Epithelial–Fibroblast Crosstalk Protects against Acidosis-Induced Inflammatory and Fibrotic Alterations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Experimental Setup
2.3. Immunofluorescence
2.4. FITC-Dextran Diffusion
2.5. Collagen Direct ELISA
2.6. Western Blot
2.7. Quantitative PCR
2.8. Data Analysis
3. Results
3.1. Extracellular Acidosis Reduces Cytosolic pH but Does Not Influence Tubule or Fibroblast Cell Viability
3.2. Renal Epithelial Cells
3.2.1. Markers of Cellular Differentiation
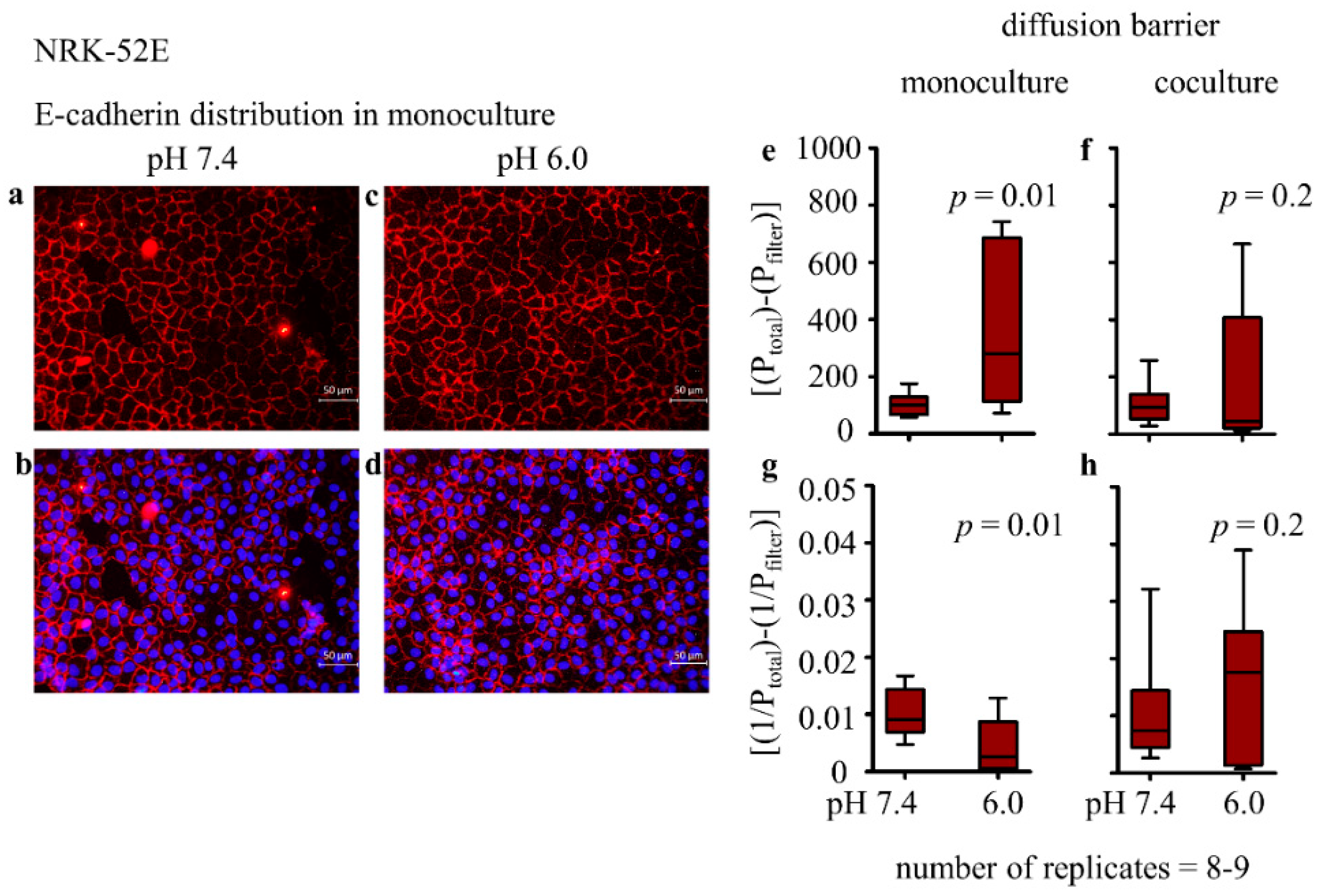
3.2.2. Distribution of E-Cadherin and Cellular Morphology
3.2.3. Epithelial Permeability and Transepithelial Electrical Resistance (TEER)
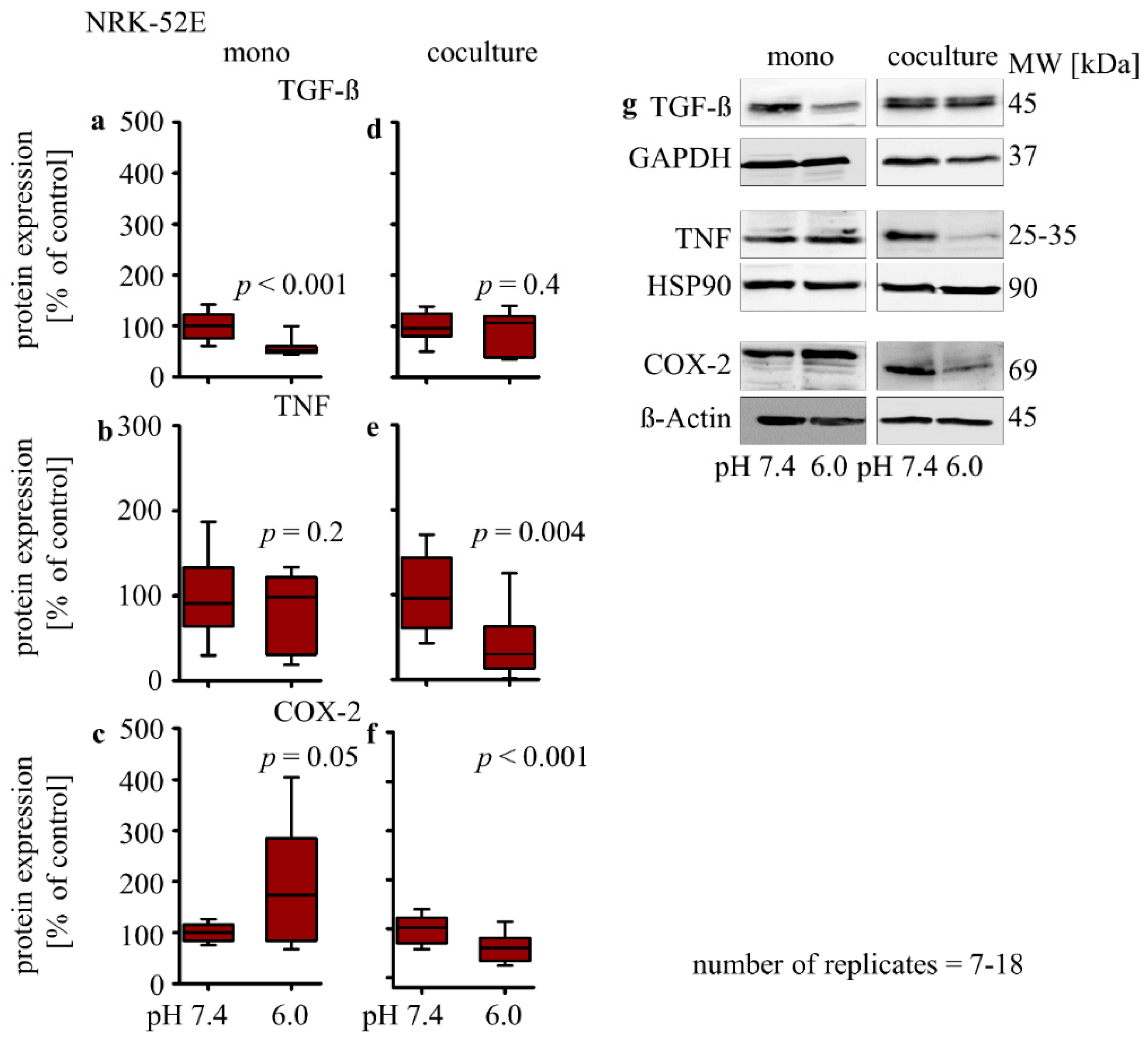
3.2.4. Influence of Extracellular Acidosis on Inflammation Marker
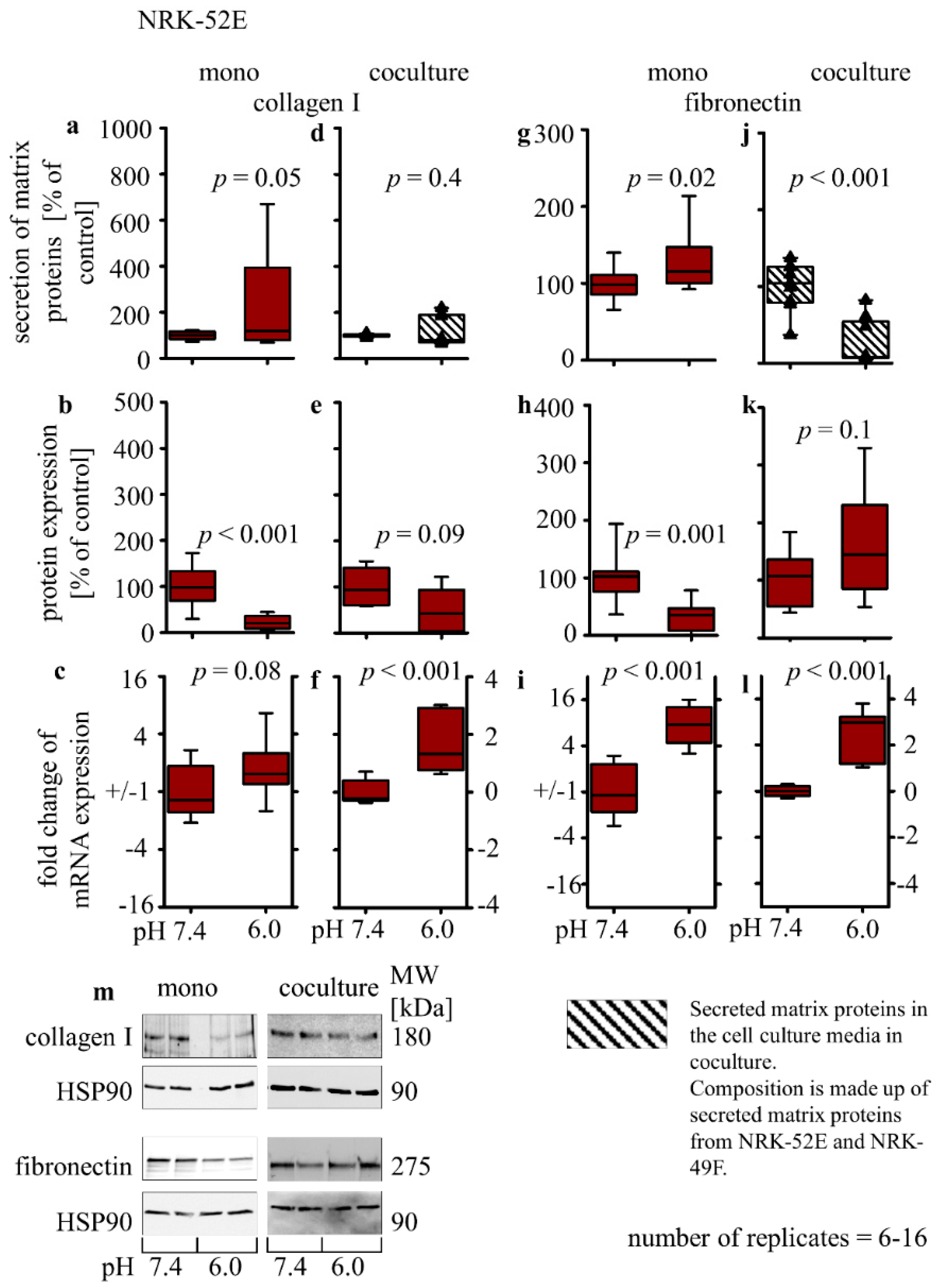
3.2.5. Collagen I and Fibronectin Expression
3.3. Renal Fibroblast
3.3.1. Markers of Cellular Differentiation
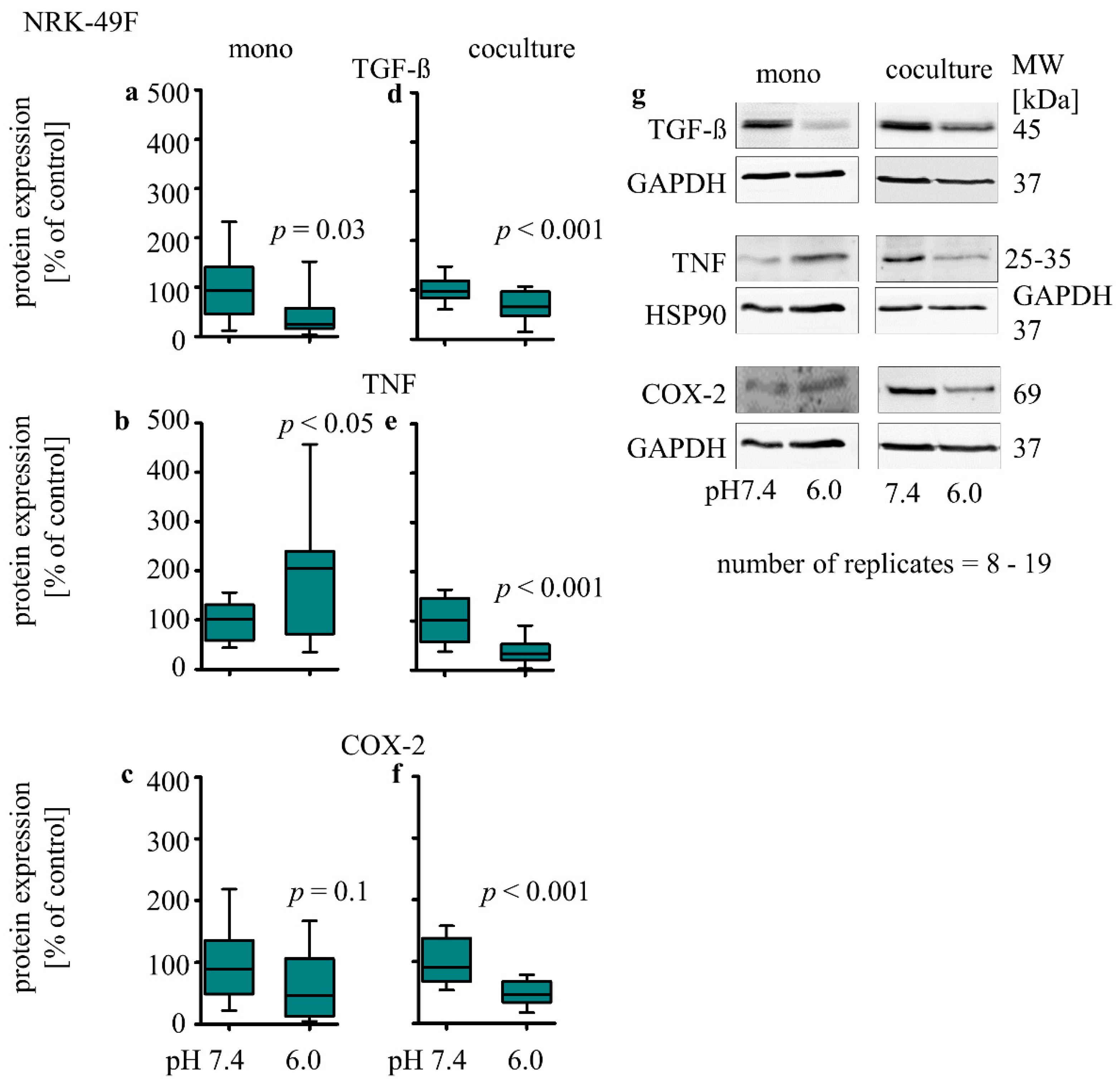
3.3.2. Influence of Extracellular Acidosis on Inflammation Marker
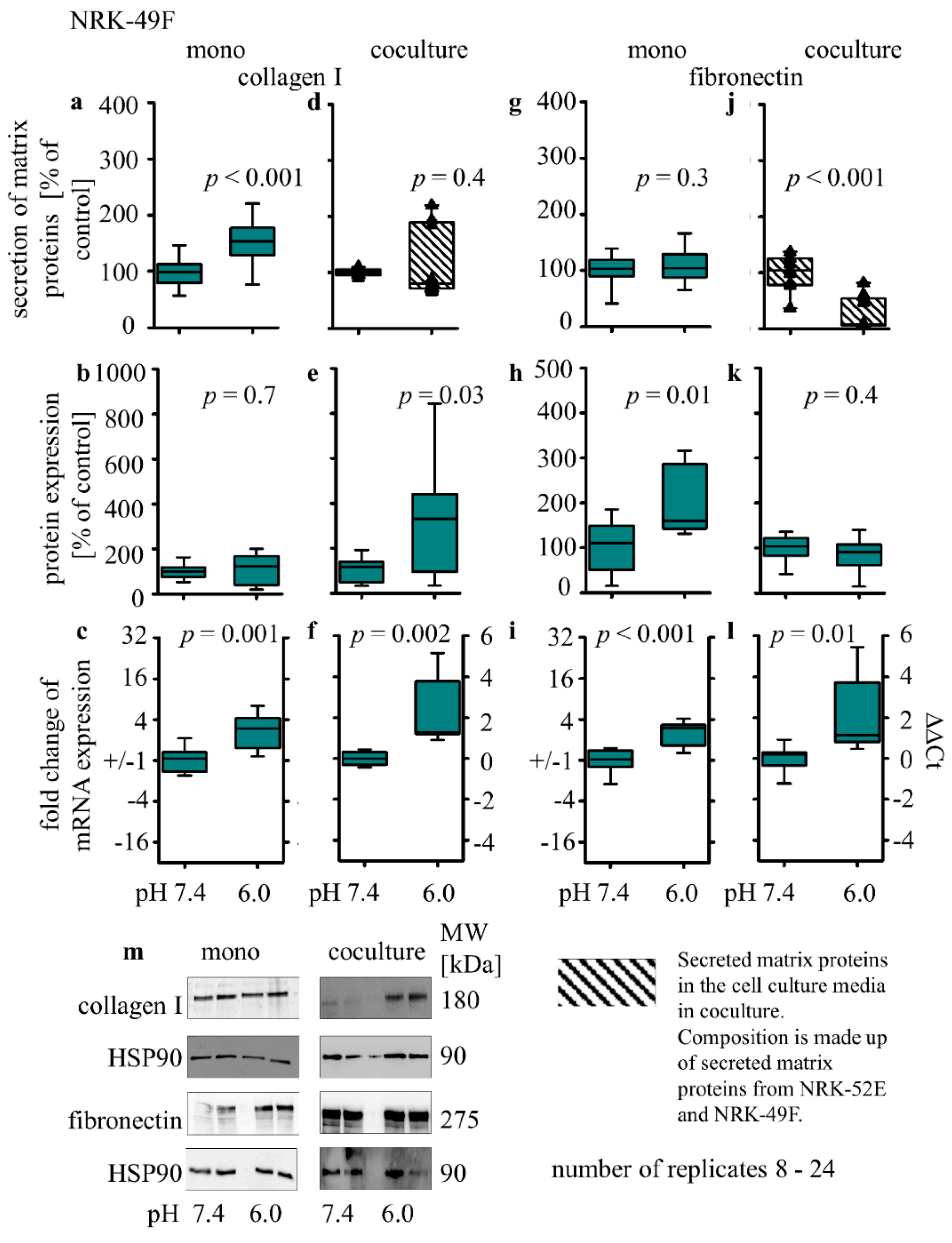
3.3.3. Collagen I and Fibronectin Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ammirati, A.L. Chronic Kidney Disease. Rev. Assoc. Med. Bras. 2020, 66, s03–s09. [Google Scholar] [CrossRef] [PubMed]
