Production and Secretion of Gelsolin by Both Human Macrophage- and Fibroblast-like Synoviocytes and GSN Modulation in the Synovial Fluid of Patients with Various Forms of Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Culture
2.2. Synovial Fluid Samples
2.3. Immunohistochemically Staining
2.4. Immunofluorescence Staining
2.5. Western Blot
2.6. ELISA
2.7. Statistics
3. Results
3.1. MLS and FLS React Positively with an Antibody against Gelsolin
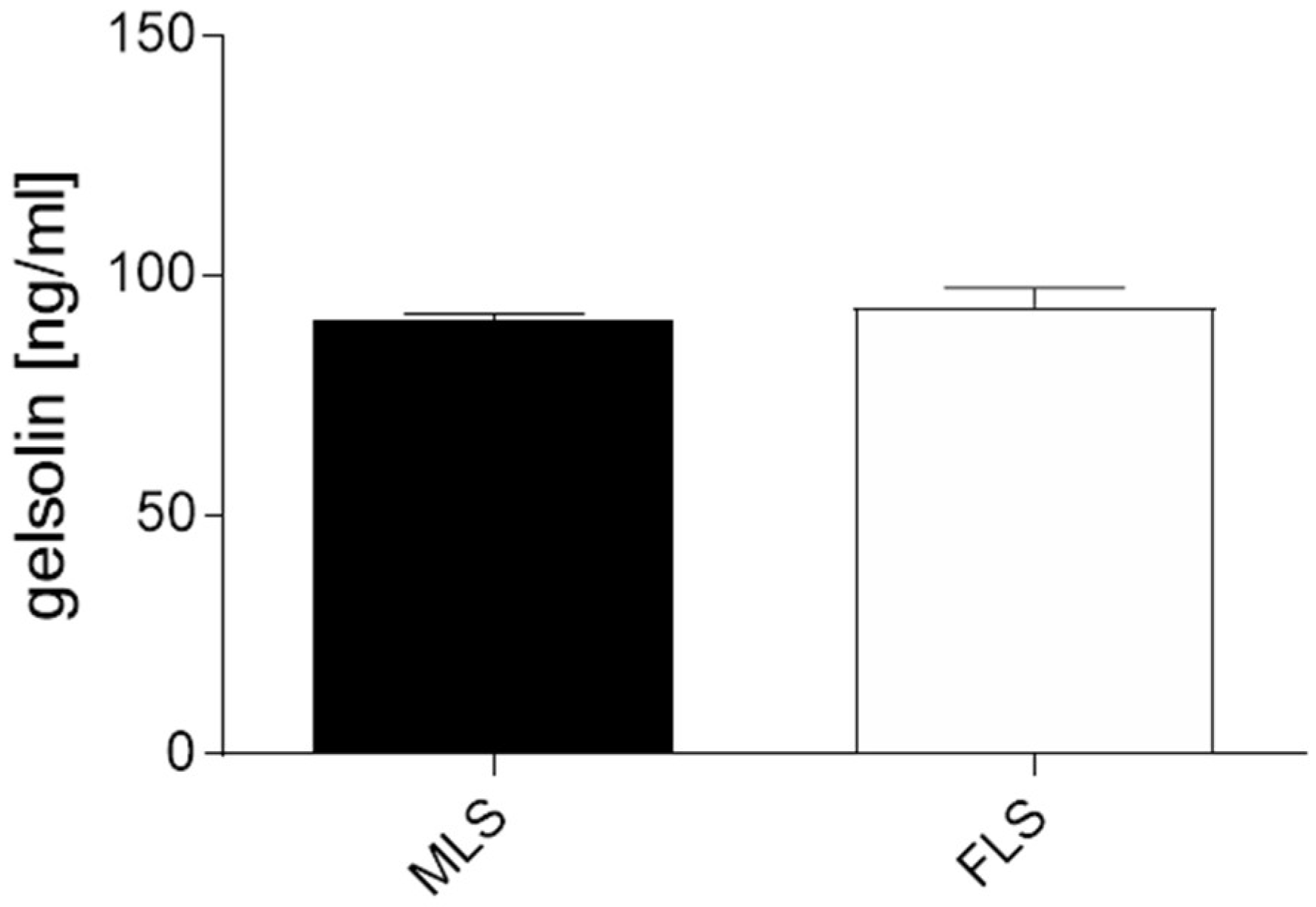
3.2. Both Isolated and Cultured MLS and FLS Produce Gelsolin
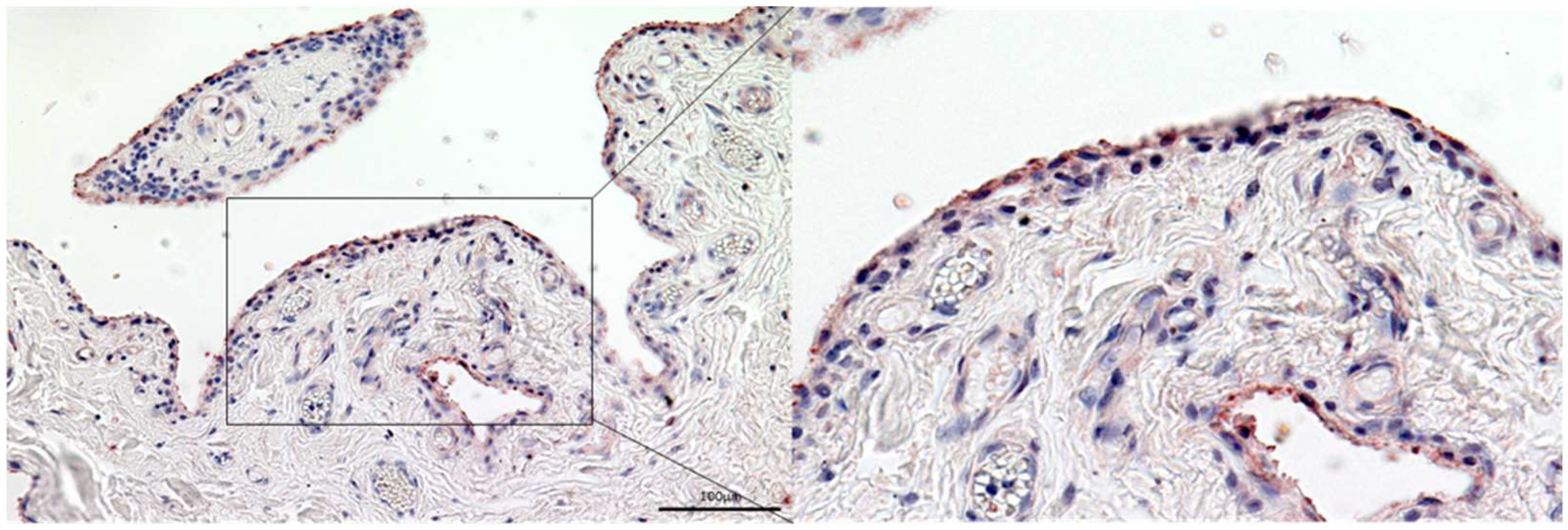
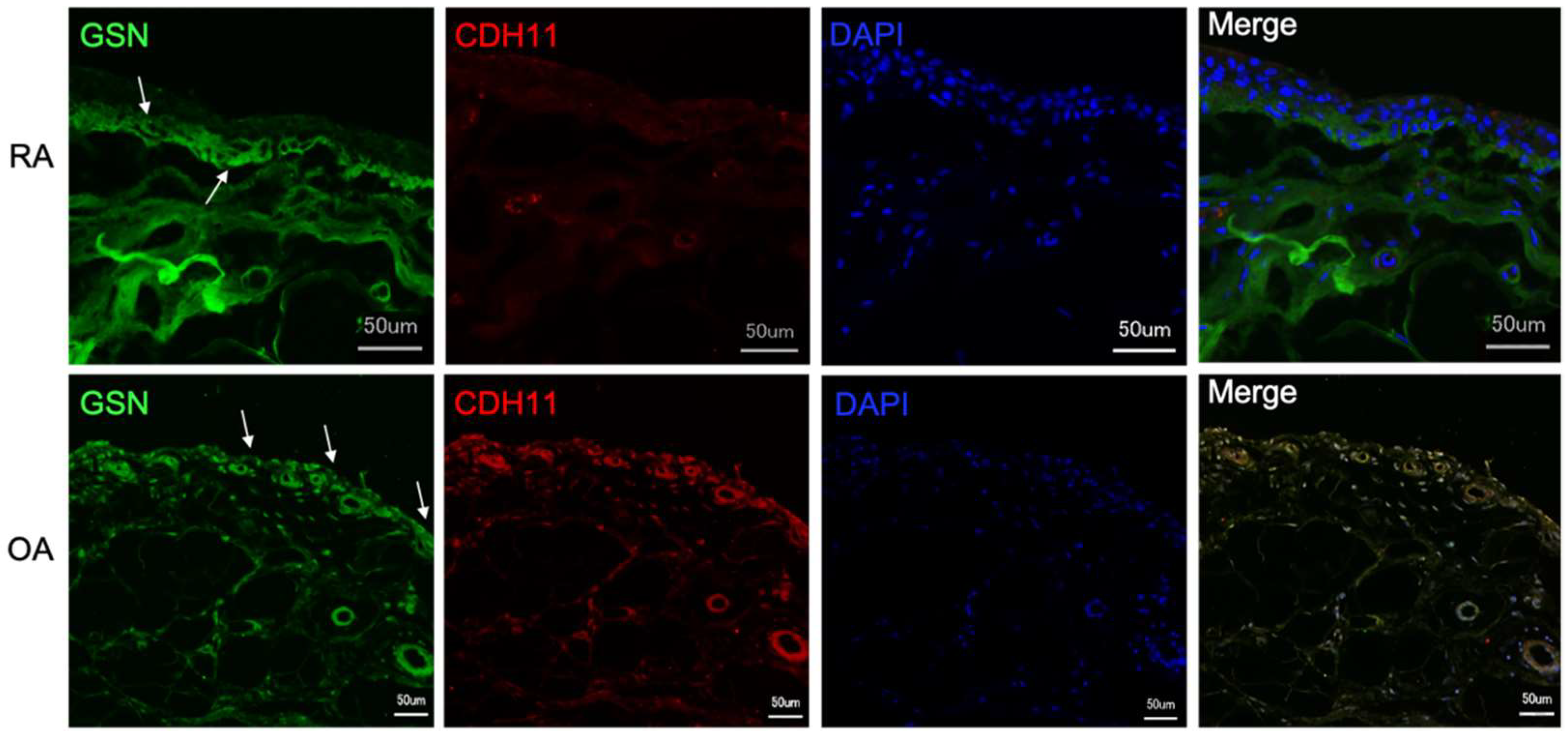
3.3. Gelsolin Is Also Detectable in the Synovial Membrane of Patients with RA and OA
3.4. Gelsolin Is Secreted into Synovia and Is Significantly Reduced in Cases of OA
3.5. Patients with Different Subtypes of Juvenile Arthritis Have Higher GSN Concentrations in the Synovial Fluid Than Older Patients or Healthy Adults
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FitzGerald, O.; Bresnihan, B. Synovial membrane cellularity and vascularity. Ann. Rheum. Dis. 1995, 54, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culemann, S.; Grüneboom, A.; Nicolás-Ávila, J.Á.; Weidner, D.; Lämmle, K.F.; Rothe, T.; Quintana, J.A.; Kirchner, P.; Krljanac, B.; Eberhardt, M.; et al. Locally renewing resident synovial macrohages provide a protective barrier for the joint. Nature 2019, 572, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Hong, W.; Zhang, P.; Wang, X.; Körner, H.; Wei, W. Ontology and function of fibroblast-like and macrophage-like synoviocytes: How do they talk to each other and can they be targeted for rheumatoid arthritis therapy? Front. Immunol. 2018, 9, 1467. [Google Scholar] [CrossRef] [PubMed]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2009, 233, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Langhe, R.P.; Gudzenko, T.; Bachmann, M.; Becker, S.F.; Gonnermann, C.; Winter, C.; Abbruzzese, G.; Alfandari, D.; Kratzer, M.