Adeno-Associated Viral Vectors as Versatile Tools for Neurological Disorders: Focus on Delivery Routes and Therapeutic Perspectives
Abstract
1. Introduction
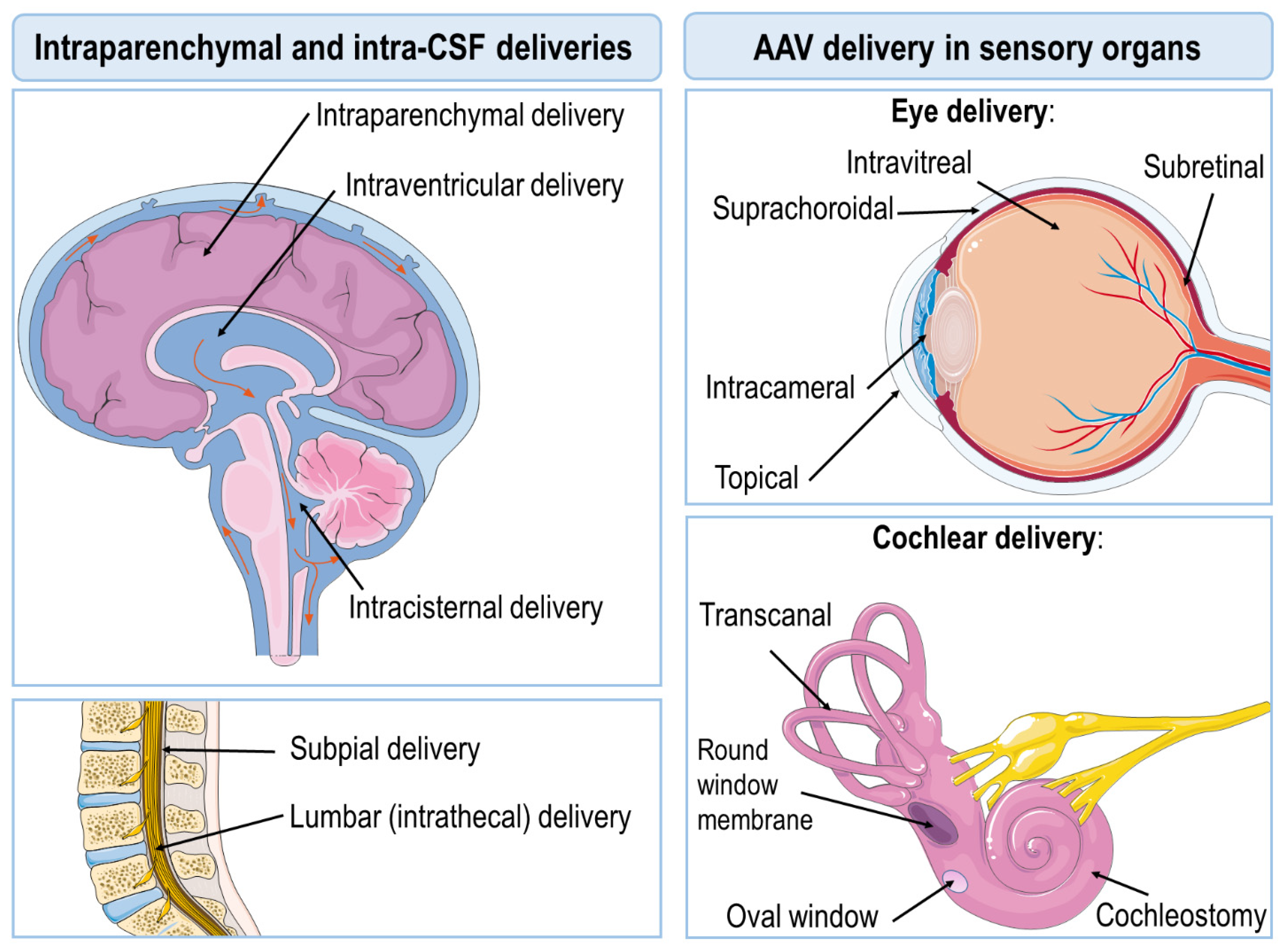
2. AAV Delivery Routes
3. Intraparenchymal Deliveries
4. Intra-CSF Deliveries
5. Intravenous Delivery Routes
6. AAV Delivery in Sensory Organs
7. AAV-Mediated Therapeutic Uses: The Path to the Clinical Scenario
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samulski, R.J.; Muzyczka, N. AAV-Mediated Gene Therapy for Research and Therapeutic Purposes. Ann. Rev. Virol. 2014, 1, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Atchison, R.W.; Casto, B.C.; Hammon, W.M. Adenovirus-associated defective virus particles. Science 1965, 149, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-S.; Agbandje-McKenna, M. Mapping the AAV Capsid Host Antibody Response toward the Development of Second Generation Gene Delivery Vectors. Front. Immunol. 2014, 5, 9. [Google Scholar] [CrossRef]
- Haberman, R.P.; McCown, T.J.; Samulski, R.J. Inducible Long-Term Gene Expression in Brain with Adeno-Associated Virus Gene Transfer. Gene Ther. 1998, 5, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Samulski, R.J.; Berns, K.I.; Tan, M.; Muzyczka, N. Cloning of Adeno-Associated Virus into PBR322: Rescue of Intact Virus from the Recombinant Plasmid in Human Cells. Proc. Natl. Acad. Sci. USA 1982, 79, 2077–2081. [Google Scholar] [CrossRef]
- Kaplitt, M.G.; Leone, P.; Samulski, R.J.; Xiao, X.; Pfaff, D.W.; O’Malley, K.L.; During, M.J. Long-Term Gene Expression and Phenotypic Correction Using Adeno-Associated Virus Vectors in the Mammalian Brain. Nat. Genet. 1994, 8, 148–154. [Google Scholar] [CrossRef]
- Conrad, C.K.; Allen, S.S.; Afione, S.A.; Reynolds, T.C.; Beck, S.E.; Fee-Maki, M.; Barrazza-Ortiz, X.; Adams, R.; Askin, F.B.; Carter, B.J.; et al. Safety of Single-Dose Administration of an Adeno-Associated Virus (AAV)-CFTR Vector in the Primate Lung. Gene Ther. 1996, 3, 658–668. [Google Scholar] [PubMed]
- Haery, L.; Deverman, B.E.; Matho, K.S.; Cetin, A.; Woodard, K.; Cepko, C.; Guerin, K.I.; Rego, M.A.; Ersing, I.; Bachle, S.M.; et al. Adeno-Associated Virus Technologies and Methods for Targeted Neuronal Manipulation. Front. Neuroanat. 2019, 13, 93. [Google Scholar] [CrossRef]
- Pignataro, D.; Sucunza, D.; Vanrell, L.; Lopez-Franco, E.; Dopeso-Reyes, I.G.; Vales, A.; Hommel, M.; Rico, A.J.; Lanciego, J.L.; Gonzalez-Aseguinolaza, G. Adeno-Associated Viral Vectors Serotype 8 for Cell-Specific Delivery of Therapeutic Genes in the Central Nervous System. Front. Neuroanat. 2017, 11, 2. [Google Scholar] [CrossRef]
- Pignataro, D.; Sucunza, D.; Rico, A.J.; Dopeso-Reyes, I.G.; Roda, E.; Rodríguez-Perez, A.I.; Labandeira-Garcia, J.L.; Broccoli, V.; Kato, S.; Kobayashi, K.; et al. Gene Therapy Approaches in the Non-Human Primate Model of Parkinson’s Disease. J. Neural. Transm. 2018, 125, 575–589. [Google Scholar] [CrossRef]
- Fajardo-Serrano, A.; Rico, A.J.; Roda, E.; Honrubia, A.; Arrieta, S.; Ariznabarreta, G.; Chocarro, J.; Lorenzo-Ramos, E.; Pejenaute, A.; Vázquez, A.; et al. Adeno-Associated Viral Vectors as Versatile Tools for Parkinson’s Research, Both for Disease Modeling Purposes and for Therapeutic Uses. Int. J. Mol. Sci. 2021, 22, 6389. [Google Scholar] [CrossRef] [PubMed]
- Elmer, B.M.; Swanson, K.A.; Bangari, D.S.; Piepenhagen, P.A.; Roberts, E.; Taksir, T.; Guo, L.; Obinu, M.-C.; Barneoud, P.; Ryan, S.; et al. Gene Delivery of a Modified Antibody to Aβ Reduces Progression of Murine Alzheimer’s Disease. PLoS ONE 2019, 14, e0226245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gottesdiener, A.J.; Parmar, M.; Li, M.; Kaminsky, S.M.; Chiuchiolo, M.J.; Sondhi, D.; Sullivan, P.M.; Holtzman, D.M.; Crystal, R.G.; et al. Intracerebral Adeno-Associated Virus Gene Delivery of Apolipoprotein E2 Markedly Reduces Brain Amyloid Pathology in Alzheimer’s Disease Mouse Models. Neurobiol. Aging 2016, 44, 159–172. [Google Scholar] [CrossRef]
- Sánchez-Sarasúa, S.; Ribes-Navarro, A.; Beltrán-Bretones, M.T.; Sánchez-Pérez, A.M. AAV Delivery of ShRNA against IRS1 in GABAergic Neurons in Rat Hippocampus Impairs Spatial Memory in Females and Male Rats. Brain Struct. Funct. 2021, 226, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, T.; Yamamoto, M.; Schroder, B.; Jacobsen, M.T.; Swan, R.J.; Lambert, M.P.; Klein, W.L.; Gendelman, H.E.; Ransohoff, R.M.; Ikezu, T. AAV1/2-Mediated CNS Gene Delivery of Dominant-Negative CCL2 Mutant Suppresses Gliosis, Beta-Amyloidosis, and Learning Impairment of APP/PS1 Mice. Mol. Ther. 2009, 17, 803–809. [Google Scholar] [CrossRef]
- Carty, N.C.; Nash, K.; Lee, D.; Mercer, M.; Gottschall, P.E.; Meyers, C.; Muzyczka, N.; Gordon, M.N.; Morgan, D. Adeno-Associated Viral (AAV) Serotype 5 Vector Mediated Gene Delivery of Endothelin-Converting Enzyme Reduces Abeta Deposits in APP + PS1 Transgenic Mice. Mol. Ther. 2008, 16, 1580–1586. [Google Scholar] [CrossRef]
- Wu, K.; Meyer, E.M.; Bennett, J.A.; Meyers, C.A.; Hughes, J.A.; King, M.A. AAV2/5-Mediated NGF Gene Delivery Protects Septal Cholinergic Neurons Following Axotomy. Brain Res. 2005, 1061, 107–113. [Google Scholar] [CrossRef]
- Mandel, R.J. CERE-110, an Adeno-Associated Virus-Based Gene Delivery Vector Expressing Human Nerve Growth Factor for the Treatment of Alzheimer’s Disease. Curr. Opin. Mol. Ther. 2010, 12, 240–247. [Google Scholar]
- Liu, W.; Zhao, L.; Blackman, B.; Parmar, M.; Wong, M.Y.; Woo, T.; Yu, F.; Chiuchiolo, M.J.; Sondhi, D.; Kaminsky, S.M.; et al. Vectored Intracerebral Immunization with the Anti-Tau Monoclonal Antibody PHF1 Markedly Reduces Tau Pathology in Mutant Tau Transgenic Mice. J. Neurosci. 2016, 36, 12425–12435. [Google Scholar] [CrossRef]
- Fukuchi, K.; Tahara, K.; Kim, H.-D.; Maxwell, J.A.; Lewis, T.L.; Accavitti-Loper, M.A.; Kim, H.; Ponnazhagan, S.; Lalonde, R. Anti-Abeta Single-Chain Antibody Delivery via Adeno-Associated Virus for Treatment of Alzheimer’s Disease. Neurobiol. Dis. 2006, 23, 502–511. [Google Scholar] [CrossRef]
- Kiyota, T.; Ingraham, K.L.; Swan, R.J.; Jacobsen, M.T.; Andrews, S.J.; Ikezu, T. AAV Serotype 2/1-Mediated Gene Delivery of Anti-Inflammatory Interleukin-10 Enhances Neurogenesis and Cognitive Function in APP+PS1 Mice. Gene Ther. 2012, 19, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Hudry, E.; Martin, C.; Gandhi, S.; György, B.; Scheffer, D.I.; Mu, D.; Merkel, S.F.; Mingozzi, F.; Fitzpatrick, Z.; Dimant, H.; et al. Exosome-Associated AAV Vector as a Robust and Convenient Neuroscience Tool. Gene Ther. 2016, 23, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Gao, C.-Y.; Yang, M.; Liu, X.-H.; Sun, Y.; Pollard, A.; Dong, X.-Y.; Wu, X.-B.; Zhong, J.-H.; Zhou, H.-D.; et al. Intramuscular Delivery of a Single Chain Antibody Gene Prevents Brain Aβ Deposition and Cognitive Impairment in a Mouse Model of Alzheimer’s Disease. Brain Behav. Immun. 2010, 24, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-H.; Wang, Y.-R.; Zhang, T.; Jiao, S.-S.; Liu, Y.-H.; Zeng, F.; Li, J.; Yao, X.-Q.; Zhou, H.-D.; Zhou, X.-F.; et al. Intramuscular Delivery of P75NTR Ectodomain by an AAV Vector Attenuates Cognitive Deficits and Alzheimer’s Disease-like Pathologies in APP/PS1 Transgenic Mice. J. Neurochem. 2016, 138, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, Y.; Tian, Y.; Wang, Y.; Wu, Z.; Lan, T.; Wang, H.; Cheng, K.; Xie, P. Different Serotypes of Adeno-Associated Virus Vector- and Lentivirus-Mediated Tropism in Choroid Plexus by Intracerebroventricular Delivery. Hum. Gene Ther. 2020, 31, 440–447. [Google Scholar] [CrossRef]