- Neuen, B.L.; Chadban, S.J.; Demaio, A.; Johnson, D.W.; Perkovic, V. Chronic kidney disease and the global NCDs agenda. BMJ Glob. Health 2017, 2, e000380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevalier, R.L. The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomeru-lotubular junction. Am. J. Physiol. Renal Physiol. 2016, 311, F145–F161. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.-W.; Bonventre, J.V. Acute kidney injury and maladaptive tubular repair leading to renal fibrosis. Curr. Opin. Nephrol. Hypertens. 2020, 29, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Akchurin, O.M.; Kaskel, F. Update on Inflammation in Chronic Kidney Disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Magno, A.L.; Herat, L.Y.; Carnagarin, R.; Schlaich, M.P.; Matthews, V.B. Current Knowledge of IL-6 Cytokine Family Members in Acute and Chronic Kidney Disease. Biomedicines 2019, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, R.; Jayakumar, C.; Ranganathan, P.V.; Ganapathy, V.; Ramesh, G. Kidney proximal tubular epithelial-specific overexpression of netrin-1 suppresses inflammation and al-buminuria through suppression of COX-2-mediated PGE2 production in streptozotocin-induced diabetic mice. Am. J. Pathol. 2012, 181, 1991–2002. [Google Scholar] [CrossRef] [Green Version]