-C.; Franz, C.M.; et al. Cadherin-11 localizes to focal adhesions and promotes cell–substrate adhesion. Nat. Commun. 2016, 7, 10909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miossec, P.; Sany, J. Cartilage and synovial membrane. Rev. Rhum. Mal. Osteo-Articul. 1989, 56, 605–608. [Google Scholar]
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Ngian, G.S. Rheumatoid arthritis. Aust. Fam. Physician 2010, 39, 626–628. [Google Scholar] [PubMed]
- Tutuncu, Z.; Kavanaugh, A. Rheumatic disease in the elderly: Rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2007, 33, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Zhang, H.; Huang, Z.; Zhang, N.; Xing, R.; Wang, P. Agnuside Alleviates Synovitis and Fibrosis in Knee Osteoarthritis through the Inhibition of HIF-1α and NLRP3 Inflammasome. Mediat. Inflamm. 2021, 2021, 5534614. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, P.M. The changing role of TGFbeta in healthy, ageing and osteoarthritic joints. Nat. Rev. Rheumatol. 2017, 13, 155–163. [Google Scholar] [CrossRef]
- Song, C.; Xu, X.; Wu, Y.; Ji, B.; Zhou, X.; Qin, L. Study of the mechanism underlying hsa-miR338-3p downregulation to promote fibrosis of the synovial tissue in osteoarthritis patients. Mol. Biol. Rep. 2019, 46, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Remst, D.F.G.; Davidson, E.N.B.; Van Der Kraan, P.M. Unravelling osteoarthritis-related synovial fibrosis: A step closer to solving joint stiffness. Rheumatology 2015, 54, 1954–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Reyes, A.; Medina-Luna, D.; Santamaría-Olmedo, M.; Martínez-Flores, K.; Zamudio-Cuevas, Y.; Fernández-Torres, J.; Martínez-Nava, G.A.; Olivos-Meza, A.; Camacho-Rea, C.; Fernández-Moreno, M.; et al. Soluble inflammatory mediators of synoviocytes stimulated by monosodium urate crystals induce the production of oxidative stress, pain, and inflammation mediators in chondrocytes. Clin. Rheumatol. 2021, 40, 3265–3271. [Google Scholar] [CrossRef]
