Effects of a 13-Week Personalized Lifestyle Intervention Based on the Diabetes Subtype for People with Newly Diagnosed Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
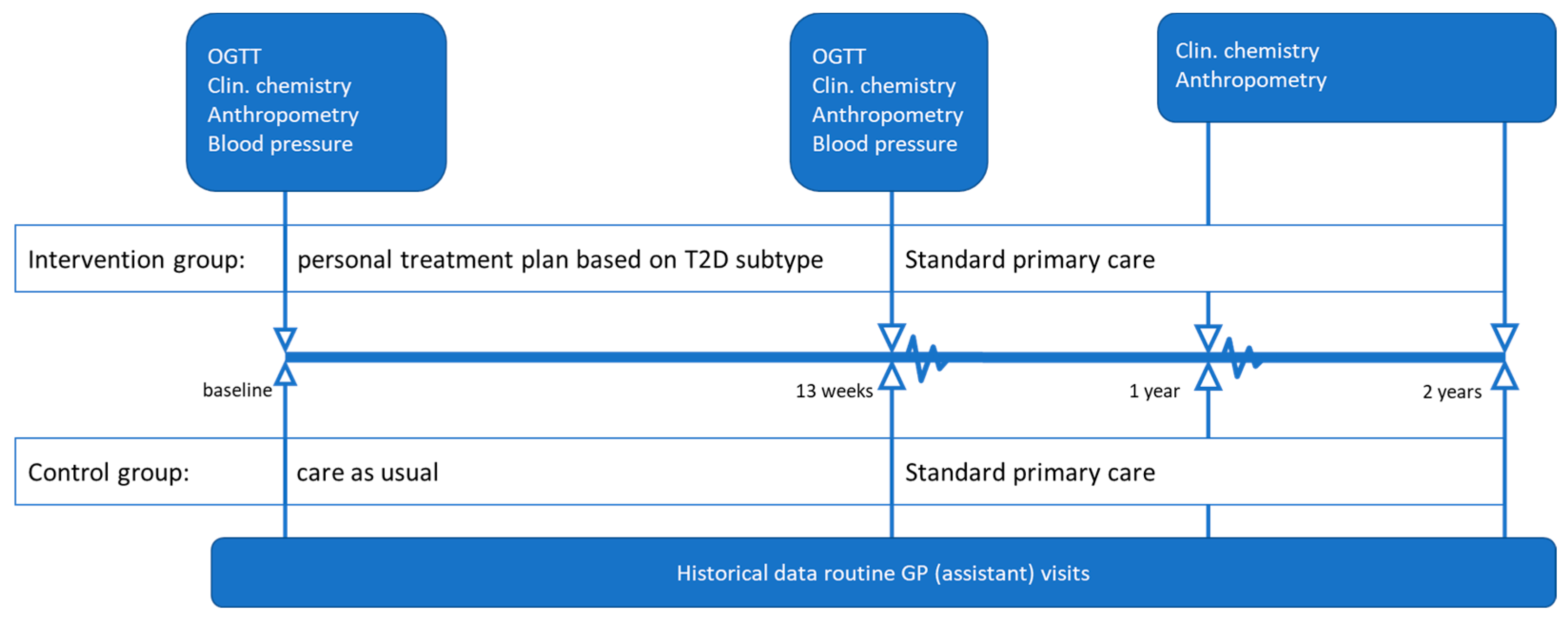
2.2. Study Design
2.3. Clinical Chemistry and OGTT
2.4. Subtyping Rationale
2.5. Interventions
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Intervention Effects Compared with Usual Care
3.3. Diabetes Remission
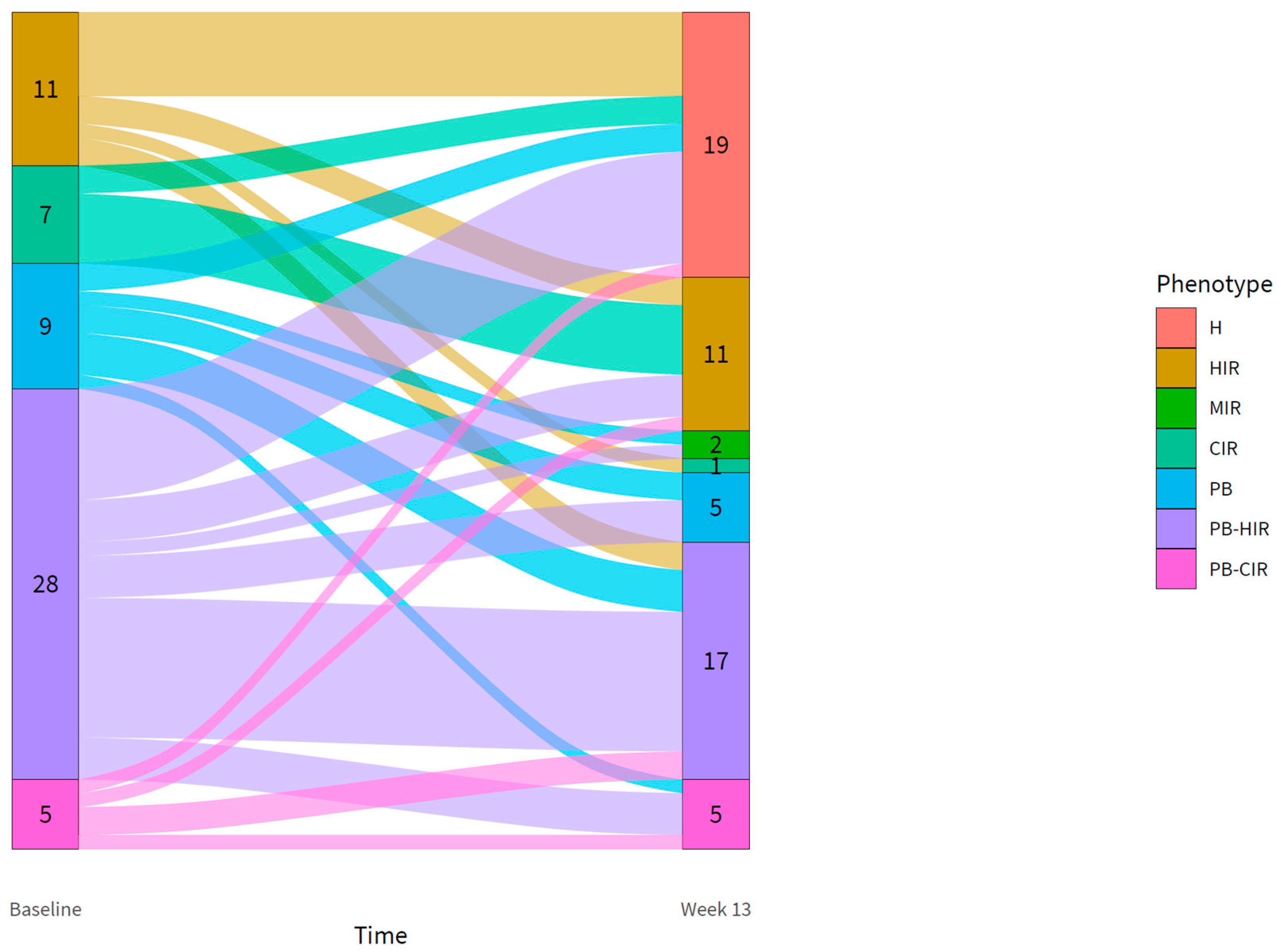
3.4. Changes in the Diabetic Phenotype in the Intervention Group
3.5. Changes in the Glucose Metabolism in the Intervention Group
3.6. Long-Term Intervention Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Defronzo, R.A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buse, J.B.; Caprio, S.; Cefalu, W.T.; Ceriello, A.; Del Prato, S.; Inzucchi, S.E.; McLaughlin, S.; Phillips, G.L.; Robertson, R.P.; Rubino, F.; et al. How Do We Define Cure of Diabetes? Diabetes Care 2009, 32, 2133–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 344–355. [Google Scholar] [CrossRef] [Green Version]
- Colberg, S.R. Physical activity: The forgotten tool for type 2 diabetes management. Front. Endocrinol. 2012, 3, 70. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.; Al-Mrabeh, A.; Zhyzhneuskaya, S.; Peters, C.; Barnes, A.C.; Aribisala, B.S.; Hollingsworth, K.G.; Mathers, J.C.; Sattar, N.; Lean, M.E.J. Erratum: Remission of Human Type 2 Diabetes Requires Decrease in Liver and Pancreas Fat Content but Is Dependent upon Capacity for β Cell Recovery. Cell Metab. 2018, 28, 667, Erratum in Cell Metab. 