- Masola, V.; Carraro, A.; Granata, S.; Signorini, L.; Bellin, G.; Violi, P.; Lupo, A.; Tedeschi, U.; Onisto, M.; Gambaro, G.; et al. In vitro effects of interleukin (IL)-1 beta inhibition on the epithelial-to-mesenchymal transition (EMT) of renal tubular and hepatic stellate cells. J. Transl. Med. 2019, 17, 12. [Google Scholar] [CrossRef] [Green Version]
- Lovisa, S.; LeBleu, V.S.; Tampe, B.; Sugimoto, H.; Vadnagara, K.; Carstens, J.L.; Wu, C.-C.; Hagos, Y.; Burckhardt, B.C.; Pentcheva-Hoang, T.; et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med. 2015, 21, 998–1009. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seccia, T.M.; Caroccia, B.; Piazza, M.; Rossi, G.P. The Key Role of Epithelial to Mesenchymal Transition (EMT) in Hypertensive Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, D.; Liu, Y. Renal fibrosis in 2015: Understanding the mechanisms of kidney fibrosis. Nat. Rev. Nephrol. 2016, 12, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Bülow, R.D.; Boor, P. Extracellular Matrix in Kidney Fibrosis: More than Just a Scaffold. J. Histochem. Cytochem. 2019, 67, 643–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meran, S.; Steadman, R. Fibroblasts and myofibroblasts in renal fibrosis. Int. J. Exp. Pathol. 2011, 92, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Ferenbach, D.A.; Bonventre, J.V. Kidney tubules: Intertubular, vascular, and glomerular cross-talk. Curr. Opin. Nephrol. Hypertens. 