu, I.; Plemel, J.R.; Caprariello, A.V.; Nave, K.A.; Stys, P.K. Axo-Myelinic Neurotransmission: A Novel Mode of Cell Signalling in the Central Nervous System. Nat. Rev. Neurosci. 2018, 19, 49–57. [Google Scholar] [CrossRef]
- Ivanova, M.V.; Zakharova, M.N. Antibodies against Myelin Lipids in Multiple Sclerosis. Hum. Physiol. 2017, 43, 875–880. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Baumgarth, N. Natural IgM and the Development of B Cell-Mediated Autoimmune Diseases. Crit. Rev. Immunol. 2016, 36, 163–177. [Google Scholar] [CrossRef]
- Rodriguez, M.; Miller, D.J. A Monoclonal Autoantibody That Promotes Central Nervous System Remyelination in a Model of Multiple Sclerosis Is a Natural Autoantibody Encoded by Germline Immunoglobulin Genes. J. Immunol. 1995, 154, 2460–2469. [Google Scholar] [CrossRef]
- Warrington, A.E.; Bieber, A.J.; Keulen, V.V.; Rodriguez, M. A Recombinant Human IgM Promotes Myelin Repair After a Single, Very Low Dose. J. Neurosci. Res. 2007, 85, 967–976. [Google Scholar] [CrossRef]
- Wootla, B.; Denic, A.; Warrington, A.E.; Rodriguez, M. A Monoclonal Natural Human IgM Protects Axons in the Absence of Remyelination. J. Neuroinflammation 2016, 13, 1–9. [Google Scholar] [CrossRef]
- Brenner, D.; Brüstle, A.; Lin, G.H.Y.; Lang, P.A.; Duncan, G.S.; Knobbe-Thomsen, C.B.; Paul, M.S.; Reardon, C.; Tusche, M.W.; Snow, B.; et al. Toso Controls Encephalitogenic Immune Responses by Dendritic Cells and Regulatory T Cells. Proc. Natl. Acad. Sci. USA 2014, 111, 1060–1065. [Google Scholar] [CrossRef]
- Blandino, R.; Baumgarth, N. Secreted IgM: New Tricks for an Old Molecule. J. Leukoc. Biol. 2019, 106, 1021–1034. [Google Scholar] [CrossRef]
- Wright, B.R.; Warrington, A.E.; Edberg, D.E.; Rodriguez, M. Cellular Mechanisms of Central Nervous System Repair by Natural Autoreactive Monoclonal Antibodies. Arch. Neurol. 2009, 66, 1456. [Google Scholar] [CrossRef]
- Gold, M.; Pul, R.; Bach, J.P.; Stangel, M.; Dodel, R. Pathogenic and Physiological Autoantibodies in the Central Nervous System. Immunol. Rev. 2012, 248, 68–86. [Google Scholar] [CrossRef]
- Beltrán, E.; Obermeier, B.; Moser, M.; Coret, F.; Simó-Castelló, M.; Boscá, I.; Pérez-Miralles, F.; Villar, L.M.; Senel, M.; Tumani, H.; et al. Intrathecal Somatic Hypermutation of IgM in Multiple Sclerosis and Neuroinflammation. Brain 2014, 137, 2703–2714. [Google Scholar] [CrossRef]
- Avrameas, S.; Alexopoulos, H.; Moutsopoulos, H.M. Natural Autoantibodies: An Undersugn Hero of the Immune System and Autoimmune Disorders—A Point of View. Front. Immunol. 2018, 9, 1–4. [Google Scholar] [CrossRef]
- Pipi, E.; Nayar, S.; Gardner, D.H.; Colafrancesco, S.; Smith, C.; Barone, F. Tertiary Lymphoid Structures: Autoimmunity Goes Local. Front. Immunol. 2018, 9, 1–21. [Google Scholar] [CrossRef]
- Villar, L.M.; Espiño, M.; Cavanillas, M.L.; Roldán, E.; Urcelay, E.; De la Concha, E.G.; Sádaba, M.C.; Arroyo, R.; González-Porqué, P.; Álvarez-Cermeño, J.C. Immunological Mechanisms That Associate with Oligoclonal IgM Band Synthesis in Multiple Sclerosis. Clin. Immunol. 2010, 137, 51–59. [Google Scholar] [CrossRef]
