NLRP3 Inhibition Reduces rt-PA Induced Endothelial Dysfunction under Ischemic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Oxygen and Glucose Deprivation (OGD)
2.4. Treatment Regimes
2.5. xCELLigence Assay
2.6. Immunofluorescence Microscopy
2.7. Statistics
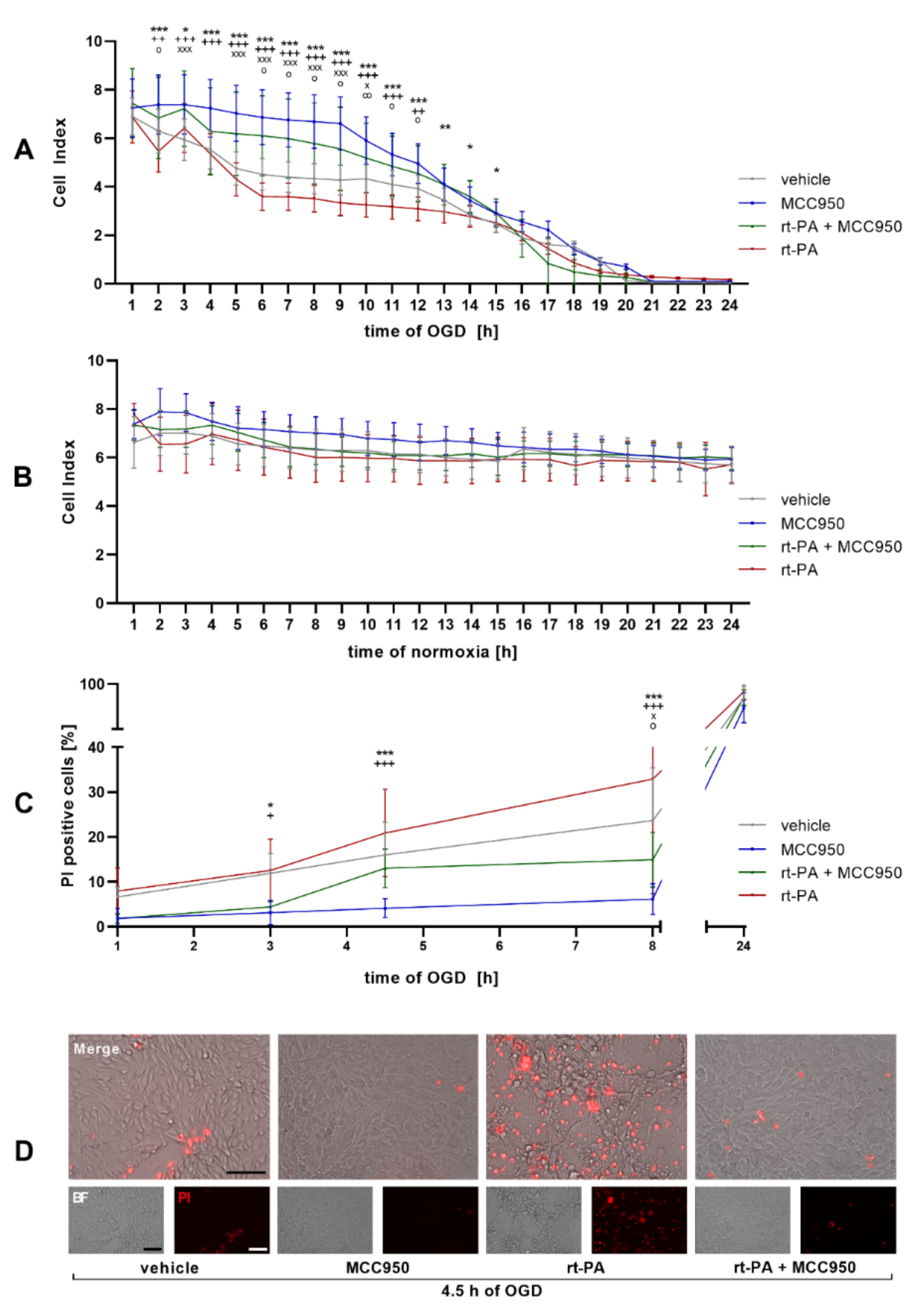
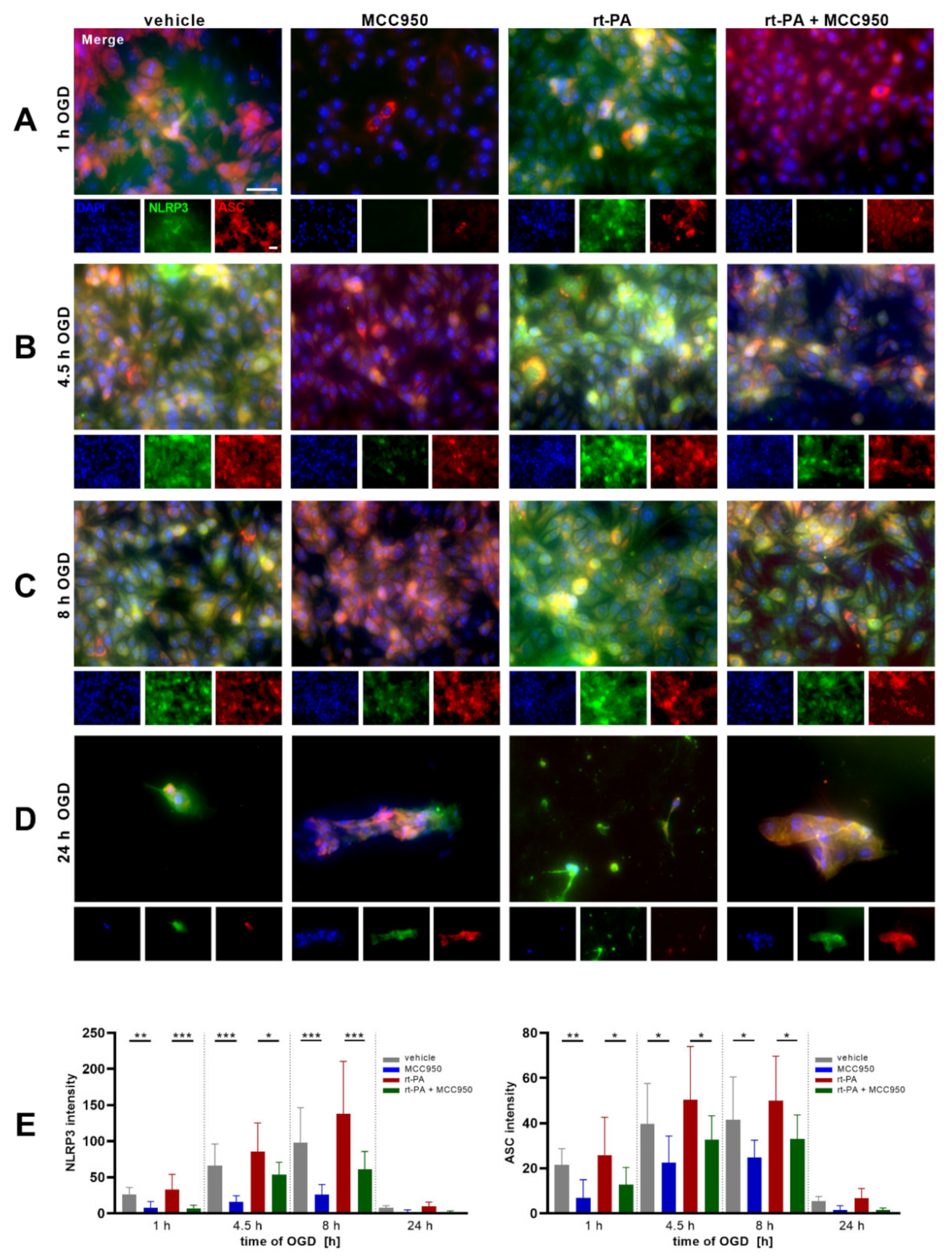
3. Results
NLRP3 Inhibition Improves Endothelial Barrier Function and EC Survival after rt-PA Administration under OGD
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Abbruscato, T.J.; Davis, T.P. Combination of Hypoxia/Aglycemia Compromises in Vitro Blood-Brain Barrier Integrity. J. Pharmacol. Exp. Ther. 1999, 289, 668–675. [Google Scholar] [PubMed]
- Del Zoppo, G.J.; Saver, J.L.; Jauch, E.C.; Adams, H.P. Expansion of the Time Window for Treatment of Acute Ischemic Stroke With Intravenous Tissue Plasminogen Activator: A Science Advisory From the American Heart Association/American Stroke Association. Stroke 2009, 40, 2945–2948. [Google Scholar] [CrossRef]
- Wang, X. Mechanisms of Hemorrhagic Transformation After Tissue Plasminogen Activator Reperfusion Therapy for Ischemic Stroke. Stroke 2004, 35, 2726–2730. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Xie, J.; Sun, S.; Li, H.; Li, T.; Jiang, C.; Chen, X.; Wang, J.; Le, A.; Wang, J.; et al. Hemorrhagic Transformation After Tissue Plasminogen Activator Treatment in Acute Ischemic Stroke. Cell. Mol. Neurobiol. 2020, 42, 621–646. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and Functions of Inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Schuhmann, M.K.; Kollikowski, A.M.; März, A.G.; Bieber, M.; Pham, M.; Stoll, G. Danger-Associated Molecular Patterns Are Locally Released during Occlusion in Hyper-Acute Stroke. Brain Behav. Immun. Health 2021, 15, 100270. [Google Scholar] [CrossRef]