- So, K.-H.; Choi, J.H.; Islam, J.; Kc, E.; Moon, H.C.; Won, S.Y.; Kim, H.K.; Kim, S.; Hyun, S.-H.; Park, Y.S. An Optimization of AAV-82Q-Delivered Rat Model of Huntington’s Disease. J. Korean Neurosurg. Soc. 2020, 63, 579–589. [Google Scholar] [CrossRef]
- Kells, A.P.; Fong, D.M.; Dragunow, M.; During, M.J.; Young, D.; Connor, B. AAV-Mediated Gene Delivery of BDNF or GDNF Is Neuroprotective in a Model of Huntington Disease. Mol. Ther. 2004, 9, 682–688. [Google Scholar] [CrossRef]
- Ekman, F.K.; Ojala, D.S.; Adil, M.M.; Lopez, P.A.; Schaffer, D.V.; Gaj, T. CRISPR-Cas9-Mediated Genome Editing Increases Lifespan and Improves Motor Deficits in a Huntington’s Disease Mouse Model. Mol. Ther. Nucleic Acids 2019, 17, 829–839. [Google Scholar] [CrossRef]
- Cheng, A.; Yang, Y.; Zhou, Y.; Maharana, C.; Lu, D.; Peng, W.; Liu, Y.; Wan, R.; Marosi, K.; Misiak, M.; et al. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016, 23, 128–142. [Google Scholar] [CrossRef]
- Zuleta, A.; Vidal, R.L.; Armentano, D.; Parsons, G.; Hetz, C. AAV-Mediated Delivery of the Transcription Factor XBP1s into the Striatum Reduces Mutant Huntingtin Aggregation in a Mouse Model of Huntington’s Disease. Biochem. Biophys. Res. Commun. 2012, 420, 558–563. [Google Scholar] [CrossRef]
- Keeler, A.M.; Sapp, E.; Chase, K.; Sottosanti, E.; Danielson, E.; Pfister, E.; Stoica, L.; DiFiglia, M.; Aronin, N.; Sena-Esteves, M. Cellular Analysis of Silencing the Huntington’s Disease Gene Using AAV9 Mediated Delivery of Artificial Micro RNA into the Striatum of Q140/Q140 Mice. J. Huntingt. Dis. 2016, 5, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Franich, N.R.; Fitzsimons, H.L.; Fong, D.M.; Klugmann, M.; During, M.J.; Young, D. AAV Vector-Mediated RNAi of Mutant Huntingtin Expression Is Neuroprotective in a Novel Genetic Rat Model of Huntington’s Disease. Mol. Ther. 2008, 16, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, I.; Fiengo, P.; Remelli, R.; Miragliotta, V.; Rossini, L.; Biotti, I.; Cappelli, A.; Petricca, L.; La Rosa, S.; Caricasole, A.; et al. Recombinant Adeno Associated Viral (AAV) Vector Type 9 Delivery of Ex1-Q138-Mutant Huntingtin in the Rat Striatum as a Short-Time Model for in Vivo Studies in Drug Discovery. Neurobiol. Dis. 2016, 86, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Agustín-Pavón, C.; Mielcarek, M.; Garriga-Canut, M.; Isalan, M. Deimmunization for Gene Therapy: Host Matching of Synthetic Zinc Finger Constructs Enables Long-Term Mutant Huntingtin Repression in Mice. Mol. Neurodegener. 2016, 11, 64. [Google Scholar] [CrossRef]
- Lubansu, A.; Abeloos, L.; Bockstael, O.; Lehtonen, E.; Blum, D.; Brotchi, J.; Levivier, M.; Tenenbaum, L. Recombinant AAV Viral Vectors Serotype 1, 2, and 5 Mediate Differential Gene Transfer Efficiency in Rat Striatal Fetal Grafts. Cell Transplant. 2007, 16, 1013–1020. [Google Scholar] [CrossRef]
- Ramaswamy, S.; McBride, J.L.; Han, I.; Berry-Kravis, E.M.; Zhou, L.; Herzog, C.D.; Gasmi, M.; Bartus, R.T.; Kordower, J.H. Intrastriatal CERE-120 (AAV-Neurturin) Protects Striatal and Cortical Neurons and Delays Motor Deficits in a Transgenic Mouse Model of Huntington’s Disease. Neurobiol. Dis. 2009, 34, 40–50. [Google Scholar] [CrossRef]
- Monteys, A.M.; Spengler, R.M.; Dufour, B.D.; Wilson, M.S.; Oakley, C.K.; Sowada, M.J.; McBride, J.L.; Davidson, B.L. Single Nucleotide Seed Modification Restores in Vivo Tolerability of a Toxic Artificial MiRNA Sequence in the Mouse Brain. Nucleic Acids Res. 2014, 42, 13315–13327. [Google Scholar] [CrossRef]
- Birolini, G.; Verlengia, G.; Talpo, F.; Maniezzi, C.; Zentilin, L.; Giacca, M.; Conforti, P.; Cordiglieri, C.; Caccia, C.; Leoni, V.; et al. SREBP2 Gene Therapy Targeting Striatal Astrocytes Ameliorates Huntington’s Disease Phenotypes. Brain 2021, 144, 3175–3190. [Google Scholar] [CrossRef]
- van der Bom, I.M.J.; Moser, R.P.; Gao, G.; Mondo, E.; O’Connell, D.; Gounis, M.J.; McGowan, S.; Chaurette, J.; Bishop, N.; Sena-Esteves, M.S.; et al. Finding the Striatum in Sheep: Use of a Multi-Modal Guided Approach for Convection Enhanced Delivery. J. Huntingt. Dis. 2013, 2, 41–45. [Google Scholar] [CrossRef]
- Dufour, B.D.; Smith, C.A.; Clark, R.L.; Walker, T.R.; McBride, J.L. Intrajugular Vein Delivery of AAV9-RNAi Prevents Neuropathological Changes and Weight Loss in Huntington’s Disease Mice. Mol. Ther. 2014, 22, 797–810. [Google Scholar] [CrossRef]
- Li, C.; Xiao, P.; Gray, S.J.; Weinberg, M.S.; Samulski, R.J. Combination Therapy Utilizing ShRNA Knockdown and an Optimized Resistant Transgene for Rescue of Diseases Caused by Misfolded Proteins. Proc. Natl. Acad. Sci. USA 2011, 108, 14258–14263. [Google Scholar] [CrossRef] [PubMed]
- Pfister, E.L.; DiNardo, N.; Mondo, E.; Borel, F.; Conroy, F.; Fraser, C.; Gernoux, G.; Han, X.; Hu, D.; Johnson, E.; et al. Artificial MiRNAs Reduce Human Mutant Huntingtin Throughout the Striatum in a Transgenic Sheep Model of Huntington’s Disease. Hum. Gene Ther. 2018, 29, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Jara, J.H.; Stanford, M.J.; Zhu, Y.; Tu, M.; Hauswirth, W.W.; Bohn, M.C.; DeVries, S.H.; Özdinler, P.H. Healthy and Diseased Corticospinal Motor Neurons Are Selectively Transduced upon Direct AAV2-2 Injection into the Motor Cortex. Gene Ther. 2016, 23, 272–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biferi, M.G.; Cohen-Tannoudji, M.; Cappelletto, A.; Giroux, B.; Roda, M.; Astord, S.; Marais, T.; Bos, C.; Voit, T.; Ferry, A.; et al. A New AAV10-U7-Mediated Gene Therapy Prolongs Survival and Restores Function in an ALS Mouse Model. Mol. Ther. 2017, 25, 2038–2052. [Google Scholar] [CrossRef]
- Wang, W.; Wen, D.; Duan, W.; Yin, J.; Cui, C.; Wang, Y.; Li, Z.; Liu, Y.; Li, C. Systemic Administration of ScAAV9-IGF1 Extends Survival in SOD1G93A ALS Mice via Inhibiting P38 MAPK and the JNK-Mediated Apoptosis Pathway. Brain Res. Bull. 2018, 139, 203–210. [Google Scholar] [CrossRef]
- Thomsen, G.M.; Alkaslasi, M.; Vit, J.-P.; Lawless, G.; Godoy, M.; Gowing, G.; Shelest, O.; Svendsen, C.N. Systemic Injection of AAV9-GDNF Provides Modest Functional Improvements in the SOD1G93A ALS Rat but Has Adverse Side Effects. Gene Ther. 2017, 24, 245–252. [Google Scholar] [CrossRef]
- Eykens, C.; Rossaert, E.; Duqué, S.; Rué, L.; Bento-Abreu, A.; Hersmus, N.; Lenaerts, A.; Kerstens, A.; Corthout, N.; Munck, S.; et al. AAV9-Mediated Gene Delivery of MCT1 to Oligodendrocytes Does Not Provide a Therapeutic Benefit in a Mouse Model of ALS. Mol. Ther. Methods Clin. Dev. 2021, 20, 508–519. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, N.; Kim, S.; Lee, J.; Choi, W.; Yu, S.S.; Kim, J.H.; Kim, S. Intramuscular Delivery of HGF-Expressing Recombinant AAV Improves Muscle Integrity and Alleviates Neurological Symptoms in the Nerve Crush and SOD1-G93A Transgenic Mouse Models. Biochem. Biophys. Res. Commun. 2019, 517, 452–457. [Google Scholar] [CrossRef]
- Lin, H.; Hu, H.; Duan, W.; Liu, Y.; Tan, G.; Li, Z.; Liu, Y.; Deng, B.; Song, X.; Wang, W.; et al. Intramuscular Delivery of ScAAV9-HIGF1 Prolongs Survival in the HSOD1G93A ALS Mouse Model via Upregulation of D-Amino Acid Oxidase. Mol. Neurobiol. 2018, 55, 682–695. [Google Scholar] [CrossRef]
- Lu, Y.-Y.; Wang, L.-J.; Muramatsu, S.; Ikeguchi, K.; Fujimoto, K.; Okada, T.; Mizukami, H.; Matsushita, T.; Hanazono, Y.; Kume, A.; et al. Intramuscular Injection of AAV-GDNF Results in Sustained Expression of Transgenic GDNF, and Its Delivery to Spinal Motoneurons by Retrograde Transport. Neurosci. Res. 2003, 45, 33–40. [Google Scholar] [CrossRef]