2018, 28, 547–556.e3. [Google Scholar] [CrossRef] [Green Version]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Steven, S.; Hollingsworth, K.G.; Al-Mrabeh, A.; Avery, L.; Aribisala, B.; Caslake, M.; Taylor, R. Very Low-Calorie Diet and 6 Months of Weight Stability in Type 2 Diabetes: Pathophysiological Changes in Responders and Nonresponders. Diabetes Care 2016, 39, 808–815. [Google Scholar] [CrossRef] [Green Version]
- Ried-Larsen, M.; Johansen, M.Y.; MacDonald, C.S.; Hansen, K.B.; Christensen, R.; Wedell-Neergaard, A.; Pilmark, N.S.; Langberg, H.; Vaag, A.A.; Pedersen, B.K.; et al. Type 2 diabetes remission 1 year after an intensive lifestyle intervention: A secondary analysis of a randomized clinical trial. Diabetes Obes. Metab. 2019, 21, 2257–2266. [Google Scholar] [CrossRef]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef] [Green Version]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B.; Muller, D.C.; Nathan, D.M.; Blake, D.R.; Andres, R.; Baltimore Longitudinal Study of Aging. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes 2003, 52, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Pearson, E.R. Type 2 diabetes: A multifaceted disease. Diabetologia 2019, 62, 1107–1112. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Ghani, M.A.; Tripathy, D.; DeFronzo, R.A. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006, 29, 1130–1139. [Google Scholar] [CrossRef]
- Van Der Kolk, B.W.; Kalafati, M.; Adriaens, M.; Van Greevenbroek, M.M.J.; Vogelzangs, N.; Saris, W.H.M.; Astrup, A.; Valsesia, A.; Langin, D.; Van Der Kallen, C.J.H.; et al. Subcutaneous adipose tissue and systemic inflammation are associated with peripheral but not hepatic insulin resistance in humans. Diabetes 2019, 68, 2247–2258. [Google Scholar] [CrossRef]
