HPLC-MS/MS Oxylipin Analysis of Plasma from Amyotrophic Lateral Sclerosis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Oxylipin Analysis
2.4. Quantitation
2.5. General LOD and LOQ Determination
2.6. Specilized Pro-Resolving Lipid Mediators LOD and LOQ
2.7. Statistical Analysis
3. Results
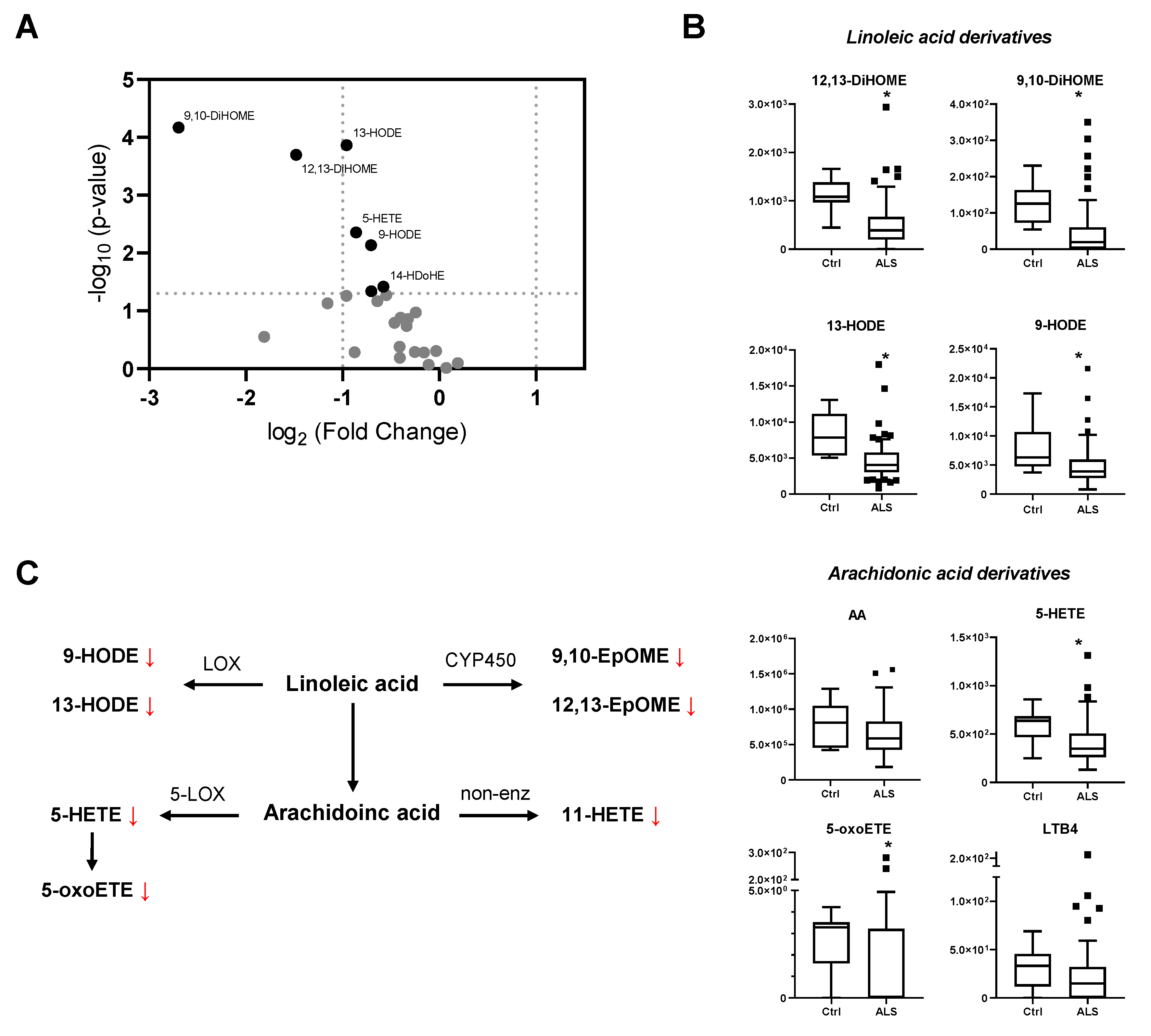
3.1. Oxylipin Analysis
3.2. Individual Oxylipin Analysis
3.3. Multivariate Analysis
3.4. Correlation Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Prim. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, O.; Al Chalabi, A.; Brayne, C.; Beghi, E.; van den Berg, L.H.; Chio, A.; Martin, S.; Logroscino, G.; Rooney, J. The changing picture of amyotrophic lateral sclerosis: Lessons from European registers. J. Neurol. Neurosurg. Psychiatry 2017, 88, 557–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2017, 337, 162–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, M.C.; Couch, Y.; Sibson, N.; Turner, M.R. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol. Cell. Neurosci. 2013, 53, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Camu, W.; Mickunas, M.; Veyrune, J.-L.; Payan, C.; Garlanda, C.; Locati, M.; Juntas-Morales, R.; Pageot, N.; Malaspina, A.; Andreasson, U.; et al. Repeated 5-day cycles of low dose aldesleukin in amyotrophic lateral sclerosis (IMODALS): A phase 2a randomised, double-blind, placebo-controlled trial. EBioMedicine 2020, 59, 102844. [Google Scholar] [CrossRef] [PubMed]
- Gille, B.; De Schaepdryver, M.; Dedeene, L.; Goossens, J.; Claeys, K.G.; Van Den Bosch, L.; Tournoy, J.; Van Damme, P.; Poesen, K. Inflammatory markers in cerebrospinal fluid: Independent prognostic biomarkers in amyotrophic lateral sclerosis? J. Neurol. Neurosurg. Psychiatry 2019, 90, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Béland, L.-C.; Markovinovic, A.; Jakovac, H.; De Marchi, F.; Bilic, E.; Mazzini, L.; Kriz, J.; Munitic, I. Immunity in amyotrophic lateral sclerosis: Blurred lines between excessive inflammation and inefficient immune responses. Brain Commun. 2020, 2, fcaa124. [Google Scholar] [CrossRef]
- Lyon, M.S.; Wosiski-Kuhn, M.; Gillespie, R.; Caress, J.; Milligan, C. Inflammation, Immunity, and amyotrophic lateral sclerosis: I. Etiology and pathology. Muscle Nerve 2019, 59, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Appel, S.H.; Beers, D.R.; Zhao, W. Amyotrophic lateral sclerosis is a systemic disease. Curr. Opin. Neurol. 2021, 34, 765–772. [Google Scholar] [CrossRef]
- Lu, C.-H.; Allen, K.; Oei, F.; Leoni, E.; Kuhle, J.; Tree, T.; Fratta, P.; Sharma, N.; Sidle, K.; Howard, R.; et al. Systemic inflammatory response and neuromuscular involvement in amyotrophic lateral sclerosis. Neurol.-Neuroimmunol. Neuroinflam. 2016, 3, e244. [Google Scholar] [CrossRef] [Green Version]