- Wang, L.-J.; Lu, Y.-Y.; Muramatsu, S.; Ikeguchi, K.; Fujimoto, K.; Okada, T.; Mizukami, H.; Matsushita, T.; Hanazono, Y.; Kume, A.; et al. Neuroprotective Effects of Glial Cell Line-Derived Neurotrophic Factor Mediated by an Adeno-Associated Virus Vector in a Transgenic Animal Model of Amyotrophic Lateral Sclerosis. J. Neurosci. 2002, 22, 6920–6928. [Google Scholar] [CrossRef] [PubMed]
- Ayers, J.I.; Fromholt, S.; Sinyavskaya, O.; Siemienski, Z.; Rosario, A.M.; Li, A.; Crosby, K.W.; Cruz, P.E.; DiNunno, N.M.; Janus, C.; et al. Widespread and Efficient Transduction of Spinal Cord and Brain Following Neonatal AAV Injection and Potential Disease Modifying Effect in ALS Mice. Mol. Ther. 2015, 23, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Towne, C.; Setola, V.; Schneider, B.L.; Aebischer, P. Neuroprotection by Gene Therapy Targeting Mutant SOD1 in Individual Pools of Motor Neurons Does Not Translate into Therapeutic Benefit in FALS Mice. Mol. Ther. 2011, 19, 274–283. [Google Scholar] [CrossRef]
- Kaspar, B.K.; Lladó, J.; Sherkat, N.; Rothstein, J.D.; Gage, F.H. Retrograde Viral Delivery of IGF-1 Prolongs Survival in a Mouse ALS Model. Science 2003, 301, 839–842. [Google Scholar] [CrossRef]
- Wen, D.; Cui, C.; Duan, W.; Wang, W.; Wang, Y.; Liu, Y.; Li, Z.; Li, C. The Role of Insulin-like Growth Factor 1 in ALS Cell and Mouse Models: A Mitochondrial Protector. Brain Res. Bull. 2019, 144, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Hala, T.J.; Seetharam, S.; Poulsen, D.J.; Wright, M.C.; Lepore, A.C. GLT1 Overexpression in SOD1(G93A) Mouse Cervical Spinal Cord Does Not Preserve Diaphragm Function or Extend Disease. Neurobiol. Dis. 2015, 78, 12–23. [Google Scholar] [CrossRef]
- Lim, C.K.W.; Gapinske, M.; Brooks, A.K.; Woods, W.S.; Powell, J.E.; Zeballos, C.; Winter, J.; Perez-Pinera, P.; Gaj, T. Treatment of a Mouse Model of ALS by In Vivo Base Editing. Mol. Ther. 2020, 28, 1177–1189. [Google Scholar] [CrossRef]
- Herranz-Martin, S.; Chandran, J.; Lewis, K.; Mulcahy, P.; Higginbottom, A.; Walker, C.; Valenzuela, I.M.-P.Y.; Jones, R.A.; Coldicott, I.; Iannitti, T.; et al. Viral Delivery of C9orf72 Hexanucleotide Repeat Expansions in Mice Leads to Repeat-Length-Dependent Neuropathology and Behavioural Deficits. Dis. Model. Mech. 2017, 10, 859–868. [Google Scholar] [CrossRef]
- Rashnonejad, A.; Amini Chermahini, G.; Gündüz, C.; Onay, H.; Aykut, A.; Durmaz, B.; Baka, M.; Su, Q.; Gao, G.; Özkınay, F. Fetal Gene Therapy Using a Single Injection of Recombinant AAV9 Rescued SMA Phenotype in Mice. Mol. Ther. 2019, 27, 2123–2133. [Google Scholar] [CrossRef]
- Besse, A.; Astord, S.; Marais, T.; Roda, M.; Giroux, B.; Lejeune, F.-X.; Relaix, F.; Smeriglio, P.; Barkats, M.; Biferi, M.G. AAV9-Mediated Expression of SMN Restricted to Neurons Does Not Rescue the Spinal Muscular Atrophy Phenotype in Mice. Mol. Ther. 2020, 28, 1887–1901. [Google Scholar] [CrossRef]
- Passini, M.A.; Bu, J.; Richards, A.M.; Treleaven, C.M.; Sullivan, J.A.; O’Riordan, C.R.; Scaria, A.; Kells, A.P.; Samaranch, L.; San Sebastian, W.; et al. Translational Fidelity of Intrathecal Delivery of Self-Complementary AAV9–Survival Motor Neuron 1 for Spinal Muscular Atrophy. Hum. Gene Ther. 2014, 25, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, N.; Lattanzi, A.; Jeavons, M.; Van Wittenberghe, L.; Gjata, B.; Marais, T.; Martin, S.; Vignaud, A.; Voit, T.; Mavilio, F.; et al. Efficacy and Biodistribution Analysis of Intracerebroventricular Administration of an Optimized ScAAV9-SMN1 Vector in a Mouse Model of Spinal Muscular Atrophy. Mol. Ther. Methods Clin. Dev. 2016, 3, 16060. [Google Scholar] [CrossRef] [PubMed]
- Kaifer, K.A.; Villalón, E.; Smith, C.E.; Simon, M.E.; Marquez, J.; Hopkins, A.E.; Morcos, T.I.; Lorson, C.L. AAV9-DOK7 Gene Therapy Reduces Disease Severity in Smn2B/- SMA Model Mice. Biochem. Biophys. Res. Commun. 2020, 530, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, C.; Katz, N.; Buza, E.L.; Dyer, C.; Goode, T.; Bell, P.; Richman, L.K.; Wilson, J.M. Severe Toxicity in Nonhuman Primates and Piglets Following High-Dose Intravenous Administration of an Adeno-Associated Virus Vector Expressing Human SMN. Hum. Gene Ther. 2018, 29, 285–298. [Google Scholar] [CrossRef]
- Benkhelifa-Ziyyat, S.; Besse, A.; Roda, M.; Duque, S.; Astord, S.; Carcenac, R.; Marais, T.; Barkats, M. Intramuscular ScAAV9-SMN Injection Mediates Widespread Gene Delivery to the Spinal Cord and Decreases Disease Severity in SMA Mice. Mol. Ther. 2013, 21, 282–290. [Google Scholar] [CrossRef]
- Donadon, I.; Bussani, E.; Riccardi, F.; Licastro, D.; Romano, G.; Pianigiani, G.; Pinotti, M.; Konstantinova, P.; Evers, M.; Lin, S.; et al. Rescue of Spinal Muscular Atrophy Mouse Models with AAV9-Exon-Specific U1 SnRNA. Nucleic Acids Res. 2019, 47, 7618–7632. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, D.; Qiao, T.; Yang, C.; Su, Q.; Gao, G.; Xu, Z. A Single Injection of Recombinant Adeno-Associated Virus into the Lumbar Cistern Delivers Transgene Expression Throughout the Whole Spinal Cord. Mol. Neurobiol. 2016, 53, 3235–3248. [Google Scholar] [CrossRef]
- Song, L.; Llanga, T.; Conatser, L.M.; Zaric, V.; Gilger, B.C.; Hirsch, M.L. Serotype Survey of AAV Gene Delivery via Subconjunctival Injection in Mice. Gene Ther. 2018, 25, 402–414. [Google Scholar] [CrossRef]
- Moreno, A.M.; Fu, X.; Zhu, J.; Katrekar, D.; Shih, Y.-R.V.; Marlett, J.; Cabotaje, J.; Tat, J.; Naughton, J.; Lisowski, L.; et al. In Situ Gene Therapy via AAV-CRISPR-Cas9-Mediated Targeted Gene Regulation. Mol. Ther. 2018, 26, 1818–1827. [Google Scholar] [CrossRef]
- Wang, S.K.; Xue, Y.; Cepko, C.L. Microglia Modulation by TGF-Β1 Protects Cones in Mouse Models of Retinal Degeneration. J. Clin. Investig. 2020, 130, 4360–4369. [Google Scholar] [CrossRef]
- Ramachandran, P.S.; Lee, V.; Wei, Z.; Song, J.Y.; Casal, G.; Cronin, T.; Willett, K.; Huckfeldt, R.; Morgan, J.I.W.; Aleman, T.S.; et al. Evaluation of Dose and Safety of AAV7m8 and AAV8BP2 in the Non-Human Primate Retina. Hum. Gene Ther. 2017, 28, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.; Yeh, W.-H.; Pendse, N.; Davis, J.R.; Hennessey, E.; Butcher, R.; Koblan, L.W.; Comander, J.; Liu, Q.; Liu, D.R. Cytosine and Adenine Base Editing of the Brain, Liver, Retina, Heart and Skeletal Muscle of Mice via Adeno-Associated Viruses. Nat. Biomed. Eng. 2020, 4, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Tornabene, P.; Trapani, I.; Minopoli, R.; Centrulo, M.; Lupo, M.; de Simone, S.; Tiberi, P.; Dell’Aquila, F.; Marrocco, E.; Iodice, C.; et al. Intein-Mediated Protein Trans-Splicing Expands Adeno-Associated Virus Transfer Capacity in the Retina. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wing, K.; Wang, J.-H.; Luu, C.D.; Bender, J.A.; Chen, J.; Wang, Q.; Lu, Q.; Nguyen Tran, M.T.; Young, K.M.; et al. Comparison of CRISPR/Cas Endonucleases for in Vivo Retinal Gene Editing. Front. Cell. Neurosci. 2020, 14, eaav4523. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Wang, J.-H.; Barathi, V.A.; Prea, S.M.; He, Z.; Lee, J.H.; Bender, J.; King, A.E.; Logan, G.J.; Alexander, I.E.; et al. AAV-Mediated Gene Delivery of the Calreticulin Anti-Angiogenic Domain Inhibits Ocular Neovascularization. Angiogenesis 2018, 21, 95–109. [Google Scholar] [CrossRef]
- Byrne, L.C.; Day, T.P.; Visel, M.; Strazzeri, J.A.; Fortuny, C.; Dalkara, D.; Merigan, W.H.; Schaffer, D.V.; Flannery, J.G. In Vivo-Directed Evolution of Adeno-Associated Virus in the Primate Retina. JCI Insight 2020, 5, e135112. [Google Scholar] [CrossRef]
- Dalkara, D.; Byrne, L.C.; Klimczak, R.R.; Visel, M.; Yin, L.; Merigan, W.H.; Flannery, J.G.; Schaffer, D.V. In Vivo-Directed Evolution of a New Adeno-Associated Virus for Therapeutic Outer Retinal Gene Delivery from the Vitreous. Sci. Transl. Med. 2013, 5, 189ra76. [Google Scholar] [CrossRef]