- Sacitharan, P.K. Ageing and Osteoarthritis. Subcell. Biochem. 2019, 91, 123–159. [Google Scholar] [PubMed]
- Petty, R.E.; Southwood, T.R.; Baum, J.; Bhettay, E.; Glass, D.N.; Manners, P.; Maldonado-Cocco, J.; Suarez-Almazor, M.; Orozco-Alcala, J.; Prieur, A.M. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J. Rheumatol. 1998, 25, 1991–1994. [Google Scholar]
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.M.; et al. International League of Associations for, R., International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar]
- Martini, A.; Ravelli, A.; Avcin, T.; Beresford, M.W.; Burgos-Vargas, R.; Cuttica, R.; Ilowite, N.T.; Khubchandani, R.; Laxer, R.M.; Lovell, D.J.; et al. Toward New Classification Criteria for Juvenile Idiopathic Arthritis: First Steps, Pediatric Rheumatology International Trials Organization International Consensus. J. Rheumatol. 2018, 46, 190–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, E.; Barrett, J.H.; Donn, R.P.; Thomson, W.; Southwood, T.R.; The British Paediatric Rheumatology Group. Subtyping of juvenile idiopathic arthritis using latent class analysis. Arthritis Care Res. 2000, 43, 1496–1503. [Google Scholar] [CrossRef]
- Finnegan, S.; Clarke, S.; Gibson, D.; McAllister, C.; Rooney, M. Synovial membrane immunohistology in early untreated juvenile idiopathic arthritis: Differences between clinical subgroups. Ann. Rheum. Dis. 2011, 70, 1842–1850. [Google Scholar] [CrossRef] [Green Version]
- Giancane, G.; Alongi, A.; Ravelli, A. Update on the pathogenesis and treatment of juvenile idiopathic arthritis. Curr. Opin. Rheumatol. 2017, 29, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Niehues, T.; Feyen, O.; Telieps, T. Concepts on the pathogenesis of juvenile idiopathic arthritis. Z. Rheumatol. 2008, 67, 111–116, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Budd, R.C.; McInnes, I.B.; O’Dell, J.R.; Gabriel, S.E.; Firestein, G.S.; Kelley, W.N. Kelley’s Textbook of Rheumatology; WB Saunders Company: Philadelphia, PA, USA, 2013; Volume 2. [Google Scholar]