- Kiernan, M.C.; Vucic, S.; Talbot, K.; McDermott, C.J.; Hardiman, O.; Shefner, J.M.; Al-Chalabi, A.; Huynh, W.; Cudkowicz, M.; Talman, P.; et al. Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2021, 17, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, Y.; Cao, Z.; Liu, Q.; Cheng, Y. Cerebrospinal Fluid Inflammatory Cytokine Aberrations in Alzheimer’s Disease, Parkinson’s Disease and Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Front. Immunol. 2018, 9, 2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iłżecka, J. Prostaglandin E2 is increased in amyotrophic lateral sclerosis patients. Acta Neurol. Scand. 2003, 108, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Almer, G.; Guégan, C.; Teismann, P.; Naini, A.; Rosoklija, G.; Hays, A.P.; Chen, C.; Przedborski, S. Increased expression of the pro-inflammatory enzyme cyclooxygenase-2 in amyotrophic lateral sclerosis. Ann. Neurol. 2001, 49, 176–185. [Google Scholar] [CrossRef]
- Almer, G.; Teismann, P.; Stevic, Z.; Halaschek–Wiener, J.; Deecke, L.; Kostic, V.; Przedborski, S. Increased levels of the pro-inflammatory prostaglandin PGE2 in CSF from ALS patients. Neurology 2002, 58, 1277–1279. [Google Scholar] [CrossRef]
- Henriques, A.; Croixmarie, V.; Priestman, D.A.; Rosenbohm, A.; Dirrig-Grosch, S.; D’Ambra, E.; Huebecker, M.; Hussain, G.; Boursier-Neyret, C.; Echaniz-Laguna, A.; et al. Amyotrophic lateral sclerosis and denervation alter sphingolipids and up-regulate glucosylceramide synthase. Hum. Mol. Genet. 2015, 24, 7390–7405. [Google Scholar] [CrossRef] [Green Version]
- Blasco, H.; Veyrat-Durebex, C.; Bocca, C.; Patin, F.; Vourc’h, P.; Kouassi Nzoughet, J.; Lenaers, G.; Andres, C.R.; Simard, G.; Corcia, P.; et al. Lipidomics Reveals Cerebrospinal-Fluid Signatures of ALS. Sci. Rep. 2017, 7, 17652. [Google Scholar] [CrossRef]
- Area-Gomez, E.; Larrea, D.; Yun, T.; Xu, Y.; Hupf, J.; Zandkarimi, F.; Chan, R.B.; Mitsumoto, H. Lipidomics study of plasma from patients suggest that ALS and PLS are part of a continuum of motor neuron disorders. Sci. Rep. 2021, 11, 13562. [Google Scholar] [CrossRef]
- O’Reilly, É.J.; Bjornevik, K.; Furtado, J.D.; Kolonel, L.N.; Le Marchand, L.; McCullough, M.L.; Stevens, V.L.; Shadyab, A.H.; Snetselaar, L.; Manson, J.A.E.; et al. Prediagnostic plasma polyunsaturated fatty acids and the risk of amyotrophic lateral sclerosis. Neurology 2020, 94, e811–e819. [Google Scholar] [CrossRef]
- Fernández-Eulate, G.; Ruiz-Sanz, J.I.; Riancho, J.; Zufiría, M.; Gereñu, G.; Fernández-Torrón, R.; Poza-Aldea, J.J.; Ondaro, J.; Espinal, J.B.; González-Chinchón, G.; et al. A comprehensive serum lipidome profiling of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 252–262. [Google Scholar] [CrossRef]