- Khabou, H.; Desrosiers, M.; Winckler, C.; Fouquet, S.; Auregan, G.; Bemelmans, A.-P.; Sahel, J.-A.; Dalkara, D. Insight into the Mechanisms of Enhanced Retinal Transduction by the Engineered AAV2 Capsid Variant-7m8. Biotechnol. Bioeng. 2016, 113, 2712–2724. [Google Scholar] [CrossRef]
- György, B.; Nist-Lund, C.; Pan, B.; Asai, Y.; Karavitaki, K.D.; Kleinstiver, B.P.; Garcia, S.P.; Zaborowski, M.P.; Solanes, P.; Spataro, S.; et al. Allele-Specific Gene Editing Prevents Deafness in a Model of Dominant Progressive Hearing Loss. Nat. Med. 2019, 25, 1123–1130. [Google Scholar] [CrossRef]
- Taiber, S.; Cohen, R.; Yizhar-Barnea, O.; Sprinzak, D.; Holt, J.R.; Avraham, K.B. Neonatal AAV Gene Therapy Rescues Hearing in a Mouse Model of SYNE4 Deafness. EMBO Mol. Med. 2021, 13, e13259. [Google Scholar] [CrossRef]
- Kang, W.; Zhao, X.; Sun, Z.; Dong, T.; Jin, C.; Tong, L.; Zhu, W.; Tao, Y.; Wu, H. Adeno-Associated Virus Vector Enables Safe and Efficient Cas9 Activation in Neonatal and Adult Cas9 Knockin Murine Cochleae. Gene Ther. 2020, 27, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Chai, R.; Guo, L.; Dong, B.; Li, W.; Shu, Y.; Huang, X.; Li, H. Transduction of Adeno-Associated Virus Vectors Targeting Hair Cells and Supporting Cells in the Neonatal Mouse Cochlea. Front. Cell. Neurosci. 2019, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Huang, M.; Shu, Y.; Ruprecht, A.; Wang, H.; Tang, Y.; Vandenberghe, L.H.; Wang, Q.; Gao, G.; Kong, W.J.; et al. Delivery of Adeno-Associated Virus Vectors in Adult Mammalian Inner-Ear Cell Subtypes Without Auditory Dysfunction. Hum. Gene Ther. 2018, 29, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jin, C.; Dong, T.; Sun, Z.; Zheng, X.; Feng, B.; Cheng, Z.; Li, X.; Tao, Y.; Wu, H. Characterization of Promoters for Adeno-Associated Virus Mediated Efficient Cas9 Activation in Adult Cas9 Knock-in Murine Cochleae. Hear. Res. 2020, 394, 107999. [Google Scholar] [CrossRef]
- Cooper, L.B.; Chan, D.K.; Roediger, F.C.; Shaffer, B.R.; Fraser, J.F.; Musatov, S.; Selesnick, S.H.; Kaplitt, M.G. AAV-Mediated Delivery of the Caspase Inhibitor XIAP Protects against Cisplatin Ototoxicity. Otol. Neurotol. 2006, 27, 484–490. [Google Scholar] [CrossRef]
- György, B.; Meijer, E.J.; Ivanchenko, M.V.; Tenneson, K.; Emond, F.; Hanlon, K.S.; Indzhykulian, A.A.; Volak, A.; Karavitaki, K.D.; Tamvakologos, P.I.; et al. Gene Transfer with AAV9-PHP.B Rescues Hearing in a Mouse Model of Usher Syndrome 3A and Transduces Hair Cells in a Non-Human Primate. Mol. Ther. Methods Clin. Dev. 2019, 13, 1–13. [Google Scholar] [CrossRef]
- Pan, B.; Askew, C.; Galvin, A.; Heman-Ackah, S.; Asai, Y.; Indzhykulian, A.A.; Jodelka, F.M.; Hastings, M.L.; Lentz, J.J.; Vandenberghe, L.H.; et al. Gene Therapy Restores Auditory and Vestibular Function in a Mouse Model of Usher Syndrome Type 1c. Nat. Biotechnol. 2017, 35, 264–272. [Google Scholar] [CrossRef]
- György, B.; Sage, C.; Indzhykulian, A.A.; Scheffer, D.I.; Brisson, A.R.; Tan, S.; Wu, X.; Volak, A.; Mu, D.; Tamvakologos, P.I.; et al. Rescue of Hearing by Gene Delivery to Inner-Ear Hair Cells Using Exosome-Associated AAV. Mol. Ther. 2017, 25, 379–391. [Google Scholar] [CrossRef]
- Kim, M.-A.; Ryu, N.; Kim, H.-M.; Kim, Y.-R.; Lee, B.; Kwon, T.-J.; Bok, J.; Kim, U.-K. Targeted Gene Delivery into the Mammalian Inner Ear Using Synthetic Serotypes of Adeno-Associated Virus Vectors. Mol. Ther. Methods Clin. Dev. 2019, 13, 197–204. [Google Scholar] [CrossRef]
- Lee, J.; Nist-Lund, C.; Solanes, P.; Goldberg, H.; Wu, J.; Pan, B.; Schneider, B.L.; Holt, J.R. Efficient Viral Transduction in Mouse Inner Ear Hair Cells with Utricle Injection and AAV9-PHP.B. Hear. Res. 2020, 394, 107882. [Google Scholar] [CrossRef]
- Miyanohara, A.; Kamizato, K.; Juhas, S.; Juhasova, J.; Navarro, M.; Marsala, S.; Lukacova, N.; Hruska-Plochan, M.; Curtis, E.; Gabel, B.; et al. Potent Spinal Parenchymal AAV9-Mediated Gene Delivery by Subpial Injection in Adult Rats and Pigs. Mol. Ther. Methods Clin. Dev. 2016, 3, 16046. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Hernández, M.; Tadokoro, T.; Marsala, M. Subpial AAV Delivery for Spinal Parenchymal Gene Regulation in Adult Mammals. Methods Mol. Biol. 2019, 1950, 209–233. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Uytingco, C.R.; Green, W.W.; McIntyre, J.C.; Ukhanov, K.; Zimmerman, A.D.; Shively, D.T.; Zhang, L.; Nishimura, D.Y.; Sheffield, V.C.; et al. Gene Therapeutic Reversal of Peripheral Olfactory Impairment in Bardet-Biedl Syndrome. Mol. Ther. 2017, 25, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Lonser, R.R.; Akhter, A.S.; Zabek, M.; Elder, J.B.; Bankiewicz, K.S. Direct Convective Delivery of Adeno-Associated Virus Gene Therapy for Treatment of Neurological Disorders. J. Neurosurg. 2020, 134, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef]
- Samaranch, L.; Blits, B.; San Sebastian, W.; Hadaczek, P.; Bringas, J.; Sudhakar, V.; Macayan, M.; Pivirotto, P.J.; Petry, H.; Bankiewicz, K.S. MR-Guided Parenchymal Delivery of Adeno-Associated Viral Vector Serotype 5 in Non-Human Primate Brain. Gene Ther. 2017, 24, 253–261. [Google Scholar] [CrossRef]
- Tervo, D.G.R.; Hwang, B.-Y.; Viswanathan, S.; Gaj, T.; Lavzin, M.; Ritola, K.D.; Lindo, S.; Michael, S.; Kuleshova, E.; Ojala, D.; et al. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 2016, 92, 372–382. [Google Scholar] [CrossRef]
- Tordo, J.; O’Leary, C.; Antunes, A.S.L.M.; Palomar, N.; Aldrin-Kirk, P.; Basche, M.; Bennett, A.; D’Souza, Z.; Gleitz, H.; Godwin, A.; et al. A Novel Adeno-Associated Virus Capsid with Enhanced Neurotropism Corrects a Lysosomal Transmembrane Enzyme Deficiency. Brain 2018, 141, 2014–2031. [Google Scholar] [CrossRef]
- Davidsson, M.; Wang, G.; Aldrin-Kirk, P.; Cardoso, T.; Nolbrant, S.; Hartnor, M.; Mudannayake, J.; Parmar, M.; Björklund, T. A Systematic Capsid Evolution Approach Performed in Vivo for the Design of AAV Vectors with Tailored Properties and Tropism. Proc. Natl. Acad. Sci. USA 2019, 116, 27053–27062. [Google Scholar] [CrossRef]
- Hinderer, C.; Bell, P.; Katz, N.; Vite, C.H.; Louboutin, J.-P.; Bote, E.; Yu, H.; Zhu, Y.; Casal, M.L.; Bagel, J.; et al. Evaluation of Intrathecal Routes of Administration for Adeno-Associated Viral Vectors in Large Animals. Hum. Gene Ther. 2018, 29, 15–24. [Google Scholar] [CrossRef]
- Hordeaux, J.; Buza, E.L.; Dyer, C.; Goode, T.; Mitchell, T.W.; Richman, L.; Denton, N.; Hinderer, C.; Katz, N.; Schmid, R.; et al. Adeno-Associated Virus-Induced Dorsal Root Ganglion Pathology. Hum. Gene Ther. 2020, 31, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Deverman, B.E.; Pravdo, P.L.; Simpson, B.P.; Kumar, S.R.; Chan, K.Y.; Banerjee, A.; Wu, W.-L.; Yang, B.; Huber, N.; Pasca, S.P.; et al. Cre-Dependent Selection Yields AAV Variants for Widespread Gene Transfer to the Adult Brain. Nat. Biotechnol. 2016, 34, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Galvan, A.; Petkau, T.L.; Hill, A.M.; Korecki, A.J.; Lu, G.; Choi, D.; Rahman, K.; Simpson, E.; Leavitt, B.R.; Smith, Y. Intracerebroventricular Administration of AAV9-PHP.B SYN1-EmGFP Induces Widespread Transgene Expression in the Mouse and Monkey CNS. Hum. Gene Ther. 2021, 32, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Hordeaux, J.; Wang, Q.; Katz, N.; Buza, E.L.; Bell, P.; Wilson, J.M. The Neurotropic Properties of AAV-PHP.B Are Limited to C57BL/6J Mice. Mol. Ther. 2018, 26, 664–668. [Google Scholar] [CrossRef]
- Flotte, T.R.; Carter, B.J. Adeno-Associated Virus Vectors for Gene Therapy. Gene Ther. 1995, 2, 357–362. [Google Scholar]