- Guillaume, S.; Prieur, A.-M.; Coste, J.; Job-Deslandre, C. Long-term outcome and prognosis in oligoarticular-onset juvenile idiopathic arthritis. Arthritis Care Res. 2000, 43, 1858–1865. [Google Scholar] [CrossRef] [Green Version]
- Glerup, M.; Rypdal, V.; Arnstad, E.D.; Ekelund, M.; Peltoniemi, S.; Aalto, K.; Rygg, M.; Toftedal, P.; Nielsen, S.; Fasth, A.; et al. Long-Term Outcomes in Juvenile Idiopathic Arthritis: Eighteen Years of Follow-Up in the Population-Based Nordic Juvenile Idiopathic Arthritis Cohort. Arthritis Care Res. 2020, 72, 507–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minden, K.; Niewerth, M.; Listing, J.; Biedermann, T.; Bollow, M.; Schöntube, M.; Zink, A. Long-term outcome in patients with juvenile idiopathic arthritis. Arthritis Care Res. 2002, 46, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, A. Toward an understanding of the long-term outcome of juvenile idiopathic arthritis. Clin. Exp. Rheumatol. 2004, 22, 271–275. [Google Scholar] [PubMed]
- Mitra, S.; Samui, P.P.; Samanta, M.; Mondal, R.K.; Hazra, A.; Mandal, K.; Sabui, T.K. Ultrasound detected changes in joint cartilage thickness in juvenile idiopathic arthritis. Int. J. Rheum. Dis. 2019, 22, 1263–1270. [Google Scholar] [CrossRef]
- Choida, V.; Hall-Craggs, M.; Jebson, B.R.; Fisher, C.; Leandro, M.; Wedderburn, L.R.; Ciurtin, C. Biomarkers of Response to Biologic Therapy in Juvenile Idiopathic Arthritis. Front. Pharmacol. 2021, 11, 635823. [Google Scholar] [CrossRef] [PubMed]
- Nees, T.A.; Schiltenwolf, M. Pharmacological treatment of osteoarthritis-related pain. Schmerz 2019, 33, 30–48. [Google Scholar] [CrossRef]