- Mastrogiovanni, M.; Ifrán, E.; Trostchansky, A.; Rubbo, H. Lipidomic Analysis of Oxygenated Polyunsaturated Fatty Acid–Derived Inflammatory Mediators in Neurodegenerative Diseases. In Metabolimics—Neuromethods 159; Wood, P., Ed.; Springer Science+Business Media: New York, NY, USA, 2021; pp. 121–141. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2021. Available online: https://www.r-project.org (accessed on 27 January 2022).
- Wang, X.; Zhu, M.; Hjorth, E.; Cortés-Toro, V.; Eyjolfsdottir, H.; Graff, C.; Nennesmo, I.; Palmblad, J.; Eriksdotter, M.; Sambamurti, K.; et al. Resolution of inflammation is altered in Alzheimer’s disease. Alzheimer Dement. 2015, 11, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooij, G.; Troletti, C.D.; Leuti, A.; Norris, P.C.; Riley, I.; Albanese, M.; Ruggieri, S.; Libreros, S.; van der Pol, S.M.A.; van het Hof, B.; et al. Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction. Haematologica 2020, 105, 2056–2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Fernández, A.; Zandee, S.; Mastrogiovanni, M.; Charabati, M.; Rubbo, H.; Prat, A.; López-Vales, R. Administration of Maresin-1 ameliorates the physiopathology of experimental autoimmune encephalomyelitis. J. Neuroinflam. 2022, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Polinski, K.J.; Armstrong, M.; Manke, J.; Seifert, J.; Crume, T.; Yang, F.; Clare-Salzler, M.; Holers, V.M.; Reisdorph, N.; Norris, J.M. Collection and storage of human plasma for measurement of oxylipins. Metabolites 2021, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Kutzner, L.; Rund, K.M.; Ostermann, A.I.; Hartung, N.M.; Galano, J.-M.; Balas, L.; Durand, T.; Balzer, M.S.; David, S.; Schebb, N.H. Development of an Optimized LC-MS Method for the Detection of Specialized Pro-Resolving Mediators in Biological Samples. Front. Pharmacol. 2019, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Gladine, C.; Ostermann, A.I.; Newman, J.W.; Schebb, N.H. MS-based targeted metabolomics of eicosanoids and other oxylipins: Analytical and inter-individual variabilities. Free Radic. Biol. Med. 2019, 144, 72–89. [Google Scholar] [CrossRef]
- Dalli, J.; Colas, R.A.; Walker, M.E.; Serhan, C.N. Lipid mediator metabolomics via LC-MS/MS profiling and analysis. In Clinical Metabolomics: Methods and Protocols; Giera, M., Ed.; Humana Press: Totova, NJ, USA, 2018; pp. 59–72. ISBN 9781493975921. [Google Scholar]
- Dumlao, D.S.; Buczynski, M.W.; Norris, P.C.; Harkewicz, R.; Dennis, E.A. High-throughput lipidomic analysis of fatty acid derived eicosanoids and N-acylethanolamines. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2011, 1811, 724–736. [Google Scholar] [CrossRef] [Green Version]