- Wagner, J.A.; Messner, A.H.; Moran, M.L.; Daifuku, R.; Kouyama, K.; Desch, J.K.; Manley, S.; Norbash, A.M.; Conrad, C.K.; Friborg, S.; et al. Safety and Biological Efficacy of an Adeno-Associated Virus Vector-Cystic Fibrosis Transmembrane Regulator (AAV-CFTR) in the Cystic Fibrosis Maxillary Sinus. Laryngoscope 1999, 109, 266–274. [Google Scholar] [CrossRef]
- Salegio, E.; Samaranch, L.; Kells, A.; Mittermeyer, G.; San Sebastián, W.; Zhou, S.; Beyer, J.; Forsayeth, J.; Bankiewicz, K. Axonal Transport of Adeno-Associated Viral Vectors Is Serotype-Dependent. Gene Ther. 2012, 20, 348–352. [Google Scholar] [CrossRef]
- Kells, A.P.; Forsayeth, J.; Bankiewicz, K.S. Glial-Derived Neurotrophic Factor Gene Transfer for Parkinson’s Disease: Anterograde Distribution of AAV2 Vectors in the Primate Brain. Neurobiol. Dis. 2012, 48, 228–235. [Google Scholar] [CrossRef]
- Green, F.; Samaranch, L.; Zhang, H.S.; Manning-Bog, A.; Meyer, K.; Forsayeth, J.; Bankiewicz, K.S. Axonal Transport of AAV9 in Nonhuman Primate Brain. Gene Ther. 2016, 23, 520–526. [Google Scholar] [CrossRef]
- Mandel, T.E.; Koulmanda, M.; Cozzi, E.; Waterworth, P.; Tolan, M.; Langford, G.; White, D.J. Transplantation of Normal and DAF-Transgenic Fetal Pig Pancreas into Cynomolgus Monkeys. Transplant. Proc. 1997, 29, 940. [Google Scholar] [CrossRef]
- Bartus, R.T.; Weinberg, M.S.; Samulski, R.J. Parkinson’s Disease Gene Therapy: Success by Design Meets Failure by Efficacy. Mol. Ther 2014, 22, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Christine, C.W.; Bankiewicz, K.S.; Van Laar, A.D.; Richardson, R.M.; Ravina, B.; Kells, A.P.; Boot, B.; Martin, A.J.; Nutt, J.; Thompson, M.E.; et al. Magnetic Resonance Imaging-Guided Phase 1 Trial of Putaminal AADC Gene Therapy for Parkinson’s Disease. Ann. Neurol. 2019, 85, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, S.; Fujimoto, K.; Kato, S.; Mizukami, H.; Asari, S.; Ikeguchi, K.; Kawakami, T.; Urabe, M.; Kume, A.; Sato, T.; et al. A Phase I Study of Aromatic L-Amino Acid Decarboxylase Gene Therapy for Parkinson’s Disease. Mol. Ther. 2010, 18, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- McFarthing, K.; Prakash, N.; Simuni, T. Clinical trial highlights: 1. Gene therapy for Parkinson´s, 2. Phase 3 study in focus intec pharma´s accordion pill, 3. Clinial trials resources. J. Parkinsons Dis. 2019, 9, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Bäckman, C.M.; Shan, L.; Zhang, Y.J.; Hoffer, B.J.; Leonard, S.; Troncoso, J.C.; Vonsatel, P.; Tomac, A.C. Gene Expression Patterns for GDNF and Its Receptors in the Human Putamen Affected by Parkinson’s Disease: A Real-Time PCR Study. Mol. Cell. Endocrinol. 2006, 252, 160–166. [Google Scholar] [CrossRef]
- Airaksinen, M.S.; Saarma, M. The GDNF Family: Signalling, Biological Functions and Therapeutic Value. Nat. Rev. Neurosci. 2002, 3, 383–394. [Google Scholar] [CrossRef]
- Björklund, A.; Kirik, D.; Rosenblad, C.; Georgievska, B.; Lundberg, C.; Mandel, R.J. Towards a Neuroprotective Gene Therapy for Parkinson’s Disease: Use of Adenovirus, AAV and Lentivirus Vectors for Gene Transfer of GDNF to the Nigrostriatal System in the Rat Parkinson Model. Brain Res. 2000, 886, 82–98. [Google Scholar] [CrossRef]
- Marks, W.J.; Ostrem, J.L.; Verhagen, L.; Starr, P.A.; Larson, P.S.; Bakay, R.A.; Taylor, R.; Cahn-Weiner, D.A.; Stoessl, A.J.; Olanow, C.W.; et al. Safety and Tolerability of Intraputaminal Delivery of CERE-120 (Adeno-Associated Virus Serotype 2-Neurturin) to Patients with Idiopathic Parkinson’s Disease: An Open-Label, Phase I Trial. Lancet Neurol. 2008, 7, 400–408. [Google Scholar] [CrossRef]
- Bartus, R.T.; Baumann, T.L.; Siffert, J.; Herzog, C.D.; Alterman, R.; Boulis, N.; Turner, D.A.; Stacy, M.; Lang, A.E.; Lozano, A.M.; et al. Safety/Feasibility of Targeting the Substantia Nigra with AAV2-Neurturin in Parkinson Patients. Neurology 2013, 80, 1698–1701. [Google Scholar] [CrossRef]
- LeWitt, P.A.; Rezai, A.R.; Leehey, M.A.; Ojemann, S.G.; Flaherty, A.W.; Eskandar, E.N.; Kostyk, S.K.; Thomas, K.; Sarkar, A.; Siddiqui, M.S.; et al. AAV2-GAD Gene Therapy for Advanced Parkinson’s Disease: A Double-Blind, Sham-Surgery Controlled, Randomised Trial. Lancet Neurol. 2011, 10, 309–319. [Google Scholar] [CrossRef]
- Kaplitt, M.G.; Feigin, A.; Tang, C.; Fitzsimons, H.L.; Mattis, P.; Lawlor, P.A.; Bland, R.J.; Young, D.; Strybing, K.; Eidelberg, D.; et al. Safety and Tolerability of Gene Therapy with an Adeno-Associated Virus (AAV) Borne GAD Gene for Parkinson’s Disease: An Open Label, Phase I Trial. Lancet 2007, 369, 2097–2105. [Google Scholar] [CrossRef]
- Feigin, A.; Kaplitt, M.G.; Tang, C.; Lin, T.; Mattis, P.; Dhawan, V.; During, M.J.; Eidelberg, D. Modulation of Metabolic Brain Networks after Subthalamic Gene Therapy for Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2007, 104, 19559–19564. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, M.; Tang, C.C.; LeWitt, P.A.; Rezai, A.R.; Leehey, M.A.; Ojemann, S.G.; Flaherty, A.W.; Eskandar, E.N.; Kostyk, S.K.; Sarkar, A.; et al. Long-Term Follow-up of a Randomized AAV2-GAD Gene Therapy Trial for Parkinson’s Disease. JCI Insight 2017, 2, e90133. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Kaplitt, M.G.; Fitzsimons, H.L.; Zuzga, D.S.; Liu, Y.; Oshinsky, M.L.; During, M.J. Subthalamic GAD Gene Therapy in a Parkinson’s Disease Rat Model. Science 2002, 298, 425–429. [Google Scholar] [CrossRef]
- Blandini, F.; Cilia, R.; Cerri, S.; Pezzoli, G.; Schapira, A.H.V.; Mullin, S.; Lanciego, J.L. Glucocerebrosidase Mutations and Synucleinopathies: Toward a Model of Precision Medicine. Mov. Disord. 2019, 34, 9–21. [Google Scholar] [CrossRef]
- Rocha, E.M.; Smith, G.A.; Park, E.; Cao, H.; Brown, E.; Hayes, M.A.; Beagan, J.; McLean, J.R.; Izen, S.C.; Perez-Torres, E.; et al. Glucocerebrosidase Gene Therapy Prevents α-Synucleinopathy of Midbrain Dopamine Neurons. Neurobiol. Dis. 2015, 82, 495–503. [Google Scholar] [CrossRef]
- Morabito, G.; Giannelli, S.G.; Ordazzo, G.; Bido, S.; Castoldi, V.; Indrigo, M.; Cabassi, T.; Cattaneo, S.; Luoni, M.; Cancellieri, C.; et al. AAV-PHP.B-Mediated Global-Scale Expression in the Mouse Nervous System Enables GBA1 Gene Therapy for Wide Protection from Synucleinopathy. Mol. Ther. 2017, 25, 2727–2742. [Google Scholar] [CrossRef]
- Sucunza, D.; Rico, A.J.; Roda, E.; Collantes, M.; González-Aseguinolaza, G.; Rodríguez-Pérez, A.I.; Peñuelas, I.; Vázquez, A.; Labandeira-García, J.L.; Broccoli, V.; et al. Glucocerebrosidase Gene Therapy Induces Alpha-Synuclein Clearance and Neuroprotection of Midbrain Dopaminergic Neurons in Mice and Macaques. Int. J. Mol. Sci. 2021, 22, 4825. [Google Scholar] [CrossRef]
- Xu, R.; Camboni, M.; Martin, P.T. Postnatal Overexpression of the CT GalNAc Transferase Inhibits Muscular Dystrophy in Mdx Mice without Altering Muscle Growth or Neuromuscular Development. Neuromuscul. Disord. 2007, 17, 209–220. [Google Scholar] [CrossRef]
- Fischell, J.M.; Fishman, P.S. A Multifaceted Approach to Optimizing AAV Delivery to the Brain for the Treatment of Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 1235. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.A.; Lehman, K.J.; McColly, M.; Lowes, L.P.; Alfano, L.N.; Reash, N.F.; Iammarino, M.A.; Church, K.R.; Kleyn, A.; et al. Five-Year Extension Results of the Phase 1 START Trial of Onasemnogene Abeparvovec in Spinal Muscular Atrophy. JAMA Neurol. 2021, 78, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Zolgensma. FDA. C. for B.E. and ZOLGENSMA. FDA. 2021. Available online: https://www.fda.gov/vaccines-blood-biologics/zolgensma (accessed on 20 March 2022).