- Abulafia, D.P.; de Rivero Vaccari, J.P.; Lozano, J.D.; Lotocki, G.; Keane, R.W.; Dietrich, W.D. Inhibition of the Inflammasome Complex Reduces the Inflammatory Response after Thromboembolic Stroke in Mice. J. Cereb. Blood Flow Metab. 2009, 29, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Agostini, L.; Martinon, F.; Burns, K.; McDermott, M.F.; Hawkins, P.N.; Tschopp, J. NALP3 Forms an IL-1β-Processing Inflammasome with Increased Activity in Muckle-Wells Autoinflammatory Disorder. Immunity 2004, 20, 319–325. [Google Scholar] [CrossRef]
- Alishahi, M.; Farzaneh, M.; Ghaedrahmati, F.; Nejabatdoust, A.; Sarkaki, A.; Khoshnam, S.E. NLRP3 Inflammasome in Ischemic Stroke: As Possible Therapeutic Target. Int. J. Stroke 2019, 14, 574–591. [Google Scholar] [CrossRef]
- Lu, A.; Magupalli, V.G.; Ruan, J.; Yin, Q.; Atianand, M.K.; Vos, M.R.; Schröder, G.F.; Fitzgerald, K.A.; Wu, H.; Egelman, E.H. Unified Polymerization Mechanism for the Assembly of ASC-Dependent Inflammasomes. Cell 2014, 156, 1193–1206. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Bellut, M.; Papp, L.; Bieber, M.; Kraft, P.; Stoll, G.; Schuhmann, M.K. NLPR3 Inflammasome Inhibition Alleviates Hypoxic Endothelial Cell Death in Vitro and Protects Blood–Brain Barrier Integrity in Murine Stroke. Cell Death Dis. 2021, 13, 20. [Google Scholar] [CrossRef]
- Franke, M.; Bieber, M.; Kraft, P.; Weber, A.N.R.; Stoll, G.; Schuhmann, M.K. The NLRP3 Inflammasome Drives Inflammation in Ischemia/Reperfusion Injury after Transient Middle Cerebral Artery Occlusion in Mice. Brain Behav. Immun. 2020, 92, 221–231. [Google Scholar] [CrossRef]
- Essig, F.; Kollikowski, A.M.; Müllges, W.; Stoll, G.; Haeusler, K.G.; Schuhmann, M.K.; Pham, M. Local Cerebral Recombinant Tissue Plasminogen Activator Concentrations During Acute Stroke. JAMA Neurol. 2021, 78, 615–617. [Google Scholar] [CrossRef]
- Bischoff, I.; Hornburger, M.C.; Mayer, B.A.; Beyerle, A.; Wegener, J.; Fürst, R. Pitfalls in Assessing Microvascular Endothelial Barrier Function: Impedance-Based Devices versus the Classic Macromolecular Tracer Assay. Sci. Rep. 2016, 6, 23671. [Google Scholar] [CrossRef] [Green Version]