- Hjorth, M.F.; Astrup, A.; Zohar, Y.; Urban, L.E.; Sayer, R.D.; Patterson, B.W.; Herring, S.J.; Klein, S.; Zemel, B.S.; Foster, G.D.; et al. Personalized nutrition: Pretreatment glucose metabolism determines individual long-term weight loss responsiveness in individuals with obesity on low-carbohydrate versus low-fat diet. Int. J. Obes. 2019, 43, 2037–2044. [Google Scholar] [CrossRef]
- Blanco-Rojo, R.; Alcala-Diaz, J.F.; Wopereis, S.; Perez-Martinez, P.; Quintana-Navarro, G.M.; Marin, C.; Ordovas, J.M.; van Ommen, B.; Perez-Jimenez, F.; Delgado-Lista, J.; et al. The insulin resistance phenotype (muscle or liver) interacts with the type of diet to determine changes in disposition index after 2 years of intervention: The CORDIOPREV-DIAB randomised clinical trial. Diabetologia 2016, 59, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Kirwan, J.P.; Solomon, T.P.J.; Wojta, D.M.; Staten, M.A.; Holloszy, J.O. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am. J. Physiol. Metab. 2009, 297, E151–E156. [Google Scholar] [CrossRef] [Green Version]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef] [Green Version]
- Van Ommen, B.; Wopereis, S.; van Empelen, P.; van Keulen, H.M.; Otten, W.; Kasteleyn, M.; Molema, J.J.W.; de Hoogh, I.M.; Chavannes, N.H.; Numans, M.E.; et al. From diabetes care to diabetes cure-the integration of systems biology, ehealth, and behavioral change. Front. Endocrinol. 2018, 8, 381. [Google Scholar] [CrossRef]
- Barents, E.; Bilo, H.; Donk, M.; Hart, H.; Verburg-Oorthuizen, A.; Wiersma, T. NHG-Standaard Diabetes Mellitus Type 2-Pagina 1 NHG-Standaard Diabetes Mellitus Type 2 (M01). 2018. Available online: https://richtlijnen.nhg.org/standaarden/diabetes-mellitus-type-2 (accessed on 19 January 2022).