- Richardson, R.M.; Kells, A.P.; Rosenbluth, K.H.; Salegio, E.A.; Fiandaca, M.S.; Larson, P.S.; Starr, P.A.; Martin, A.J.; Lonser, R.R.; Federoff, H.J.; et al. Interventional MRI-Guided Putaminal Delivery of AAV2-GDNF for a Planned Clinical Trial in Parkinson’s Disease. Mol. Ther. 2011, 19, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G.; Curtze, C.; Hiller, A.; Anderson, S.; Larson, P.S.; Van Laar, A.D.; Richardson, R.M.; Thompson, M.E.; Sedkov, A.; Leinonen, M.; et al. Aromatic L-Amino Acid Decarboxylase Gene Therapy Enhances Levodopa Response in Parkinson’s Disease. Mov. Disord. 2020, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Rafii, M.S.; Tuszynski, M.H.; Thomas, R.G.; Barba, D.; Brewer, J.B.; Rissman, R.A.; Siffert, J.; Aisen, P.S. AAV2-NGF Study Team Adeno-Associated Viral Vector (Serotype 2)-Nerve Growth Factor for Patients with Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, A.H.; Merrill, D.A.; Coppola, G.; Tsukada, S.; Schroeder, B.E.; Shaked, G.M.; Wang, L.; Blesch, A.; Kim, A.; Conner, J.M.; et al. Neuroprotective Effects of Brain-Derived Neurotrophic Factor in Rodent and Primate Models of Alzheimer’s Disease. Nat. Med. 2009, 15, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, A.H.; Tuszynski, M.H. Potential Therapeutic Uses of BDNF in Neurological and Psychiatric Disorders. Nat. Rev. Drug Discov. 2011, 10, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.B.; Wild, E.J. Huntington’s Disease Clinical Trials Corner: April 2020. J. Huntingt. Dis. 2020, 9, 185–197. [Google Scholar] [CrossRef]
- Day, J.W.; Finkel, R.S.; Chiriboga, C.A.; Connolly, A.M.; Crawford, T.O.; Darras, B.T.; Iannaccone, S.T.; Kuntz, N.L.; Peña, L.D.M.; Shieh, P.B.; et al. Onasemnogene Abeparvovec Gene Therapy for Symptomatic Infantile-Onset Spinal Muscular Atrophy in Patients with Two Copies of SMN2 (STR1VE): An Open-Label, Single-Arm, Multicentre, Phase 3 Trial. Lancet Neurol. 2021, 20, 284–293. [Google Scholar] [CrossRef]
- Bevan, A.K.; Duque, S.; Foust, K.D.; Morales, P.R.; Braun, L.; Schmelzer, L.; Chan, C.M.; McCrate, M.; Chicoine, L.G.; Coley, B.D.; et al. Systemic Gene Delivery in Large Species for Targeting Spinal Cord, Brain, and Peripheral Tissues for Pediatric Disorders. Mol. Ther. 2011, 19, 1971–1980. [Google Scholar] [CrossRef]
- Foust, K.D.; Wang, X.; McGovern, V.L.; Braun, L.; Bevan, A.K.; Haidet, A.M.; Le, T.T.; Morales, P.R.; Rich, M.M.; Burghes, A.H.M.; et al. Rescue of the Spinal Muscular Atrophy Phenotype in a Mouse Model by Early Postnatal Delivery of SMN. Nat. Biotechnol. 2010, 28, 271–274. [Google Scholar] [CrossRef]
- Ashtari, M.; Cyckowski, L.L.; Monroe, J.F.; Marshall, K.A.; Chung, D.C.; Auricchio, A.; Simonelli, F.; Leroy, B.P.; Maguire, A.M.; Shindler, K.S.; et al. The Human Visual Cortex Responds to Gene Therapy-Mediated Recovery of Retinal Function. J. Clin. Investig. 2011, 121, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, F.; Maguire, A.M.; Testa, F.; Pierce, E.A.; Mingozzi, F.; Bennicelli, J.L.; Rossi, S.; Marshall, K.; Banfi, S.; Surace, E.M.; et al. Gene Therapy for Leber’s Congenital Amaurosis Is Safe and Effective through 1.5 Years after Vector Administration. Mol. Ther. 2010, 18, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Acland, G.M.; Aguirre, G.D.; Bennett, J.; Aleman, T.S.; Cideciyan, A.V.; Bennicelli, J.; Dejneka, N.S.; Pearce-Kelling, S.E.; Maguire, A.M.; Palczewski, K.; et al. Long-Term Restoration of Rod and Cone Vision by Single Dose RAAV-Mediated Gene Transfer to the Retina in a Canine Model of Childhood Blindness. Mol. Ther. 2005, 12, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, J.W.B.; Smith, A.J.; Barker, S.S.; Robbie, S.; Henderson, R.; Balaggan, K.; Viswanathan, A.; Holder, G.E.; Stockman, A.; Tyler, N.; et al. Effect of Gene Therapy on Visual Function in Leber’s Congenital Amaurosis. N. Engl. J. Med. 2008, 358, 2231–2239. [Google Scholar] [CrossRef]
- Cideciyan, A.V.; Aleman, T.S.; Boye, S.L.; Schwartz, S.B.; Kaushal, S.; Roman, A.J.; Pang, J.-J.; Sumaroka, A.; Windsor, E.A.M.; Wilson, J.M.; et al. Human Gene Therapy for RPE65 Isomerase Deficiency Activates the Retinoid Cycle of Vision but with Slow Rod Kinetics. Proc. Natl. Acad. Sci. USA 2008, 105, 15112–15117. [Google Scholar] [CrossRef]
- Hauswirth, W.W.; Aleman, T.S.; Kaushal, S.; Cideciyan, A.V.; Schwartz, S.B.; Wang, L.; Conlon, T.J.; Boye, S.L.; Flotte, T.R.; Byrne, B.J.; et al. Treatment of Leber Congenital Amaurosis Due to RPE65 Mutations by Ocular Subretinal Injection of Adeno-Associated Virus Gene Vector: Short-Term Results of a Phase I Trial. Hum. Gene Ther. 2008, 19, 979–990. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Boye, S.L.; Aleman, T.S.; Conlon, T.J.; Zeiss, C.J.; Roman, A.J.; Cideciyan, A.V.; Schwartz, S.B.; Komaromy, A.M.; Doobrajh, M.; et al. Safety in Nonhuman Primates of Ocular AAV2-RPE65, a Candidate Treatment for Blindness in Leber Congenital Amaurosis. Hum. Gene Ther. 2006, 17, 845–858. [Google Scholar] [CrossRef]
- Feuer, W.J.; Schiffman, J.C.; Davis, J.L.; Porciatti, V.; Gonzalez, P.; Koilkonda, R.D.; Yuan, H.; Lalwani, A.; Lam, B.L.; Guy, J. Gene Therapy for Leber Hereditary Optic Neuropathy: Initial Results. Ophthalmology 2016, 123, 558–570. [Google Scholar] [CrossRef]
- Newman, N.J.; Yu-Wai-Man, P.; Carelli, V.; Biousse, V.; Moster, M.L.; Vignal-Clermont, C.; Sergott, R.C.; Klopstock, T.; Sadun, A.A.; Girmens, J.-F.; et al. Intravitreal Gene Therapy vs. Natural History in Patients with Leber Hereditary Optic Neuropathy Carrying the m.11778G>A ND4 Mutation: Systematic Review and Indirect Comparison. Front. Neurol. 2021, 12, 662838. [Google Scholar] [CrossRef]
- Yang, S.; Ma, S.-Q.; Wan, X.; He, H.; Pei, H.; Zhao, M.-J.; Chen, C.; Wang, D.-W.; Dong, X.-Y.; Yuan, J.-J.; et al. Long-Term Outcomes of Gene Therapy for the Treatment of Leber’s Hereditary Optic Neuropathy. EBioMedicine 2016, 10, 258–268. [Google Scholar] [CrossRef]
- Ran, R.; Yang, S.; He, H.; Ma, S.; Chen, Z.; Li, B. A Retrospective Analysis of Characteristics of Visual Field Damage in Patients with Leber’s Hereditary Optic Neuropathy. Springerplus 2016, 5, 843. [Google Scholar] [CrossRef] [PubMed]
- Parinot, C.; Nandrot, E.F. A Comprehensive Review of Mutations in the MERTK Proto-Oncogene. Adv. Exp. Med. Biol. 2016, 854, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Cehajic-Kapetanovic, J.; Xue, K.; Martinez-Fernandez de la Camara, C.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R.; et al. Initial Results from a First-in-Human Gene Therapy Trial on X-Linked Retinitis Pigmentosa Caused by Mutations in RPGR. Nat. Med. 2020, 26, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Petit, L.; Lhériteau, E.; Weber, M.; Le Meur, G.; Deschamps, J.-Y.; Provost, N.; Mendes-Madeira, A.; Libeau, L.; Guihal, C.; Colle, M.-A.; et al. Restoration of Vision in the Pde6β-Deficient Dog, a Large Animal Model of Rod-Cone Dystrophy. Mol. Ther. 2012, 20, 2019–2030. [Google Scholar] [CrossRef]
- Pichard, V.; Provost, N.; Mendes-Madeira, A.; Libeau, L.; Hulin, P.; Tshilenge, K.-T.; Biget, M.; Ameline, B.; Deschamps, J.-Y.; Weber, M.; et al. AAV-Mediated Gene Therapy Halts Retinal Degeneration in PDE6β-Deficient Dogs. Mol. Ther. 2016, 24, 867–876. [Google Scholar] [CrossRef]
- Simunovic, M.P.; Jolly, J.K.; Xue, K.; Edwards, T.L.; Groppe, M.; Downes, S.M.; MacLaren, R.E. The Spectrum of CHM Gene Mutations in Choroideremia and Their Relationship to Clinical Phenotype. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6033–6039. [Google Scholar] [CrossRef]
- Xue, K.; Oldani, M.; Jolly, J.K.; Edwards, T.L.; Groppe, M.; Downes, S.M.; MacLaren, R.E. Correlation of Optical Coherence Tomography and Autofluorescence in the Outer Retina and Choroid of Patients with Choroideremia. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3674–3684. [Google Scholar] [CrossRef]
- Seitz, I.P.; Zhour, A.; Kohl, S.; Llavona, P.; Peter, T.; Wilhelm, B.; Zrenner, E.; Ueffing, M.; Bartz-Schmidt, K.U.; Fischer, M.D. Multimodal Assessment of Choroideremia Patients Defines Pre-Treatment Characteristics. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 2143–2150. [Google Scholar] [CrossRef]
- MacLaren, R.E.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Seymour, L.; Clark, K.R.; During, M.J.; Cremers, F.P.M.; Black, G.C.M.; et al. Retinal Gene Therapy in Patients with Choroideremia: Initial Findings from a Phase 1/2 Clinical Trial. Lancet 2014, 383, 1129–1137. [Google Scholar] [CrossRef]
- Davis, J.L. The Blunt End: Surgical Challenges of Gene Therapy for Inherited Retinal Diseases. Am. J. Ophthalmol. 2018, 196, 25–29. [Google Scholar] [CrossRef]