- Villar, L.M.; Espiño, M.; Roldán, E.; Marín, N.; Costa-Frossard, L.; Muriel, A.; Álvarez-Cermeño, J.C. Increased Peripheral Blood CD5+ B Cells Predict Earlier Conversion to MS in High-Risk Clinically Isolated Syndromes. Mult. Scler. J. 2011, 17, 690–694. [Google Scholar] [CrossRef]
- Arneth, B.M. Impact of B Cells to the Pathophysiology of Multiple Sclerosis. J. Neuroinflamm. 2019, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mix, E.; Olsson, T.; Corraele, J.; Baig, S.; Kostulas, V.; Olsson, O.; Link, H. B Cells Expressing CD5 Are Increased in Cerebrospinal Fluidof Patients with Multiple Sclerosis. Clin. Exp. Immunol. 1990, 79, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.; García-Barragán, N.; Espiño, M.; Roldán, E.; Sádaba, M.; Gómez-Rial, J.; González-Porqué, P.; Álvarez-Cermeño, J. Influence of Oligoclonal IgM Specificity in Multiple. Mult. Scler. 2008, 14, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s Disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Grönwall, C.; Vas, J.; Silverman, G.J. Protective Roles of Natural IgM Antibodies. Front. Immunol. 2012, 3, 1–10. [Google Scholar] [CrossRef]
- McKay, J.T.; Haro, M.A.; Daly, C.A.; Yammani, R.D.; Pang, B.; Swords, W.E.; Haas, K.M. PD-L2 Regulates B-1 Cell Antibody Production against Phosphorylcholine through an IL-5–Dependent Mechanism. J. Immunol. 2017, 199, 2020–2029. [Google Scholar] [CrossRef]
- Novikova, N.S.; Diatlova, A.S.; Derevtsova, K.Z.; Korneva, E.A.; Viktorovna, F.T.; Ostrinki, Y.; Abraham, L.; Quinn, S.; Segal, Y.; Churilov, L.; et al. Tuftsin-Phosphorylcholine Attenuate Experimental Autoimmune Encephalomyelitis. J. Neuroimmunol. 2019, 337, 577070. [Google Scholar] [CrossRef]
- Vo, H. Dysregulation of Phospholipid-Specific Phagocytosis by B1 B Cells in Diet-Induced Obese Mice. Master’s Thesis, Boston University, Boston, MA, USA, 2014. Available online: https://hdl.handle.net/2144/14388 (accessed on 26 January 2022).
- Luchicchi, A.; Hart, B.; Frigerio, I.; Van Dam, A.M.; Perna, L.; Offerhaus, H.L.; Stys, P.K.; Schenk, G.J.; Geurts, J.J.G. Axon-Myelin Unit Blistering as Early Event in MS Normal Appearing White Matter. Ann. Neurol. 2021, 89, 711–725. [Google Scholar] [CrossRef]
- Poitelon, Y.; Kopec, A.M.; Belin, S. Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells 2020, 9, 812. [Google Scholar] [CrossRef]
- Evans, M.J.; Finean, J.B. The Lipid Composition of Myelin from Brain and Peripheral Nerve. J. Neurochem. 1965, 12, 729–734. [Google Scholar] [CrossRef]
- Elvington, A.; Atkinson, C.; Kulik, L.; Zhu, H.; Yu, J.; Kindy, M.S.; Holers, V.M.; Tomlinson, S. Pathogenic Natural Antibodies Propagate Cerebral Injury Following Ischemic Stroke in Mice. J. Immunol. 2012, 188, 1460–1468. [Google Scholar] [CrossRef]
