Blockade of TASK-1 Channel Improves the Efficacy of Levetiracetam in Chronically Epileptic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Chemicals
2.2. Generation of Chronically Epileptic Rats
2.3. Electrode Implantation and ML365 Infusion
2.4. Drug Trial Protocols
2.4.1. Experiment I
2.4.2. Experiment II
2.5. Western Blot
2.6. Immunohistochemistry
2.7. Data Analysis
3. Results
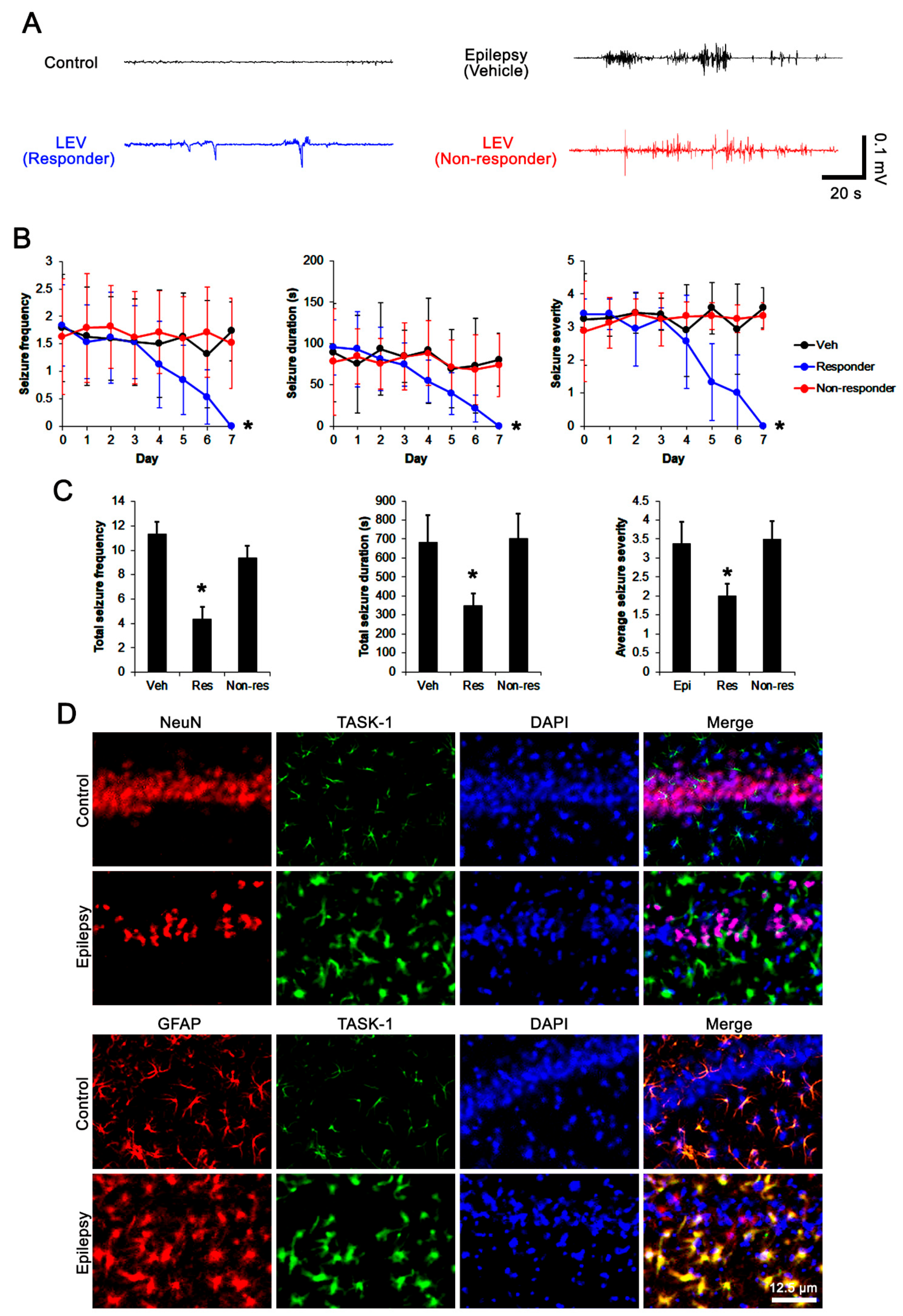