- Christensen, R.; Bartels, E.M.; Astrup, A.; Bliddal, H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2006, 66, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, A.; Green, R.; Semciw, A.; Kingsley, M.; Pizzari, T. Efficacy of rehabilitation programs for improving muscle strength in people with hip or knee osteoarthritis: A systematic review with meta-analysis. Osteoarthr. Cartil. 2014, 22, 1752–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, G.-I. Perspective on Intra-articular Injection Cell Therapy for Osteoarthritis Treatment. Tissue Eng. Regen. Med. 2019, 16, 357–363. [Google Scholar] [CrossRef] [PubMed]
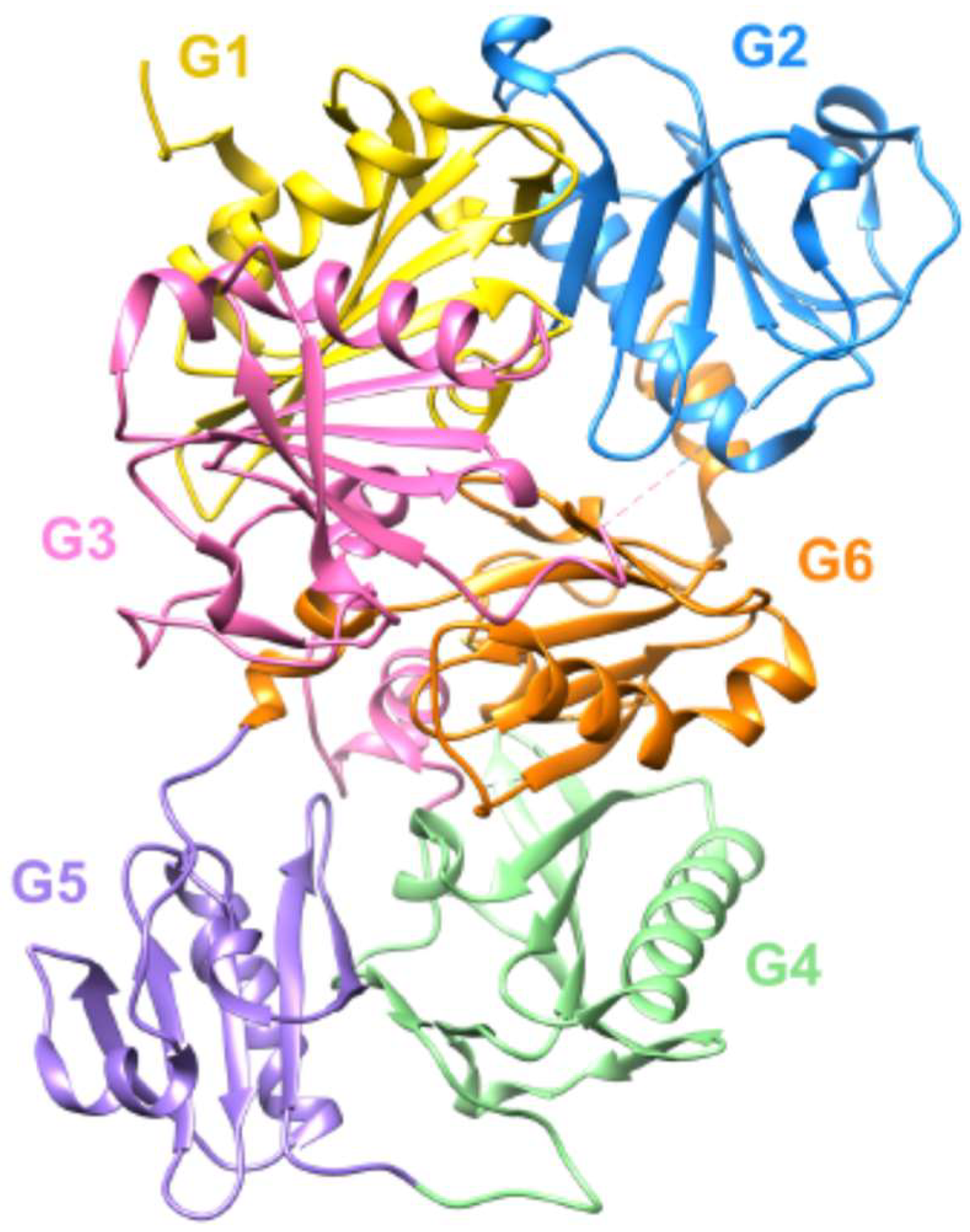
- Feldt, J.; Schicht, M.; Garreis, F.; Welss, J.; Schneider, U.W.; Paulsen, F. Structure, regulation and related diseases of the actin-binding protein gelsolin. Expert Rev. Mol. Med. 2018, 20, e7. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, J.; Dieckow, J.; Schröder, H.; Hampel, U.; Garreis, F.; Jacobi, C.; Milczarek, A.; Hsieh, K.L.; Pulli, B.; Chen, J.W.; et al. Plasma gelsolin promotes re-epithelialization. Sci. Rep. 2018, 8, 13140. [Google Scholar] [CrossRef] [PubMed]
- Feldt, J.; Welss, J.; Schropp, V.; Gelse, K.; Tsokos, M.; Paulsen, F. Recombinant human gelsolin promotes the migration of human articular cartilage chondrocytes by regulating gene expression in vitro. Osteoarthr. Cartil. Open 2020, 2, 100124. [Google Scholar] [CrossRef]
- Nag, S.; Ma, Q.; Wang, H.; Chumnarnsilpa, S.; Lee, W.L.; Larsson, M.; Kannan, B.; Hernandez-Valladares, M.; Burtnick, L.D.; Robinson, R.C. Ca2+ binding by domain 2 plays a critical role in the activation and stabilization of gelsolin. Proc. Natl. Acad. Sci. USA 2009, 106, 13713–13718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Mun, S.; Kim, D.; Lee, Y.; Sheen, D.; Ihm, C.; Lee, S.H.; Kang, H. Proteomics Analysis for Verification of Rheumatoid Arthritis Biomarker Candidates Using Multiple Reaction Monitoring. Proteom.–Clin. Appl. 2019, 13, e1800011. [Google Scholar] [CrossRef]