- Colas, R.A.; Shinohara, M.; Dalli, J.; Chiang, N.; Serhan, C.N. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. AJP Cell Physiol. 2014, 307, C39–C54. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol. Aspects Med. 2017, 58, 1–11. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef]
- Murphy, R.C. Specialized pro-resolving mediators: Do they circulate in plasma? J. Lipid Res. 2015, 56, 1641–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mainka, M.; Dalle, C.; Pétéra, M.; Dalloux-Chioccioli, J.; Kampschulte, N.; Ostermann, A.I.; Rothe, M.; Bertrand-Michel, J.; Newman, J.W.; Gladine, C.; et al. Harmonized procedures lead to comparable quantification of total oxylipins across laboratories. J. Lipid Res. 2020, 61, 1424–1436. [Google Scholar] [CrossRef] [PubMed]
- Vangaveti, V.N.; Jansen, H.; Kennedy, R.L.; Malabu, U.H. Hydroxyoctadecadienoic acids: Oxidised derivatives of linoleic acid and their role in inflammation associated with metabolic syndrome and cancer. Eur. J. Pharmacol. 2016, 785, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yoshikawa, A.; Kinumi, T.; Ogawa, Y.; Saito, Y.; Ohara, K.; Yamamoto, H.; Imai, Y.; Niki, E. Hydroxyoctadecadienoic acid and oxidatively modified peroxiredoxins in the blood of Alzheimer’s disease patients and their potential as biomarkers. Neurobiol. Aging 2009, 30, 174–185. [Google Scholar] [CrossRef]
- Nieman, D.C.; Meaney, M.P.; John, C.S.; Knagge, K.J.; Chen, H. 9- and 13-Hydroxy-octadecadienoic acids (9 + 13 HODE) are inversely related to granulocyte colony stimulating factor and IL-6 in runners after 2 h running. Brain. Behav. Immun. 2016, 56, 246–252. [Google Scholar] [CrossRef]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1111–1120.e3. [Google Scholar] [CrossRef] [Green Version]
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: A review of recent studies. J. Nutr. Biochem. 2020, 86, 108484. [Google Scholar] [CrossRef]

| ALS (n = 78) | Control (n = 9) | |
|---|---|---|
| Gender | ||
| Female | 31 | 7 |
| Male | 47 | 2 |
| BMIa | 25.2 (+/− 3.8) | 23.2 (+/− 1.8) b |
| Site at onset | ||
| Spinal | 42 | - |
| Bulbar | 28 | - |
| Other | 8 | - |
| Age | ||
| at onset (years) a | 62.2 (+/− 11.8) | - |
| sample collection (years) a | 63.8 (+/− 11.1) | 47.3 (+/− 15.8) |
| Time since onset (months)a | 23.1 (+/− 30.9) | - |
| ALSFRS-r slope at last visit (n = 65) | 1.35 (+/− 1.61) | - |
| SP | 28 | - |
| NP | 17 | - |
| FP | 24 | - |
| Metabolites | Control (n = 9) | ALS (n = 74) | p-Value b | ||
|---|---|---|---|---|---|
| Median (pg/mL) | Percentile a | Median (pg/mL) | Percentile | ||
| LA (18:2n-6) metabolome | |||||
| 12,13-DiHOME | 1084 | (969.3; 1381) | 389 | (201; 666) | 0.0002 |
| 9,10-DiHOME | 126 | (73.1; 163) | 19.4 | (1.20; 60.0) | <0.0001 |
| 13-HODE | 7865 | (5375; 11,132) | 4049 | (3044; 5773) | 0.0001 |