2016, 25, 194–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, Z.; Leffler, N.R.; Asch, A.S.; Chi, J.-T.; Yang, L.V. Acidosis Activation of the Proton-Sensing GPR4 Receptor Stimulates Vascular Endothelial Cell Inflammatory Responses Revealed by Transcriptome Analysis. PLoS ONE 2013, 8, e61991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellum, J.A.; Song, M.; Li, J. Science review: Extracellular acidosis and the immune response: Clinical and physiologic implications. Crit. Care 2004, 8, 331–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesson, D.E.; Buysse, J.M.; Bushinsky, D.A. Mechanisms of Metabolic Acidosis–Induced Kidney Injury in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2020, 31, 469–482. [Google Scholar] [CrossRef] [Green Version]
- de Larco, J.E.; Todaro, G.J. Epithelioid and fibroblastic rat kidney cell clones: Epidermal growth factor (EGF) receptors and the effect of mouse sarcoma virus transformation. J. Cell. Physiol. 1978, 94, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Best, C.J.M.; Tanzer, L.R.; Phelps, P.C.; Merriman, R.L.; Boder, G.G.; Trump, B.F.; Elliget, K.A. H-ras-transformed NRK-52E renal epithelial cells have altered growth, morphology, and cytoskeletal structure that correlates with renal cell carcinoma in vivo. In Vitro Cell. Dev. Biol.