- Osborn, T.M.; Verdrengh, M.; Stossel, T.P.; Tarkowski, A.; Bokarewa, M. Decreased levels of the gelsolin plasma isoform in patients with rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneva, M.K.; Greco, K.V.; Headland, S.E.; Montero-Melendez, T.; Mori, P.; Greenslade, K.; Pitzalis, C.; Moore, A.; Perretti, M. Identification of Novel Chondroprotective Mediators in Resolving Inflammatory Exudates. J. Immunol. 2017, 198, 2876–2885. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Sharma, S.; Saroha, A.; Bhakuni, D.S.; Malhotra, R.; Zahur, M.; Oellerich, M.; Das, H.R.; Asif, A.R. Identification of Novel Autoantigen in the Synovial Fluid of Rheumatoid Arthritis Patients Using an Immunoproteomics Approach. PLoS ONE 2013, 8, e56246. [Google Scholar] [CrossRef] [Green Version]
- Arai, M.; Kwiatkowski, D.J. Differential developmentally regulated expression of gelsolin family members in the mouse. Dev. Dyn. 1999, 215, 297–307. [Google Scholar] [CrossRef]
- Chan, B.; Parreno, J.; Glogauer, M.; Wang, Y.; Kandel, R. Adseverin, an actin binding protein, regulates articular chondrocyte phenotype. J. Tissue Eng. Regen. Med. 2019, 13, 1438–1452. [Google Scholar] [CrossRef] [PubMed]
- Sohn, D.H.; Sokolove, J.; Sharpe, O.; Erhart, J.C.; Chandra, P.E.; Lahey, L.J.; Lindstrom, T.M.; Hwang, I.; Boyer, K.A.; Andriacchi, T.P.; et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 2012, 14, R7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateos, J.; Lourido, L.; Fernández-Puente, P.; Calamia, V.; Fernández-López, C.; Oreiro, N.; Ruiz-Romero, C.; Blanco, F.J. Differential protein profiling of synovial fluid from rheumatoid arthritis and osteoarthritis patients using LC-MALDI TOF/TOF. J. Proteom. 2012, 75, 2869–2878. [Google Scholar] [CrossRef]
- Ebata, T.; Terkawi, M.A.; Hamasaki, M.; Matsumae, G.; Onodera, T.; Aly, M.K.; Yokota, S.; Alhasan, H.; Shimizu, T.; Takahashi, D.; et al. Flightless I is a catabolic factor of chondrocytes that promotes hypertrophy and cartilage degeneration in osteoarthritis. iScience 2021, 24, 102643. [Google Scholar] [CrossRef] [PubMed]