- Yamanishi, S.; Iizumi, T.; Watanabe, E.; Shimizu, M.; Kamiya, S.; Nagata, K.; Kumagai, Y.; Fukunaga, Y.; Takahashi, H. Implications for Induction of Autoimmimity via Activation of B-1 Cells by Helicobacter Pylori Urease. Infect. Immun. 2006, 74, 248–256. [Google Scholar] [CrossRef]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost All about Citrulline in Mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef]
- Breuillard, C.; Cynober, L.; Moinard, C. Citrulline and Nitrogen Homeostasis: An Overview. Amino Acids 2015, 47, 685–691. [Google Scholar] [CrossRef]
- ’t Hart, B.A.; Dunham, J.; Faber, B.W.; Laman, J.D.; Van Horssen, J.; Bauer, J.; Kap, Y.S. A B Cell-Driven Autoimmune Pathway Leading to Pathological Hallmarks of Progressive Multiple Sclerosis in the Marmoset Experimental Autoimmune Encephalomyelitis Model. Front. Immunol. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Schellekens, G.A.; De Jong, B.A.W.; Van Den Hoogen, F.H.J.; Van De Putte, L.B.A.; Van Venrooij, W.J. Citrulline Is an Essential Constituent of Antigenic Determinants Recognized by Rheumatoid Arthritis-Specific Autoantibodies. J. Immunol. 2015, 195, 8–16. [Google Scholar] [CrossRef]
- Ken S Rosenthal. Why Don’t We Have a Vaccine Against Autoimmune Diseases?—A Review. J. Clin. Cell Immunol. 2019, 10, 574. [Google Scholar] [CrossRef]
- Jagessar, S.A.; Holtman, I.R.; Hofman, S.; Morandi, E.; Heijmans, N.; Laman, J.D.; Gran, B.; Faber, B.W.; Van Kasteren, S.I.; Eggen, B.J.L.; et al. Lymphocryptovirus Infection of Nonhuman Primate B Cells Converts Destructive into Productive Processing of the Pathogenic CD8 T Cell Epitope in Myelin Oligodendrocyte Glycoprotein. J. Immunol. 2016, 197, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- Vyver, M.V.; Beelen, R.; De Keyser, J.; Nagels, G.; Van Binst, A.M.; Verborgh, C.; D’haeseleer, M. Plasma Citrulline Levels Are Increased in Patients with Multiple Sclerosis. J. Neurol. Sci. 2018, 387, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.M.; Ramos, I.; Cross, A.K.; Haddock, G.; McQuaid, S.; Nicholas, A.P.; Woodroofe, M.N. Localisation of Citrullinated Proteins in Normal Appearing White Matter and Lesions in the Central Nervous System in Multiple Sclerosis. J. Neuroimmunol. 2014, 273, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Moscarello; Mastronardi; Wood. The Role of Citrullinated Proteins Suggests a Novel Mechanism in the Pathogenesis of Multiple Sclerosis. Neurochem. Res. 2007, 32, 251–256. [Google Scholar] [CrossRef]
- Nicholas, A.P.; Sambandam, T.; Echols, J.D.; Tourtellotte, W.W. Increased Citrullinated Glial Fibrillary Acidic Protein in Secondary Progressive Multiple Sclerosis. J. Comp. Neurol. 2004, 473, 128–136. [Google Scholar] [CrossRef]
- Seidi, O.A.; Semra, Y.K.; Sharief, M.K. Expression of CD5 on B Lymphocytes Correlates with Disease Activity in Patients with Multiple Sclerosis. J. Neuroimmunol. 2002, 133, 205–210. [Google Scholar] [CrossRef]
- Moscarello, M.A.; Pritzker, L.; Mastronardi, F.G.; Wood, D.D. Peptidylarginine Deiminase: A Candidate Factor in Demyelinating Disease. J. Neurochem. 2002, 81, 335–343. [Google Scholar] [CrossRef]