—Anim. 1999, 35, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Edwards, J.R.; Lamar, P.C.; Smith, C.S. Epithelial barrier characteristics and expression of cell adhesion molecules in proximal tubule-derived cell lines commonly used for in vitro toxicity studies. Toxicol. In Vitro 2006, 20, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Sauvant, C.; Holzinger, H.; Gekle, M. Prostaglandin E2Inhibits Its Own Renal Transport by Downregulation of Organic Anion Transporters rOAT1 and rOAT. J. Am. Soc. Nephrol. 2006, 17, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Cilleros, D.; Martín, M.; Ramos, S. (-)-Epicatechin and the Colonic 2,3-Dihydroxybenzoic Acid Metabolite Regulate Glucose Uptake, Glucose Production, and Improve Insulin Signaling in Renal NRK-52E Cells. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.P.; Norman, J.T. Differential response of activated versus non-activated renal fibroblasts to tubular epithelial cells: A model of initiation and progression of fibrosis? Exp. Nephrol. 1998, 6, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Kaissling, B.; Le Hir, M. The renal cortical interstitium: Morphological and functional aspects. Histochem. Cell Biol. 2008, 130, 247–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwerdt, G.; Holzinger, H.; Sauvant, C.; Königs, M.; Humpf, H.-U.; Gekle, M. Long-term effects of ochratoxin A on fibrosis and cell death in human proximal tubule or fibroblast cells in primary culture. Toxicology 2007, 232, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Gekle, M.; Golenhofen, N.; Oberleithner, H.; Oberleithner, H. Rapid activation of Na+/H+ exchange by aldosterone in renal epithelial cells requires Ca2+ and stimulation of a plasma membrane proton conductance. Proc. Natl. Acad. Sci. USA 1996, 93, 10500–10504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gekle, M.; Serrano, O.K.; Drumm, K.; Mildenberger, S.; Freudinger, R.; Gassner, B.; Jansen, H.W.; Jansen, H.W. NHE3 serves, as a molecular tool for cAMP-mediated regulation of receptor-mediated endocytosis. Am. J. Physiol. Renal Physiol. 2002, 283, F549–F558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, Y.; Chen, F.; Chang, R.; Trivedi, M.; Green, K.J.; Cryns, V.L. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ. 2001, 8, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.; Gassner, B.; Hauck, C.R.; Arnold, H.; Mally, S.; Eble, J.A.; Dieterich, P.; Schwab, A. Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. J. Physiol. 2005, 567, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Deutsch Gesellschaft für Nephrologie. Tubulointerstielle Erkrankungen. Available online: https://www.dgfn.eu/nephrologische-diagnosen.html (accessed on 13 July 2021).