- Bergman, R.N.; Phillips, L.S.; Cobelli, C. Physiologic evaluation of factors controlling glucose tolerance in man: Measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J. Clin. Investig. 1981, 68, 1456–1467. [Google Scholar] [CrossRef] [Green Version]
- Kahn, S.E.; Prigeon, R.L.; McCulloch, D.K.; Boyko, E.J.; Bergman, R.N.; Schwartz, M.W.; Neifing, J.L.; Ward, W.K.; Beard, J.C.; Palmer, J.P. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993, 42, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Breda, E.; Cavaghan, M.K.; Toffolo, G.; Polonsky, K.S.; Cobelli, C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001, 50, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Matsuda, M.; Balas, B.; DeFronzo, R.A. Muscle and Liver Insulin Resistance Indexes Derived from the Oral Glucose Tolerance Test. Diabetes Care 2007, 30, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Larsen, T.M.; Dalskov, S.; van Baak, M.; Jebb, S.; Kafatos, A.; Pfeiffer, A.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunešová, M.; Holst, C.; et al. The Diet, Obesity and Genes (Diogenes) Dietary Study in eight European countries—A comprehensive design for long-term intervention. Obes. Rev. 2010, 11, 76–91. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Perez-Martinez, P.; Garcia-Rios, A.; Alcala-Diaz, J.F.; Perez-Caballero, A.I.; Gomez-Delgado, F.; Fuentes, F.; Quintana-Navarro, G.; Lopez-Segura, F.; Ortiz-Morales, A.M.; et al. CORonary Diet Intervention with Olive oil and cardiovascular PREVention study (the CORDIOPREV study): Rationale, methods, and baseline characteristics. Am. Heart J. 2016, 177, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Wopereis, S.; Stroeve, J.H.M.; Stafleu, A.; Bakker, G.C.M.; Burggraaf, J.; van Erk, M.J.; Pellis, L.; Boessen, R.; Kardinaal, A.A.F.; van Ommen, B. Multi-parameter comparison of a standardized mixed meal tolerance test in healthy and type 2 diabetic subjects: The PhenFlex challenge. Genes Nutr. 2017, 12, 21. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; SAGE Publications: Thousand Oaks, CA, USA, 2019; ISBN 9781544336473. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing [Internet]. 2019. Available online: http://www.r-project.org/index.html (accessed on 19 January 2022).
- Yubero-Serrano, E.M.; Delgado-Lista, J.; Tierney, A.C.; Perez-Martinez, P.; Garcia-Rios, A.; Alcala-Diaz, J.F.; Castaño, J.P.; Tinahones, F.J.; Drevon, C.A.; Defoort, C.; et al. Insulin resistance determines a differential response to changes in dietary fat modification on metabolic syndrome risk factors: The LIPGENE study. Am. J. Clin. Nutr. 2015, 102, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Blaak, E.E. Current metabolic perspective on malnutrition in obesity: Towards more subgroup-based nutritional approaches? Proc. Nutr. Soc. 2020, 32, 331–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trouwborst, I.; Bowser, S.M.; Goossens, G.H.; Blaak, E.E. Ectopic Fat Accumulation in Distinct Insulin Resistant Phenotypes; Targets for Personalized Nutritional Interventions. Front. Nutr. 2018, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Pasman, W.J.; Memelink, R.G.; de Vogel-Van den Bosch, J.; Begieneman, M.P.V.; van den Brink, W.J.; Weijs, P.J.M.; Wopereis, S. Obese Older Type 2 Diabetes Mellitus Patients with Muscle Insulin Resistance Benefit from an Enriched Protein Drink during Combined Lifestyle Intervention: The PROBE Study. Nutrients 2020, 12, 2979. [Google Scholar] [CrossRef] [PubMed]