- Cui, C.; Wang, J.; Mullin, A.P.; Caggiano, A.O.; Parry, T.J.; Colburn, R.W.; Pavlopoulos, E. The Antibody RHIgM22 Facilitates Hippocampal Remyelination and Ameliorates Memory Deficits in the Cuprizone Mouse Model of Demyelination. Brain Res. 2018, 1694, 73–86. [Google Scholar] [CrossRef]
- Perwein, M.K.; Smestad, J.A.; Warrington, A.E.; Heider, R.M.; Kaczor, M.W.; Maher, L.J.; Wootla, B.; Kunbaz, A.; Rodriguez, M. A Comparison of Human Natural Monoclonal Antibodies and Aptamer Conjugates for Promotion of CNS Remyelination: Where Are We Now and What Comes Next? Expert Opin. Biol. Ther. 2018, 18, 545–560. [Google Scholar] [CrossRef]
- Pirko, I.; Ciric, B.; Gamez, J.; Bieber, A.J.; Warrington, A.E.; Johnson, A.J.; Hanson, D.P.; Pease, L.R.; Macura, S.I.; Rodriguez, M. A Human Antibody That Promotes Remyelination Enters the CNS and Decreases Lesion Load as Detected by T2-weighted Spinal Cord MRI in a Virus-induced Murine Model of MS. FASEB J. 2004, 18, 1577–1579. [Google Scholar] [CrossRef]
- Mullin, A.P.; Cui, C.; Wang, Y.; Wang, J.; Troy, E.; Caggiano, A.O.; Parry, T.J.; Colburn, R.W.; Pavlopoulos, E. RHIgM22 Enhances Remyelination in the Brain of the Cuprizone Mouse Model of Demyelination. Neurobiol. Dis. 2017, 105, 142–155. [Google Scholar] [CrossRef]
- Zorina, Y.; Stricker, J.; Caggiano, A.O.; Button, D.C. Human IgM Antibody RHIgM22 Promotes Phagocytic Clearance of Myelin Debris by Microglia. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Lemus, H.N.; Warrington, A.E.; Denic, A.; Wootla, B.; Rodriguez, M. Treatment with a Recombinant Human IgM That Recognizes PSA-NCAM Preserves Brain Pathology in MOG-Induced Experimental Autoimmune Encephalomyelitis. Hum. Antibodies 2017, 25, 121–129. [Google Scholar] [CrossRef]
- Eisen, A.; Greenberg, B.M.; Bowen, J.D.; Arnold, D.L.; Caggiano, A.O. A Double-Blind, Placebo-Controlled, Single Ascending-Dose Study of Remyelinating Antibody RHIgM22 in People with Multiple Sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2017, 3, 205521731774309. [Google Scholar] [CrossRef]
- Xu, X.; Denic, A.; Jordan, L.R.; Wittenberg, N.J.; Warrington, A.E.; Wootla, B.; Papke, L.M.; Zoecklein, L.J.; Yoo, D.; Shaver, J.; et al. A Natural Human IgM That Binds to Gangliosides Is Therapeutic in Murine Models of Amyotrophic Lateral Sclerosis. DMM Dis. Model. Mech. 2015, 8, 831–842. [Google Scholar] [CrossRef]
- Xu, X.; Arthur, E.W.; Brent, R.W.; Bieber, A.J.; Keulen, V.V.; Pease, L.R.; Rodriguez, M. A Human IgM Signals Axon Outgrowth: Coupling Lipid Raft to Microtubules. J. Neurochem. 2011, 119, 100–112. [Google Scholar] [CrossRef]
- Consuegra-Fernández, M.; Aranda, F.; Simões, I.; Orta, M.; Sarukhan, A.; Lozano, F. CD5 as a Target for Immune-Based Therapies. Crit. Rev. Immunol. 2015, 35, 85–115. [Google Scholar] [CrossRef]
- Dauphinée, M.; Tovar, Z.; Talal, N. B Cells Expressing Cd5 Are Increased in Sjögren’s Syndrome. Arthritis Rheum. 1988, 31, 642–647. [Google Scholar] [CrossRef]