| 9-HODE | 6332 | (4769; 10,706) | 3881 | (2775; 5951) | 0.0073 |
| AA (20:4n-6) metabolome | |||||
| AA | 811,395 | (457,556; 1,046,666) | 588,400 | (429,801; 825,059) | 0.1601 |
| TxB2 | 440 | (46.9; 614) | 125 | (46.6; 412) | 0.2800 |
| PGE2 | 0 | (0; 7.73) | 0 | (0; 0) | 0.6111 |
| PGD2 | 0 | (0; 0) | 0 | (0; 0) | 0.9029 |
| isoPGF2 | 0 | (0; 0) | 0 | (0; 443) | 0.1935 |
| 5-HETE | 636 | (469; 685) | 350 | (261; 505) | 0.0044 |
| 8-HETE | 124 | (83.0; 137) | 84.7 | (58.7; 117) | 0.0531 |
| 11-HETE | 191 | (109; 224) | 117 | (87.9; 167) | 0.0459 |
| 12-HETE | 3144 | (2673; 4431) | 1616 | (771; 4125) | 0.0550 |
| 15-HETE | 247 | (181; 271) | 187 | (139; 247) | 0.1312 |
| 5-oxoETE | 3.29 | (1.61; 3.52) | 0 | (0; 3.22) | 0.0089 |
| 12-oxoETE | 36.4 | (0; 59.8) | 0 | (0; 42.3) | 0.4171 |
| 15-oxoETE | 67.9 | (0; 119) | 37.0 | (27.7; 63.5) | 0.5207 |
| 14,15-DiHETrE | 379 | (321; 446) | 320 | (269; 383) | 0.1064 |
| LTB4 | 33.2 | (11.9; 45.5) | 14.9 | (0; 32.1) | 0.0741 |
| EPA (20:5n-3) metabolome | |||||
| EPA | 198,372 | (121,928; 361,172) | 208323 | (146,448; 292,417) | 0.9713 |
| 12-HEPE | 117 | (90.1; 169) | 92.7 | (0; 157) | 0.1816 |
| DHA (22:6n-3) metabolome | |||||
| DHA | 329,956 | (189,149; 560,821) | 376709 | (261,062; 529,711) | 0.8010 |
| 4-HDoHE | 260 | (158; 297) | 196 | (147; 283) | 0.4142 |
| 7-HDoHE | 75.6 | (0; 111) | 69.9 | (0; 102) | 0.8521 |
| 10-HDoHE | 27.2 | (20.0; 33.9) | 24.3 | (18.8; 31.1) | 0.5217 |
| 11-HDoHE | 30.0 | (19.9; 39.2) | 23.9 | (16.9; 30.1) | 0.1384 |
| 13-HDoHE | 87.4 | (45.3; 125) | 56.1 | (33.9; 81.3) | 0.0675 |
| 14-HDoHE | 117 | (86.5; 222) | 78.5 | (41.1; 127) | 0.0381 |
| 16-HDoHE | 66.9 | (37.5; 102) | 56.1 | (43.4; 84.9) | 0.5114 |
| 20-HDoHE | 31.2 | (10.7; 44.0) | 23.5 | (16.1; 35.9) | 0.6488 |
| 19,20-DiHDPA | 160 | (149; 201) | 157 | (136; 193) | 0.4929 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastrogiovanni, M.; Trostchansky, A.; Naya, H.; Dominguez, R.; Marco, C.; Povedano, M.; López-Vales, R.; Rubbo, H. HPLC-MS/MS Oxylipin Analysis of Plasma from Amyotrophic Lateral Sclerosis Patients. Biomedicines 2022, 10, 674. https://doi.org/10.3390/biomedicines10030674
Mastrogiovanni M, Trostchansky A, Naya H, Dominguez R, Marco C, Povedano M, López-Vales R, Rubbo H. HPLC-MS/MS Oxylipin Analysis of Plasma from Amyotrophic Lateral Sclerosis Patients. Biomedicines. 2022; 10(3):674. https://doi.org/10.3390/biomedicines10030674
Chicago/Turabian StyleMastrogiovanni, Mauricio, Andrés Trostchansky, Hugo Naya, Raúl Dominguez, Carla Marco, Mònica Povedano, Rubèn López-Vales, and Homero Rubbo. 2022. "HPLC-MS/MS Oxylipin Analysis of Plasma from Amyotrophic Lateral Sclerosis Patients" Biomedicines 10, no. 3: 674. https://doi.org/10.3390/biomedicines10030674