- Maguire, A.M.; Simonelli, F.; Pierce, E.A.; Pugh, E.N.; Mingozzi, F.; Bennicelli, J.; Banfi, S.; Marshall, K.A.; Testa, F.; Surace, E.M.; et al. Safety and Efficacy of Gene Transfer for Leber’s Congenital Amaurosis. N. Engl. J. Med. 2008, 358, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.D.; Ochakovski, G.A.; Beier, B.; Seitz, I.P.; Vaheb, Y.; Kortuem, C.; Reichel, F.F.L.; Kuehlewein, L.; Kahle, N.A.; Peters, T.; et al. Efficacy and Safety of Retinal Gene Therapy Using Adeno-Associated Virus Vector for Patients with Choroideremia: A Randomized Clinical Trial. JAMA Ophthalmol. 2019, 137, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Banin, E.; Gootwine, E.; Obolensky, A.; Ezra-Elia, R.; Ejzenberg, A.; Zelinger, L.; Honig, H.; Rosov, A.; Yamin, E.; Sharon, D.; et al. Gene Augmentation Therapy Restores Retinal Function and Visual Behavior in a Sheep Model of CNGA3 Achromatopsia. Mol. Ther. 2015, 23, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Komáromy, A.M.; Alexander, J.J.; Rowlan, J.S.; Garcia, M.M.; Chiodo, V.A.; Kaya, A.; Tanaka, J.C.; Acland, G.M.; Hauswirth, W.W.; Aguirre, G.D. Gene Therapy Rescues Cone Function in Congenital Achromatopsia. Hum. Mol. Genet. 2010, 19, 2581–2593. [Google Scholar] [CrossRef] [PubMed]
| Disease | Delivery Routes | Target | Species | AAV Serotype | References |
|---|---|---|---|---|---|
| Alzheimer | Intraparenchymal | Aβ | Mice | AAV1 | [12] |
| Intraparenchymal | APOE2 | Mice | AAV9 and AArh10 | [13] | |
| Intraparenchymal | shIRS1 (IRS1: neuroprotective role) | Rats | AAV2/DJ8 | [14] | |
| Intraparenchymal | CCL2 (diffuse amyloid plaques) | Mice | AAV1/2 | [15] | |
| Intraparenchymal | ECE (protease involved in Aβ degradation) | Mice | AAV5 | [16] | |
| Intraparenchymal | NGF (improving cholinergic activity) | Rats | AAV2 and AAV5 | [17] | |
| Intraparenchymal | NGF | Mice | CERE-110 (AAV2) | [18] | |
| Intraparenchymal | PHF1 (anti-phospho-TAU antibody) | Mice | AAVrh10 | [19] | |
| Intraparenchymal | CascFv59 (anti-Aβ antibody) | Mice | AAV2 | [20] | |
| Intraparenchymal | IL-10 (inhibition of proinflammatory cytokines) | Mice | AAV1 | [21] | |
| Intramuscular and intravenous | GFP | Mice | AAV9, exo-AAV9 (IM) and AAV8 (IV) | [22] | |
| Intramuscular | scFv (anti-Aβ antibody) | Mice | AAV1 | [23] | |
| Intramuscular | P75NTR (protective against Aβ) | Mice | AAV8 | [24] | |
| Intracerebroventricular | GFP | Mice | AAV1, AAV5, AAV8, AAV9, AAV2-BR1 and AAV2-PHP.eB | [25] | |
| Huntington | Intraparenchymal | 82Q (mutant Htt) | Rats | AAV2 | [26] |
| Intraparenchymal | BDNF and GDNF | Rats | AAV2 | [27] | |
| Intraparenchymal | CRISPR/Cas9 (Htt) | Mice | AAV1 | [28] | |
| Intraparenchymal | SIRT3 (protective against oxidative and mitochondrial stress) | Mice | AAV-DJ | [29] | |
| Intraparenchymal | XBP1 (involved in the splicing events of Htt) | Mice | AAV2 | [30] | |
| Intraparenchymal | mRNA or siRNA (Htt) | Mice | AAV9 | [31] | |
| Intraparenchymal | iRNA (Htt) | Mice | AAV8 | [32] | |
| Intraparenchymal | Exon1-Q138 mHtt and Exon1-Q17 wildtype Htt | Mice | AAV9 | [33] | |
| Intraparenchymal | Human KRAB domain from KOX1 (ZNF10); ZNF10 represses mutant Htt expression | Mice | AAV9 | [34] | |
| Intraparenchymal | GFP | Rats | AAV1, AAV2 and AAV5 | [35] | |
| Intraparenchymal | GDNF (neurturin) | Mice | AAV8 | [36] | |
| Intraparenchymal | miHDS1 (Htt) | Mice | AAV1 | [37] | |
| Intraparenchymal | SREBP2 (to reverse synaptic defects in Huntington disease) | Mice | AAV5 | [38] | |
| Intraparenchymal | siRNA (Htt) | Sheep | AAV serotype not disclosed | [39] | |
| Intravenous | iRNA (Htt) | Mice | AAV1 | [40] | |
| Intramuscular and intravenous | shRNA (AAT) | Mice | AAV8 (IV) and AAV6 (IM) | [41] | |
| Intrathecal | miRNA based on endogenous mir155 backbone (Htt) | Sheep | AAV9 | [42] | |
| Amyotrophic lateral sclerosis | Intraparenchymal and intramuscular | GFP | Mice | AAV1, AAV2, AAV5, AAV6, AAV7, AAV8 | [43] |
| Intravenous and intracisternal | SOD1 | Mice | AAVrh10 | [44] | |
| Intravenous | IGF1 | Mice | AAV9 | [45] | |
| Intravenous | GDNF | Rat | AAV9 | [46] | |
| Intracerebroventricular | GFP | Mice | AAV9 | [47] | |
| Intramuscular | HGF in SOD1 model | Mice | AAV6 | [48] | |
| Intramuscular | hIGF1 in SOD1model | Mice | AAV9 | [49] | |
| Intramuscular | GDNF | Mice | AAV2 | [50] | |
| Intramuscular | GDNF | Mice | AAV2 | [51] | |
| Intramuscular | GFP | Mice | AAV1, AAV5, AAV8 and AAV9 | [52] | |
| Intramuscular | SOD1 | Mice | AAV6 | [53] | |
| Intramuscular | IGF1 and GDNF | Mice | AAV2 | [54] | |
| Intramuscular | IGF1 | Mice | AAV9 | [55] | |
| Intrathecal | GLT1 overexpression in SOD1 animal model | Mice | AAV8 | [56] | |
| Intrathecal | SOD1 | Mice | AAV9 | [57] | |
| Intracisternal | C9orf72 hexanucleotide repeat expansions (generates neuropathology) | Mice | AAV9 | [58] | |
| Spinal muscular atrophy | Intracerebroventricular and intraperitoneal | GFP | Mice | AAV9 | [59] |
| Intracerebroventricular | SMN1 (gene replacement strategy) | Mice | AAV9 | [60] | |
| Intracerebroventricular (mice) and intracisternal (pigs and NHP) | hSMN1 | Mice, Pigs, and NHPs | AAV9 | [61] | |
| Intracerebroventricular and intravenous | SMN1 | Mice | AAV9 | [62] | |
| Intramuscular | DOK7 (tuning down disease severity) | Mice | AAV9 | [63] | |
| Intravenous | SMN transgene | Piglets and NHPs | AAVhu68 | [64] | |
| Intramuscular | GFP | Mice | AAV9 | [65] | |
| Intrathecal | SMN2 (to rescue the SMA model) | Mice | AAV9 | [66] | |
| Intracisternal | miRNA | Mice | AAVrh10 | [67] | |
| Vision disorders | Subconjuntival | GFP | Mice | AAV2, AAV6 and AAV8 | [68] |
| Intravenous | CRISPR/Cas9 (retinitis pigmentosa) | Mice | AAV2, AAV6 and AAV8 | [69] | |
| Subretinal | TGF-β1 (retinitis pigmentosa) | Mice | AAV8 | [70] | |
| GFP | Mice and NHPs | AAV7m8 and AAV8BP2 | [71] | ||
| GFP | Mice | AAV8, AAV9. AAV-PHP.B, AAV-PHP.eB | [72] | ||
| GFP | Mice and pigs | AAV8 | [73] | ||
| Retinal | CRISPR/Cas9 (retinal editing) | Mice | AAV2 and AAV7 | [74] | |
| Intravitreal | CAD180 (endogenous inhibitor of angiogenesis) retinal neovascularization (RNV) | Mice | AAV2 | [75] | |
| GFP | NHPs | AAV2 | [76] | ||
| GFP | Mice and NHPs | AAV2 | [77] | ||
| GFP | Mice | AAV2, AAV5, AAV8 and AAV9 | [78] | ||
| Hearing disorders | Cochlear | CRISPR/Cas9 (gene editing) | Mice | AAV2 | [79] |
| SYNE4 (to rescue in a deafness model) | Mice | AAV9-PHP.B | [80] | ||
| GFP | Mice | AAV2, AAV6, AAV8, AAV/Anc80L65 | [81] | ||
| GFP | Mice and guinea pigs | AAV2, AAV9 and Anc80L65 | [82] | ||
| Canalastomy (inner ear cells) | GFP | Mice | AAV1, AAV2, AAV6.2, AAV8, AAV9, AAVrh.39, AAVrh.43 and Anc80L65 | [83] | |
| CRISPR/Cas9 (GFP, Biodistribution) | Mice | AAV8 | [84] | ||
| Round window membrane | XIAP against Cisplatin (chemotherapeutic agent) | Mice | AAV2 | [85] | |
| GFP | Mice and NHPs | AAV9-PHP.B | [86] | ||
| Harmonin-a1 and harmonin-b1 (To rescue Usher syndrome type 1c) | Mice | AAV1 and AAV/Anc80L65 | [87] | ||
| GFP | Mice | AAV1 and exo-AAV1 | [88] | ||
| GFP | Mice | AAV2/DJ, AAV2/DJ8, AAV2-PHP.B | [89] | ||
| Utricle (inner and outer cells) | GFP | Mice | AAV9-PHP-B, Anc80L65 and AAV2.7m8 | [90] |
| Disease | Clinical Trial | Duration | Phase | Target | AAV Serotype | Delivery Routes | Status | Company References | |
|---|---|---|---|---|---|---|---|---|---|
| Parkinson | NCT01973543 | 2013–2020 | I | AADC | AAV2 | IP in the Putamen | Completed | [112] University of California | |
| NCT02418598 | 2015–2018 | I/II | AADC | AAV2 | IP in the Putamen | Terminated (another clinical study for regulatory approval is planned) | [113] Jichi Medical University | ||
| NCT03065192 | 2017–2021 | I | AADC01 | AAV2 | IP in the Putamen | Active, not recruiting | Neurocrine Biosciences | ||
| NCT03562494 | 2018–2022 | II | AADC02 | AAV2 | IP | Active, not recruiting | [114] Voyager Therapeutics (Neurocrine Biosciences) | ||
| NCT03733496 | 2018–2026 | IV | AADC01 | AAV2 | IP in the Putamen | Enrolling, by invitation | [112,133,134] Voyager Therapeutics (Neurocrine Biosciences) | ||
| NCT04167540 | 2020–2022 | I | GDNF | AAV2 | IP in the Putamen | Recruiting | Ask Bio (formerly Brain Neurotherapy Bio, Inc.) | ||
| NCT01621581 | 2013–2022 | I | GDNF | AAV2 | IP in the Putamen | Completed | [114,115,116,117] National Institute of Neurological Disorders and Stroke | ||
| NCT00643890 | 2008–2010 | II | GAD | AAV2 | IP in the STN | Terminated (due to financial reasons) | [120,121,122,123] Neurologix, Inc. | ||
| NCT00195143 | 2003–2005 | I | GAD | AAV2 | IP in the STN | Completed | [121,122,123,124] Neurologix, Inc. | ||