- Prunotto, M.; Budd, D.C.; Gabbiani, G.; Meier, M.; Formentini, I.; Hartmann, G.; Pomposiello, S.; Moll, S. Epithelial-mesenchymal crosstalk alteration in kidney fibrosis. J. Pathol. 2012, 228, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.J.; Zhou, N.; Liu, Y. Signaling Crosstalk between Tubular Epithelial Cells and Interstitial Fibroblasts after Kidney Injury. Kidney Dis. 2016, 2, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, M.-C.; Gekle, M.; Schwerdt, G. Epithelial-fibroblast cross talk aggravates the impact of the nephrotoxin ochratoxin A. Biochim. Biophys. Acta 2019, 1866, 118528. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-beta-Slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.L.; Bleaken, B.M.; Romisher, A.R.; Alnwibit, A.A.; Menko, A.S. In wound repair vimentin mediates the transition of mesenchymal leader cells to a myofibroblast phenotype. Mol. Biol. Cell 2018, 29, 1555–1570. [Google Scholar] [CrossRef] [PubMed]
- Riemann, A.; Rauschner, M.; Gießelmann, M.; Reime, S.; Haupt, V.; Thews, O. Extracellular Acidosis Modulates the Expression of Epithelial-Mesenchymal Transition (EMT) Markers and Adhesion of Epithelial and Tumor Cells. Neoplasia 2019, 21, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Peppicelli, S.; Bianchini, F.; Torre, E.; Calorini, L. Contribution of acidic melanoma cells undergoing epithelial-to-mesenchymal transition to aggressiveness of non-acidic melanoma cells. Clin. Exp. Metastasis 2014, 31, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.-M.; Tao, Z.-Q.; Li, R.; Wang, Y.-Q.; Wang, X.; Zhao, J. Vimentin and post-translational modifications in cell motility during cancer—A review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2603–2606. [Google Scholar]
- Snider, N.T.; Omary, M.B. Post-translational modifications of intermediate filament proteins: Mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2014, 15, 163–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S. Stressor-driven extracellular acidosis as tumor inducer via aberrant enzyme activation: A review on the mechanisms and possible prophylaxis. Gene 2017, 626, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Ihnatko, R.; Kubes, M.; Takacova, M.; Takacova, M.; Sedlak, J.; Pastorek, J.; Pastorek, J.; Pastorekova, S. Extracellular acidosis elevates carbonic anhydrase IX in human glioblastoma cells via transcriptional mod-ulation that does not depend on hypoxia. Int. J. Oncol. 2006, 29, 1025–1033. [Google Scholar] [PubMed] [Green Version]
- Hutchins, J.B.; Casagrande, V.A. Vimentin: Changes in distribution during brain development. Glia 1989, 2, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Kulkarni, S. Butein induces intrinsic pathway of apoptosis, vimentin proteolysis, and inhibition of cancer stem cell population in a human papillary thyroid cancer cell line. Toxicol. In Vitro 2021, 77, 105244. [Google Scholar] [CrossRef] [PubMed]
- Matsuyoshi, N.; Imamura, S. Multiple Cadherins Are Expressed in Human Fibroblasts. Biochem. Biophys. Res. Commun. 1997, 235, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Labernadie, A.; Kato, T.; Brugués, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; González-Tarragó, V.; Elosegui-Artola, A.; Albertazzi, L.; et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 2017, 19, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; McLean, M.; Garcia-Melchor, E.; Crowe, L.A.; McMillan, P.; Fazzi, U.G.; Martin, D.; Arthur, A.; Reilly, J.H.; McInnes, I.B.; et al. Fibroblast activation and inflammation in frozen shoulder. PLoS ONE 2019, 14, e0215301. [Google Scholar] [CrossRef] [Green Version]
- Ihling, A.; Ihling, C.H.; Sinz, A.; Gekle, M. Acidosis-Induced Changes in Proteome Patterns of the Prostate Cancer-Derived Tumor Cell Line AT-1. J. Proteome Res. 2015, 14, 3996–4004. [Google Scholar] [CrossRef]