- Kotb, A.; Ismail, S.; Kimito, I.; Mohamed, W.; Salman, A.; Mohammed, A.A. Increased CD5+ B-Cells Are Associated with Autoimmune Phenomena in Lepromatous Leprosy Patients. J. Infect. Public Health 2019, 12, 656–659. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Daoussis, D.; Mavropoulos, A.; Liossis, S.N.; Bogdanos, D.P. Regulatory B Cells: New Players in Inflammatory and Autoimmune Rheumatic Diseases. Semin. Arthritis Rheum. 2019, 48, 1133–1141. [Google Scholar] [CrossRef]
- Deng, J.; Wang, X.; Chen, Q.; Sun, X.; Xiao, F.; Ko, K.H.; Zhang, M.; Lu, L. B1a Cells Play a Pathogenic Role in the Development of Autoimmune Arthritis. Oncotarget 2016, 7, 19299–19311. [Google Scholar] [CrossRef]
- Kasaian, M.T.; Casali, P. Autoimmunity-Prone b-l (Cd5 b) Cells, Natural Antibodies and Self Recognition. Autoimmunity 1993, 15, 315–329. [Google Scholar] [CrossRef]
- Blanco, E.; Pérez-Andrés, M.; Arriba-Méndez, S.; Contreras-Sanfeliciano, T.; Criado, I.; Pelak, O.; Serra-Caetano, A.; Romero, A.; Puig, N.; Remesal, A.; et al. Age-Associated Distribution of Normal B-Cell and Plasma Cell Subsets in Peripheral Blood. J. Allergy Clin. Immunol. 2018, 141, 2208–2219.e16. [Google Scholar] [CrossRef]
- Veneri, D.; Ortolani, R.; Franchini, M.; Tridente, G.; Pizzolo, G.; Vella, A. Expression of CD27 and CD23 on Peripheral Blood B Lymphocytes in Humans of Different Ages. Blood Transfus. 2009, 7, 29–34. [Google Scholar] [CrossRef]
- Scholz, J.L.; Alain, D.; Riley, R.L.; Cancro, M.P.; Frasca, D. Comparative Review of Ageing Mice and Human B Cells. Curr. Opin. Immunol. 2013, 25, 504–510. [Google Scholar] [CrossRef]
- Noren Hooten, N.; Longo, D.L.; Evans, M.K. Age—And Race-Related Changes in Subpopulations of Peripheral Blood Lymphocytes in Humans; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Gibson, K.L.; Wu, Y.C.; Barnett, Y.; Duggan, O.; Vaughan, R.; Kondeatis, E.; Nilsson, B.O.; Wikby, A.; Kipling, D.; Dunn-Walters, D.K. B-Cell Diversity Decreases in Old Age and Is Correlated with Poor Health Status. Aging Cell 2009, 8, 18–25. [Google Scholar] [CrossRef]
- Paganelli, R.; Quinti, I.; Fagiolo, U.; Cossarizza, A.; Ortolani, C.; Guerra, E.; Sansoni, P.; Pucillo, L.P.; Scala, E.; Cozzi, E.; et al. Changes in Circulating B Cells and Immunoglobulin Classes and Subclasses in a Healthy Aged Population. Clin. Exp. Immunol. 1992, 90, 351–354. [Google Scholar] [CrossRef]
- Bodogai, M.; Connell, J.O.; Kim, K.; Kim, Y.; Moritoh, K.; Chen, C.; Gusev, F.; Vaughan, K.; Shulzhenko, N.; Mattison, J.A.; et al. Commensal Bacteria Contribute to Insulin Resistance in Aging by Activating Innate B1a Cells. Sci. Transl. Med. 2018, 10, 1–26. [Google Scholar] [CrossRef]
- Biragyn, A.; Aliseychik, M.; Rogaev, E. Potential Importance of B Cells in Aging and Aging-Associated Neurodegenerative Diseases. Semin. Immunopathol. 2017, 39, 283–294. [Google Scholar] [CrossRef]
- Zephir, H. Phenotypic and Functional Study of 4BL B Cells in Multiple Sclerosis; NCT03796611. Available online: https://clinicaltrials.gov/ct2/show/NCT03796611 (accessed on 26 January 2022).