- Nurminsky, D.; Magee, C.; Faverman, L.; Nurminskaya, M. Regulation of chondrocyte differentiation by actin-severing protein adseverin. Dev. Biol. 2007, 302, 427–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasilopoulos, Y.; Gkretsi, V.; Armaka, M.; Aidinis, V.; Kollias, G. Actin cytoskeleton dynamics linked to synovial fibroblast activation as a novel pathogenic principle in TNF-driven arthritis. Ann. Rheum. Dis. 2007, 66 (Suppl. 3), iii23–iii28. [Google Scholar] [CrossRef]
- Heidings, J.B.; Demosthene, B.; Merlino, T.R.; Castaneda, N.; Kang, E.H. Gelsolin-mediated actin filament severing in crowded environments. Biochem. Biophys. Res. Commun. 2020, 532, 548–554. [Google Scholar] [CrossRef]
- Falconer, J.; Murphy, A.N.; Young, S.P.; Clark, A.R.; Tiziani, S.; Guma, M.; Buckley, C.D. Review: Synovial Cell Metabolism and Chronic Inflammation in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 984–999. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Arora, P.D.; Chen, Y.; McCulloch, C.A.; Liu, P. Multifunctional roles of gelsolin in health and diseases. Med. Res. Rev. 2012, 32, 999–1025. [Google Scholar] [CrossRef]


| Abbreviation | Subtyp Juvenile Arthritis (JIA) | Number of Patients |
|---|---|---|
| JIA-OA | Oligoarticular (oligoarthritis) | 7 |
| eOA | Extended oligoarthritis | 2 |
| EAA | Enthesitis associated | 1 |
| PsA | Psoriatic arthritis | 2 |
| AMA | Acute, yet unclassified monarthritis | 3 |
| PolyRF- | polyarticular, RF negative | 2 |
| Healthy (n = 6) | RA (n = 7) | OA (n = 8) | |
|---|---|---|---|
| Ø ng/mL ± SEM | 7.89 ± 1.15 | 7.11 ± 0.93 | 4.49 ± 0.22 |
| Individual ng/mL | 7.57 | 5.35 | 4.32 |
| 11.79 | 8.58 | 4.32 | |
| 8.02 | 6.52 | 5.41 | |
| 7.23 | 12.12 | 3.73 | |
| 4.26 | 5.70 | 4.01 | |
| 5.68 | 5.44 | 4.41 | |
| 6.07 | 5.50 | ||
| 4.27 |
| JIA Subtype | OA | eOA | EAA | PsA | AMA | PolyRF- |
|---|---|---|---|---|---|---|
| Ø ng/ml | 10.3 | 7.9 | 10.7 | 7.1 | 8.1 | 6.7 |
| Individual ng/mL | 10.7 | 9.1 | 10.7 | 8.2 | 8.4 | 6.8 |