- de Hoogh, I.M.; Oosterman, J.E.; Otten, W.; Krijger, A.-M.; Berbée-Zadelaar, S.; Pasman, W.J.; van Ommen, B.; Pijl, H.; Wopereis, S. The Effect of a Lifestyle Intervention on Type 2 Diabetes Pathophysiology and Remission: The Stevenshof Pilot Study. Nutrients 2021, 13, 2193. [Google Scholar] [CrossRef]
- Zhyzhneuskaya, S.V.; Al-Mrabeh, A.; Peters, C.; Barnes, A.; Aribisala, B.; Hollingsworth, K.G.; McConnachie, A.; Sattar, N.; Lean, M.E.J.; Taylor, R. Time Course of Normalization of Functional β-Cell Capacity in the Diabetes Remission Clinical Trial After Weight Loss in Type 2 Diabetes. Diabetes Care 2020, 43, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [Green Version]
- Karter, A.J.; Nundy, S.; Parker, M.M.; Moffet, H.H.; Huang, E.S. Incidence of remission in adults with type 2 diabetes: The diabetes & aging study. Diabetes Care 2014, 37, 3188–3195. [Google Scholar] [CrossRef] [Green Version]
- Dutia, R.; Brakoniecki, K.; Bunker, P.; Paultre, F.; Homel, P.; Carpentier, A.C.; McGinty, J.; Laferrere, B. Limited Recovery of β-Cell Function after Gastric Bypass Despite Clinical Diabetes Remission. Diabetes 2014, 63, 1214–1223. [Google Scholar] [CrossRef] [Green Version]
- Nagi, D.; Hambling, C.; Taylor, R. Remission of type 2 diabetes: A position statement from the Association of British Clinical Diabetologists (ABCD) and the Primary Care Diabetes Society (PCDS). Br. J. Diabetes 2019, 19, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Yu, E.A.; Le, N.A.; Stein, A.D. Measuring Postprandial Metabolic Flexibility to Assess Metabolic Health and Disease. J. Nutr. 2021, 151, 3284–3291. [Google Scholar] [CrossRef]
- Udler, M.S.; Kim, J.; von Grotthuss, M.; Bonàs-Guarch, S.; Cole, J.B.; Chiou, J.; Boehnke, M.; Laakso, M.; Atzmon, G.; Glaser, B.; et al. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: A soft clustering analysis. PLOS Med. 2018, 15, e1002654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Ahlqvist, E.; Prasad, R.B.; Groop, L. Subtypes of Type 2 Diabetes Determined From Clinical Parameters. Diabetes 2020, 69, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.; Perelman, D.; Breschi, A.; Limcaoco, P.; Kellogg, R.; McLaughlin, T.; Snyder, M. Glucotypes reveal new patterns of glucose dysregulation. PLoS Biol. 2018, 16, e2005143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelzangs, N.; van der Kallen, C.J.H.; van Greevenbroek, M.M.J.; van der Kolk, B.W.; Jocken, J.W.E.; Goossens, G.H.; Schaper, N.C.; Henry, R.M.A.; Eussen, S.J.P.M.; Valsesia, A.; et al. Metabolic profiling of tissue-specific insulin resistance in human obesity: Results from the Diogenes study and the Maastricht Study. Int. J. Obes. 2020, 44, 1376–1386. [Google Scholar] [CrossRef]
- van der Kolk, B.W.; Vogelzangs, N.; Jocken, J.W.E.; Valsesia, A.; Hankemeier, T.; Astrup, A.; Saris, W.H.M.; Arts, I.C.W.; van Greevenbroek, M.M.J.; Blaak, E.E. Plasma lipid profiling of tissue-specific insulin resistance in human obesity. Int. J. Obes. 2019, 43, 989–998. [Google Scholar] [CrossRef]