| NCT01301573 | 2011–2012 | IV | GAD | AAV2 | IP in the STN | Terminated (due to financial reasons) | Neurologix, Inc. | ||
| NCT00252850 | 2005–2007 | I | NRTN | CERE-120 (AAV2) | IP in the Putamen | Completed | [118] Ceregene | ||
| NCT00985517 | 2009–2017 | I/II | NRTN | CERE-120 (AAV2) | IP in the Putamen | Completed | [119] Sangamo Therapeutics | ||
| NCT00400634 | 2006–2008 | II | NRTN | CERE-120 (AAV2) | IP in the Putamen | Completed | [118] Ceregene | ||
| NCT04127578 | 2020–2027 | I/II | GBA1 | AAV9 | IC in the CM | Recruiting | Prevail Therapeutics | ||
| Alzheimer | NCT03634007 | 2019–2023 | I | APOE2 | AAVrh.10h | IC in the CM | Recruiting | Lexeo Therapeutics | |
| NCT04133454 | 2019–2021 | I | hTERT | N.A. | IV and IT | The status was recruiting; currently unknown | Libella Gene Therapeutics | ||
| NCT00087789 | 2004–2010 | I | NGF | CERE-110 (AAV2) | IP in the NBM | Completed | Ceregene | ||
| NCT00876863 | 2008–2015 | II | NGF | CERE-110 (AAV2) | IP in the NBM | Completed | [135] Sangamo Therapeutics | ||
| NCT05040217 | 2021–2025 | I | BDNF | AAV2 | IP | Recruiting | [136,137] | ||
| Huntington’s disease | NCT04885114 | 2021–2024 | I | miHtt | AAV1 | IP in the Putamen and TH | Withdrawn (novel AAV that may enable IV delivery) | Voyager Therapeutics | |
| NCT04120493 | 2019–2026 | I/II | miHtt | AAV5 | IP in the striatum | Recruiting | [138] UniQure Biopharma B.V. | ||
| Spinal muscular atrophy | NCT03306277 | 2017–2019 | III | SMN | AAV9 | IV | Completed | [139] Novartis Gene Therapies | |
| NCT04042025 | 2020–2035 | IV | SMN | AAV9 | IV | Enrolling by invitation | Novartis Gene Therapies | ||
| NCT03837184 | 2019–2021 | III | SMN | AAV9 | IV | Completed | Novartis Gene Therapies | ||
| NCT02122952 | 2014–2017 | I | AVXS-101 | AAV9 | IV | Completed | [140,141] | ||
| NCT03461289 | 2018–2020 | III | SMN | AAV9 | IV | Completed | Novartis Gene Therapies | ||
| NCT03381729 | 2017–2024 | I | SMN | AAV9 | IT | Completed | Novartis Gene Therapies | ||
| Vision-related diseases | Leber’s congenital amaurosis | NCT02781480 | 2016–2018 | I/II | RPE65 | AAV2/5 | SR | Completed | MeiraGTx UK II |
| NCT01496040 | 2011–2014 | I/II | RPE65 | AAV2/4 | SR | Completed | Nantes University Hospital | ||
| NCT00516477 | 2007–2018 | I | RPE65 | AAV2 | SR | Completed | Spark Therapeutics | ||
| NCT00999609 | 2012–2029 | III | RPE65 | AAV2 | SR | Active, not recruiting | [142,143] Spark Therapeutics | ||
| NCT00821340 | 2016–2017 | I | RPE65 | AAV2 | SR | Completed | [144,145] Hadassah Medical Organization | ||
| NCT00481546 | 2007–2026 | I | RPE65 | AAV2 | SR | Active, not recruiting | [146,147] University of Pennsylvania | ||
| NCT02946879 | 2016–2023 | I/II | RPE65 | AAV2/5 | SR | Recruiting | MeiraGTx UK II | ||
| NCT00749957 | 2009–2017 | I/II | RPE65 | AAV2 | SR | Completed | [144,148] Applied Genetic Technologies Corp | ||
| NCT02161380 | 2014–2023 | I | ND4 | AAV2 | IVT | Active, not recruiting | [149] University of Miami | ||
| NCT02652767 | 2016–2019 | III | ND4 | AAV2/2 | IVT | Completed | [150] GenSight Biologics | ||
| NCT02652780 | 2016–2018 | III | ND4 | AAV2/2 | IVT | Completed | [150] GenSight Biologics | ||
| NCT03153293 | 2017–2025 | II/III | ND4 | AAV2 | IVT | Active, not recruiting | [151,152] | ||
| Retinitis pigmentosa | NCT01482195 | 2011–2019 | I | MERTK | AAV2 | SR | Completed | [153] King Khaled Eye Specialist Hospital | |
| NCT03116113 | 2017–2020 | III | BIIB112 (RPGR) | AAV8 | SR | Enrolling by invitation | [154] NightstaRx, Biogen Company | ||
| NCT03252847 | 2017–2020 | I/II | RPGR | AAV2/5 | SR | Completed | MeiraGTx UK II | ||
| NCT03326336 | 2018–2025 | I/II | GS030-DP | AAV2.7m8 | IVT | Recruiting | GenSight Biologics | ||
| NCT04919473 | 2019–2020 | I/II | vMCO-I | AAV2 | IVT | Completed | Nanoscope Therapeutics | ||
| NCT03328130 | 2017–2026 | I/II | PDE6B | AAV2/5 | SR | Recruiting | [155,156] Horama | ||
| NCT04945772 | 2021–2023 | II | vMCO-010 | AAV2 | IVT | Recruiting | Nanoscope Therapeutics | ||
| NCT04850118 | 2021–2029 | II/III | RPGR | AAV2 | SR | Not yet recruiting | Applied Genetic Technologies | ||
| NCT03316560 | 2018–2026 | I/II | RPGR | AAV2 | SR | Recruiting | Applied Genetic Technologies | ||
| NCT04312672 | 2019–2023 | I/II | RPGR | AAV2 | SR | Recruiting | MeiraGTx UK II | ||
| Retinitis pigmentosa/choroideremia | NCT03584165 | 2018–2027 | III | BIIB111 (REP1) and BIIB112 (RPGR) | AAV2 and AAV8 | SR | Enrolling by invitation | NightstaRx, Biogen Company | |
| Choroideremia | NCT02161380 | 2011–2017 | I/II | REP1 | AAV2 | SR | Active, not recruiting | [157,158,159,160] University of Oxford | |
| NCT02553135 | 2015–2018 | III | REP1 | AAV2 | SR | Enrolling by invitation | [161] University of Miami | ||
| NCT03507686 | 2018–2022 | III | BIIB111 (REP1) | AAV2 | SR | Enrolling by invitation | [161] NightstaRx, Biogen Company | ||
| NCT02077361 | 2015–2025 | III | REP1 | AAV2 | SR | Enrolling by invitation | [147,162] University of Alberta | ||
| NCT02671539 | 2016–2018 | III | REP1 | AAV2 | SR | Enrolling by invitation | [163] STZ eyetrial | ||
| NCT03496012 | 2017–2020 | III | BIIB111 (REP1) | AAV2 | SR | Enrolling by invitation | [161] NightstaRx, Biogen Company | ||
| NCT02341807 | 2015–2022 | I/II | REP1 | AAV2 | SR | Active, not recruiting | Spark Therapeutics | ||
| NCT02407678 | 2016–2021 | III | REP1 | AAV2 | SR | Enrolling by invitation | University of Oxford | ||
| Achromatopsia | NCT03758404 | 2019–2021 | I/II | CNGA3 | AAV2/8 | SR | Completed | MeiraGTx UK II | |
| NCT02935517 | 2017–2025 | I/II | CNGA3 | AAV2 | SR | Recruiting | [164] Applied Genetic Technologies Corp | ||
| NCT02599922 | 2016–2025 | I/II | hCNGB3 | AAV2 | SR | Recruiting | [165] Applied Genetic Technologies Corp | ||
| NCT03001310 | 2017–2019 | I/II | CNGB3 | AAV2/8 | SR | Completed | MeiraGTx UK II | ||
| NCT03278873 | 2017–2024 | I/II | CNGB3 & CNGA3 | AAV2/8 | SR | Active, not recruiting | MeiraGTx UK II | ||
| Retinal degeneration | NCT00643747 | 2007–2014 | I/II | RPE65 | AAV2/2 | SR | Completed | [145] University College, London | |
| Retinal dystrophy | NCT04516369 | 2020–2026 | III | RPE65 | AAV2 | SR | Active, not recruiting | Novartis Pharmaceuticals | |
| Retinoschisis | NCT02416622 | 2015–2023 | I/II | RS1 | AAV2 | IVT | Active, not recruiting | Applied Genetic Technologies | |
| Age-related macular degeneration | NCT03748784 | 2018–2022 | I | aflibercept | AAV.7m8 | IVT | Active, not recruiting | Adverum Biotechnologies | |
| NCT04645212 | 2020–2025 | IV | aflibercept | AAV.7m8 | IVT | Enrolling by invitation | Adverum Biotechnologies | ||
| NCT03066258 | 2017–2021 | I/II | RGX-314 (Ab against VEGF) | AAV8 | SR | Active, not recruiting | Regenxbio | ||
| NCT04832724 | 2021–2022 | II | RGX-314 | AAV8 | SR | Recruiting | Regenxbio | ||
| Diabetic macular edema/ diabetic retinopathy | NCT04418427 | 2020–2022 | II | aflibercept | AAV.7m8 | IVT | Active, not recruiting | Adverum Biotechnologies | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fajardo-Serrano, A.; Rico, A.J.; Roda, E.; Honrubia, A.; Arrieta, S.; Ariznabarreta, G.; Chocarro, J.; Lorenzo-Ramos, E.; Pejenaute, A.; Vázquez, A.; et al. Adeno-Associated Viral Vectors as Versatile Tools for Neurological Disorders: Focus on Delivery Routes and Therapeutic Perspectives. Biomedicines 2022, 10, 746. https://doi.org/10.3390/biomedicines10040746
Fajardo-Serrano A, Rico AJ, Roda E, Honrubia A, Arrieta S, Ariznabarreta G, Chocarro J, Lorenzo-Ramos E, Pejenaute A, Vázquez A, et al. Adeno-Associated Viral Vectors as Versatile Tools for Neurological Disorders: Focus on Delivery Routes and Therapeutic Perspectives. Biomedicines. 2022; 10(4):746. https://doi.org/10.3390/biomedicines10040746
Chicago/Turabian StyleFajardo-Serrano, Ana, Alberto J. Rico, Elvira Roda, Adriana Honrubia, Sandra Arrieta, Goiaz Ariznabarreta, Julia Chocarro, Elena Lorenzo-Ramos, Alvaro Pejenaute, Alfonso Vázquez, and et al. 2022. "Adeno-Associated Viral Vectors as Versatile Tools for Neurological Disorders: Focus on Delivery Routes and Therapeutic Perspectives" Biomedicines 10, no. 4: 746. https://doi.org/10.3390/biomedicines10040746
APA StyleFajardo-Serrano, A., Rico, A. J., Roda, E., Honrubia, A., Arrieta, S., Ariznabarreta, G., Chocarro, J., Lorenzo-Ramos, E., Pejenaute, A., Vázquez, A., & Lanciego, J. L. (2022). Adeno-Associated Viral Vectors as Versatile Tools for Neurological Disorders: Focus on Delivery Routes and Therapeutic Perspectives. Biomedicines, 10(4), 746. https://doi.org/10.3390/biomedicines10040746