- Stutz, A.; Horvath, G.L.; Monks, B.G.; Latz, E. ASC Speck Formation as a Readout for Inflammasome Activation. Methods Mol. Biol. Clifton NJ 2013, 1040, 91–101. [Google Scholar] [CrossRef]
- Emberson, J.; Lees, K.R.; Lyden, P.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Effect of Treatment Delay, Age, and Stroke Severity on the Effects of Intravenous Thrombolysis with Alteplase for Acute Ischaemic Stroke: A Meta-Analysis of Individual Patient Data from Randomised Trials. Lancet 2014, 384, 1929–1935. [Google Scholar] [CrossRef]
- Nowak, K.; Derbisz, J.; Jagiełła, J.; Pułyk, R.; Popiela, T.; Słowik, A. Time from Stroke Onset to Groin Puncture Affects Rate of Recanalisation after Mechanical Thrombectomy: A Real-Life Single Centre Experience. Neurol. Neurochir. Pol. 2020, 54, 156–160. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellut, M.; Raimondi, A.T.; Haarmann, A.; Zimmermann, L.; Stoll, G.; Schuhmann, M.K. NLRP3 Inhibition Reduces rt-PA Induced Endothelial Dysfunction under Ischemic Conditions. Biomedicines 2022, 10, 762. https://doi.org/10.3390/biomedicines10040762
Bellut M, Raimondi AT, Haarmann A, Zimmermann L, Stoll G, Schuhmann MK. NLRP3 Inhibition Reduces rt-PA Induced Endothelial Dysfunction under Ischemic Conditions. Biomedicines. 2022; 10(4):762. https://doi.org/10.3390/biomedicines10040762
Chicago/Turabian StyleBellut, Maximilian, Anthony T. Raimondi, Axel Haarmann, Lena Zimmermann, Guido Stoll, and Michael K. Schuhmann. 2022. "NLRP3 Inhibition Reduces rt-PA Induced Endothelial Dysfunction under Ischemic Conditions" Biomedicines 10, no. 4: 762. https://doi.org/10.3390/biomedicines10040762
APA StyleBellut, M., Raimondi, A. T., Haarmann, A., Zimmermann, L., Stoll, G., & Schuhmann, M. K. (2022). NLRP3 Inhibition Reduces rt-PA Induced Endothelial Dysfunction under Ischemic Conditions. Biomedicines, 10(4), 762. https://doi.org/10.3390/biomedicines10040762