- Jazayeri, M.H.; Pourfathollah, A.A.; Jafari, M.E.; Rasaee, M.J.; Dargahi, Z.V. The Association between Human B-1 Cell Frequency and Aging: From Cord Blood to the Elderly. Biomed. Aging Pathol. 2013, 3, 20–22. [Google Scholar] [CrossRef]
- Michaud, E.; Mastrandrea, C.; Rochereau, N.; Paul, S. Human Secretory IgM: An Elusive Player in Mucosal Immunity. Trends Immunol. 2020, 41, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Britschgi, M.; Olin, C.E.; Johns, H.T.; Takeda-Uchimura, Y.; Lemieux, M.C.; Rufibach, K.; Rajadas, J.; Zhang, H.; Tomooka, B.; Robinson, W.H.; et al. Neuroprotective Natural Antibodies to Assemblies of Amyloidogenic Peptides Decrease with Normal Aging and Advancing Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2009, 106, 12145–12150. [Google Scholar] [CrossRef] [PubMed]
- Hillion, S.; Arleevskaya, M.I.; Blanco, P.; Bordron, A.; Brooks, W.H.; Cesbron, J.Y.; Kaveri, S.; Vivier, E.; Renaudineau, Y. The Innate Part of the Adaptive Immune System. Clin. Rev. Allergy Immunol. 2020, 58, 151–154. [Google Scholar] [CrossRef]
- Vergani, S.; Yuan, J. B-1 Cells Carry the Memory of Neonatal Immune Imprinting. Immunity 2020, 53, 11–13. [Google Scholar] [CrossRef]
- Haas, K.M.; Poe, J.C.; Steeber, D.A.; Tedder, T.F. B-1a and B-1b Cells Exhibit Distinct Developmental Requirements and Have Unique Functional Roles in Innate and Adaptive Immunity to S. Pneumoniae. Immunity 2005, 23, 7–18. [Google Scholar] [CrossRef]
- Niino, M.; Fukazawa, T.; Minami, N.; Amino, I.; Tashiro, J.; Fujiki, N.; Doi, S.; Kikuchi, S. CD5-Positive B Cell Subsets in Secondary Progressive Multiple Sclerosis. Neurosci. Lett. 2012, 523, 56–61. [Google Scholar] [CrossRef]
- Lundy, S.K. Killer B Lymphocytes: The Evidence and the Potential. Inflamm Res. 2009, 58, 345–357. [Google Scholar] [CrossRef]
- Rovituso, D.; Heller, S.; Schroeter, M.; Kleinschnitz, C.; Kuerten, S. B1 Cells Are Unaffected by Immune Modulatory Treatment in Remitting-Relapsing Multiple Sclerosis Patients. J. Neuroimmunol. 2014, 272, 86–90. [Google Scholar] [CrossRef]
- Duddy, M.; Niino, M.; Adatia, F.; Hebert, S.; Freedman, M.; Atkins, H.; Kim, H.J.; Bar-Or, A. Distinct Effector Cytokine Profiles of Memory and Naive Human B Cell Subsets and Implication in Multiple Sclerosis. J. Immunol. 2007, 178, 6092–6099. [Google Scholar] [CrossRef]
- Hirotani, M.; Niino, M.; Fukazawa, T.; Kikuchi, S.; Yabe, I.; Hamada, S.; Tajima, Y.; Sasaki, H. Decreased IL-10 Production Mediated by Toll-like Receptor 9 in B Cells in Multiple Sclerosis. J. Neuroimmunol. 2010, 221, 95–100. [Google Scholar] [CrossRef]
- Schreiner, B.; Becher, B. Perspectives on Cytokine-Directed Therapies in Multiple Sclerosis. Swiss Med. Wkly. 2015, 145, 1–9. [Google Scholar] [CrossRef]
- Warrington, A.E.; Rodriguez, M. Method of Identifying Natural Antibodies for Remyelination. J. Clin. Immunol. 2010, 30 (Suppl. 1), 1–10. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halperin, S.T.; ’t Hart, B.A.; Luchicchi, A.; Schenk, G.J. The Forgotten Brother: The Innate-like B1 Cell in Multiple Sclerosis. Biomedicines 2022, 10, 606. https://doi.org/10.3390/biomedicines10030606
Halperin ST, ’t Hart BA, Luchicchi A, Schenk GJ. The Forgotten Brother: The Innate-like B1 Cell in Multiple Sclerosis. Biomedicines. 2022; 10(3):606. https://doi.org/10.3390/biomedicines10030606
Chicago/Turabian StyleHalperin, Saar T., Bert A. ’t Hart, Antonio Luchicchi, and Geert J. Schenk. 2022. "The Forgotten Brother: The Innate-like B1 Cell in Multiple Sclerosis" Biomedicines 10, no. 3: 606. https://doi.org/10.3390/biomedicines10030606
APA StyleHalperin, S. T., ’t Hart, B. A., Luchicchi, A., & Schenk, G. J. (2022). The Forgotten Brother: The Innate-like B1 Cell in Multiple Sclerosis. Biomedicines, 10(3), 606. https://doi.org/10.3390/biomedicines10030606