| 9.3 | 6.8 | 6.0 | 7.1 | 6.6 | ||
| 12.9 | 8.8 | |||||
| 5.6 | ||||||
| 10.0 | ||||||
| 13.3 |
| Patients | GSN < 10 ng/mL | GSN ≥ 10 ng/mL | Total | Frequency |
|---|---|---|---|---|
| With Oligoarthritis | 2 | 4 | 6 | 28.6% |
| Without Oligoarthritis | 10 | 1 | 11 | 90.9% |
| Total | 12 | 5 | 17 | 70.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feldt, J.; Schicht, M.; Welss, J.; Gelse, K.; Sesselmann, S.; Tsokos, M.; Socher, E.; Garreis, F.; Müller, T.; Paulsen, F. Production and Secretion of Gelsolin by Both Human Macrophage- and Fibroblast-like Synoviocytes and GSN Modulation in the Synovial Fluid of Patients with Various Forms of Arthritis. Biomedicines 2022, 10, 723. https://doi.org/10.3390/biomedicines10030723
Feldt J, Schicht M, Welss J, Gelse K, Sesselmann S, Tsokos M, Socher E, Garreis F, Müller T, Paulsen F. Production and Secretion of Gelsolin by Both Human Macrophage- and Fibroblast-like Synoviocytes and GSN Modulation in the Synovial Fluid of Patients with Various Forms of Arthritis. Biomedicines. 2022; 10(3):723. https://doi.org/10.3390/biomedicines10030723
Chicago/Turabian StyleFeldt, Jessica, Martin Schicht, Jessica Welss, Kolja Gelse, Stefan Sesselmann, Michael Tsokos, Eileen Socher, Fabian Garreis, Thomas Müller, and Friedrich Paulsen. 2022. "Production and Secretion of Gelsolin by Both Human Macrophage- and Fibroblast-like Synoviocytes and GSN Modulation in the Synovial Fluid of Patients with Various Forms of Arthritis" Biomedicines 10, no. 3: 723. https://doi.org/10.3390/biomedicines10030723
APA StyleFeldt, J., Schicht, M., Welss, J., Gelse, K., Sesselmann, S., Tsokos, M., Socher, E., Garreis, F., Müller, T., & Paulsen, F. (2022). Production and Secretion of Gelsolin by Both Human Macrophage- and Fibroblast-like Synoviocytes and GSN Modulation in the Synovial Fluid of Patients with Various Forms of Arthritis. Biomedicines, 10(3), 723. https://doi.org/10.3390/biomedicines10030723