- Riemann, A.; Wußling, H.; Loppnow, H.; Fu, H.; Reime, S.; Thews, O. Acidosis differently modulates the inflammatory program in monocytes and macrophages. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 72–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemann, A.; Reime, S.; Thews, O. Tumor Acidosis and Hypoxia Differently Modulate the Inflammatory Program: Measurements In Vitro and In Vivo. Neoplasia 2017, 19, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Roderfeld, M. Matrix metalloproteinase functions in hepatic injury and fibrosis. Matrix Biol. 2018, 68-69, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Gicquel, T.; Victoni, T.; Valenca, S.S.; Barreto, E.; Bailly-Maître, B.; Boichot, E.; Lagente, V. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci. Rep. 2016, 36, e00360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemann, A.; Schneider, B.; Ihling, A.; Nowak, M.; Sauvant, C.; Thews, O.; Gekle, M. Acidic Environment Leads to ROS-Induced MAPK Signaling in Cancer Cells. PLoS ONE 2011, 6, e22445. [Google Scholar] [CrossRef] [PubMed]


| Target | Company | Order Number | Host | Dilution |
|---|---|---|---|---|
| anti-mouse IRDye 800CW | Li-cor Biosciences GmbH, Bad Homberg, Germany | 926-32210 | Goat | 1:40,000 |
| anti-mouse Alexa Fluor® 594 | Invitrogen by Thermo Fisher, Grand Island, NY, USA) | A21203 | donkey | 1:1000 |
| Anti-Rabbit IgG HRP | Cell Signaling, Danvers, MA, USA | 7074 | Goat | 1:1000 |
| β-actin | Cell Signaling, Danvers, MA, USA | 3700 | Mouse | 1:1000 |
| Collagen I | Biozol, Eching, Germany | PAB 17205 | Rabbit | 1:1000 |
| COX-2 | Abcam, Cambridge, UK | ab52237 | Rabbit monocl | 1:500 |
| E-Cadherin | Cell Signaling, Danvers, MA, USA | 14472 | Mouse | 1:500 |
| Fibronectin | Biomol, Hamburg, Germany | 600-401-117-0.1 | Rabbit | 1:1000 |
| GAPDH | Cell Signaling, Danvers, MA, USA | 2118 | Rabbit | 1:1000 |
| HSP90 | Cell Signaling, Danvers, MA, USA | 4874 | Rabbit | 1:1000 |
| N-cadherin | Cell Signaling, Danvers, MA, USA | 14215 | Mouse | 1:500 |
| α-SMA | Cell Signaling, Danvers, MA, USA | 14968 | Rabbit | 1:500 |
| TGF-β | Cell Signaling, Danvers, MA, USA | 3711 | Rabbit | 1:500 |
| TNF-α | Cell Signaling, Danvers, MA, USA | 6945 | Rabbit | 1:200 |
| Vimentin | Cell Signaling, Danvers, MA, USA | 5741 | Rabbit | 1:1000 |
| Gene Name | Accession Number | Forward 5′-3′ | Backward 5′-3′ | Annealing Temperature °C |
|---|---|---|---|---|
| Actb | NM_031144.2 | TGACGGTCAGGTCATCACTATC | GGCATAGAGGTCTTTACGGATG | 56 °C |
| Col1a1 | NM_053304.1 | CGGCTCCTGCTCCTCTTAG | GCCATTGTGGCAGATACAGA | 58 °C |
| Fn1 | NM_019143 | CACCGAAACCGGGAAGAG | TTGCCTAGGTAGGTCCGTTC | 58 °C |
| Vim | NM_031140.1 | AATGACCGCTTCGCCAACTA | GGTCAAGACGTGCCAGAGAA | 60 °C |
| pH 7.4 (Mean ± s.e.m.) | pH 6.0 (Mean ± s.e.m.) | p Value | |
|---|---|---|---|
| Cytosolic E-cadherin (%) | 12.3 ± 1.2 | 25.5 ± 1.6 | 0.0000001 |
| Structure index (SI) | 0.86 ± 0.01 | 0.75 ± 0.03 | 0.0000819 |
| Membrane thickness (AU) | 49 ± 0.01 | 71 ± 0.02 | 0.0000003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, M.-C.; Voß, L.; Schwerdt, G.; Gekle, M. Epithelial–Fibroblast Crosstalk Protects against Acidosis-Induced Inflammatory and Fibrotic Alterations. Biomedicines 2022, 10, 681. https://doi.org/10.3390/biomedicines10030681
Schulz M-C, Voß L, Schwerdt G, Gekle M. Epithelial–Fibroblast Crosstalk Protects against Acidosis-Induced Inflammatory and Fibrotic Alterations. Biomedicines. 2022; 10(3):681. https://doi.org/10.3390/biomedicines10030681
Chicago/Turabian StyleSchulz, Marie-Christin, Linda Voß, Gerald Schwerdt, and Michael Gekle. 2022. "Epithelial–Fibroblast Crosstalk Protects against Acidosis-Induced Inflammatory and Fibrotic Alterations" Biomedicines 10, no. 3: 681. https://doi.org/10.3390/biomedicines10030681