- World Health Organization & International Diabetes Federation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; WHO Document Production Services: Geneva, Switserland, 2006. [Google Scholar]
| Characteristic | Usual Care | Intervention | p-Value |
|---|---|---|---|
| n | 60 | 60 | |
| Men/women (n) | 34/26 | 29/31 | NS |
| Age (years) | 65.2 ± 9.7 | 63.4 ± 7.9 | 0.06 |
| Body height (m) | 1.73 ± 0.10 | 1.72 ± 0.10 | NS |
| Bodyweight (kg) | 90.4 ± 15.1 | 96.3 ± 16.1 | NS |
| BMI | 29.9 ± 5.0 | 32.6 ± 4.8 | 0.035 |
| HbA1c (%) HbA1c (mmol/mol) | 6.7 ± 3.4 | 6.0 ± 2.8 | <0.001 |
| 49.7 ± 13.9 | 42.6 ± 7.4 | ||
| FPG (mmol/L) | 8.3 ± 4.0 | 7.0 ± 1.5 | 0.005 |
| SBP (mmHg) | 136 ± 19 | 137 ± 14 | NS |
| DBP (mmHg) | 82 ± 11 | 83 ± 10 | NS |
| Total cholesterol (mmol/L) | 5.9 ± 1.9 † | 5.7 ± 1.1 | NS |
| HDL-cholesterol (mmol/L) | 1.3 ± 0.5 † | 1.3 ± 0.3 | NS |
| Triglycerides (mmol/L) | 3.5 ± 5.4 † | 2.2 ± 1.0 | NS |
| Usual Care | Intervention | |||
|---|---|---|---|---|
| (n = 41) | (%) | (n = 25) | (%) | |
| 13 weeks | 5 | 22.0 | 19 | 75.0 |
| 52 weeks | - | - | 13 | 52.4 |
| 104 weeks | - | - | 7 | 28.6 |
| Subtype | FPG | PPG | DI | HIRI | MISI |
|---|---|---|---|---|---|
| HIR (n = 11) | −1.2 ** | −1.1 | 2.19 | −1145 ** | 0.41 |
| CIR (n = 7) | −0.3 | −3.1 * | 1.44 | −619 * | −1.71 † |
| PB (n = 9) | 0.3 | 0.2 | 0.33 | 138 | 0.09 |
| PB-HIR (n = 28) | −1.2 ** | −0.3 | 0.80 * | −22 ** | 1.58 ** |
| PB-CIR (n = 5) | −0.6 | −8.4 † | 0.87 | 2525 † | −2.16 ‡ |
| HIR (n = 11) | CIR (n = 7) | PB (n = 9) | PB-HIR (n = 28) | PB-CIR (n = 5) | |
|---|---|---|---|---|---|
| Bodyweight (kg) | |||||
| Weeks 0–13 | −10.2 *** | −13.1 *** | −5.6 ** | −8.8 *** | −5.7 * |
| Weeks 0–52 | −9.1 *** | −7.3 ** | −4.8 *** | −6.0 *** | 2.0 |
| Weeks 0–104 | −8.4 *** | −7.1 ** | −2.3 * | −6.0 *** | 3.3 * |
| Fasting glucose (mmol/L) | |||||
| Weeks 0–13 | −1.1 *** | −0.3 | 0.3 | −1.1 *** | −0.5 |
| Weeks 0–52 | −1.3 *** | −0.2 | 0.0 | −0.7 *** | 0.4 |
| Weeks 0–104 | −1.0 *** | −0.2 | 0.4 | −0.7 *** | −0.3 |
| HbA1c (mmol/mol) | |||||
| Weeks 0–13 | −3.4 *** | −3.3 * | 0.0 | −6.2 *** | −2.2 |
| Weeks 0–52 | −4.3 ** | −1.3 | −1.3 | −4.9 *** | −1.5 |
| Weeks 0–104 | −2.4 * | −0.4 | 1.8 | −2.5 ** | −1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Hoogh, I.M.; Pasman, W.J.; Boorsma, A.; van Ommen, B.; Wopereis, S. Effects of a 13-Week Personalized Lifestyle Intervention Based on the Diabetes Subtype for People with Newly Diagnosed Type 2 Diabetes. Biomedicines 2022, 10, 643. https://doi.org/10.3390/biomedicines10030643
de Hoogh IM, Pasman WJ, Boorsma A, van Ommen B, Wopereis S. Effects of a 13-Week Personalized Lifestyle Intervention Based on the Diabetes Subtype for People with Newly Diagnosed Type 2 Diabetes. Biomedicines. 2022; 10(3):643. https://doi.org/10.3390/biomedicines10030643
Chicago/Turabian Stylede Hoogh, Iris M., Wilrike J. Pasman, André Boorsma, Ben van Ommen, and Suzan Wopereis. 2022. "Effects of a 13-Week Personalized Lifestyle Intervention Based on the Diabetes Subtype for People with Newly Diagnosed Type 2 Diabetes" Biomedicines 10, no. 3: 643. https://doi.org/10.3390/biomedicines10030643