3.1. Effects of LEV on Spontaneous Epileptic Seizures
3.2. Effects of LEV on TASK-1 Expression in the Epileptic Hippocampus
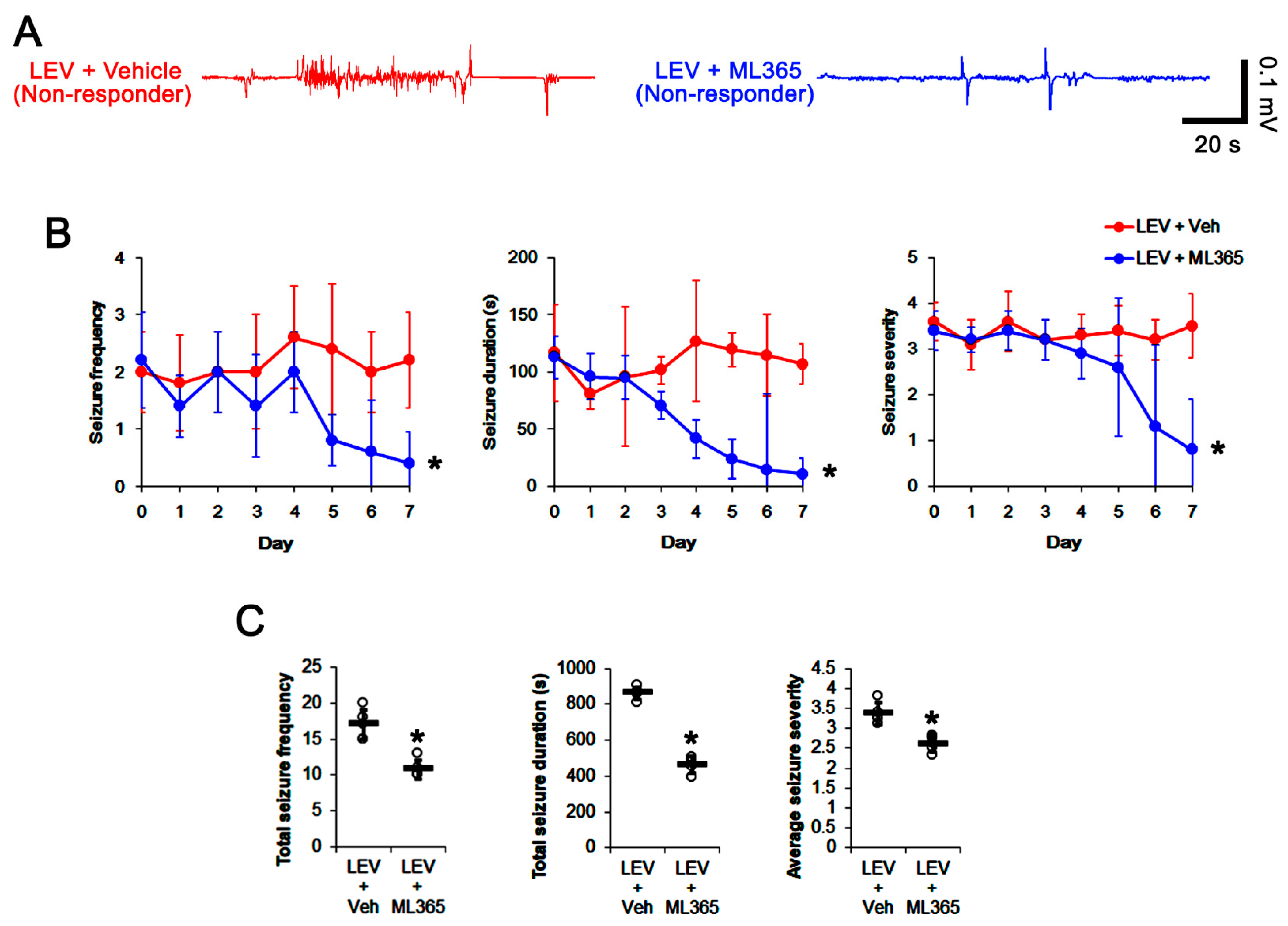
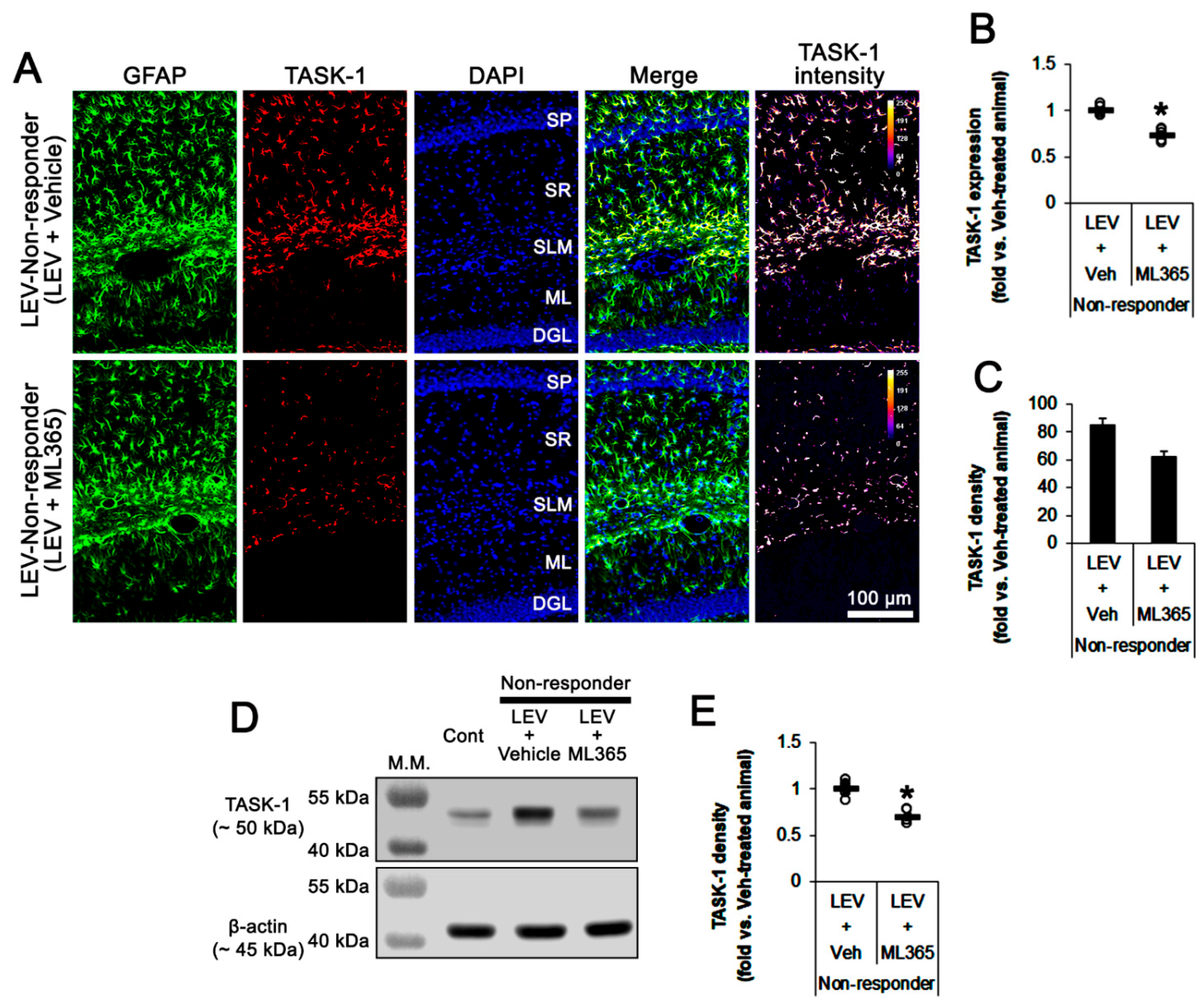
3.3. Effects of ML365 on the Spontaneous Seizures and TASK-1 Expression in Chronically Epileptic Rats
3.4. Effect of ML365 Co-Treatment on Refractory Seizures in Non-Responders to LEV
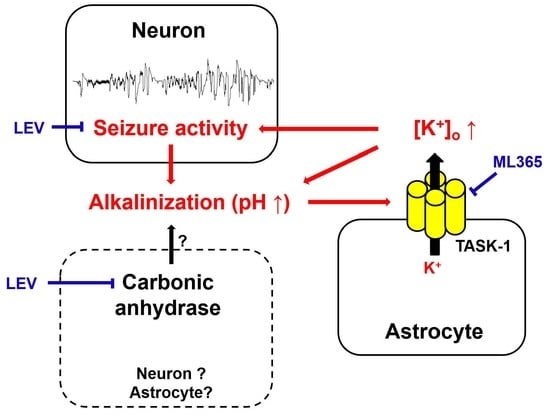
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menezes, L.F.S.; Sabiá Júnior, E.F.; Tibery, D.V.; Carneiro, L.D.A.; Schwartz, E.F. Epilepsy-Related Voltage-Gated Sodium Channelopathies: A Review. Front. Pharmacol. 2020, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Van Loo, K.M.J.; Becker, A.J. Transcriptional Regulation of Channelopathies in Genetic and Acquired Epilepsies. Front. Cell. Neurosci. 2020, 13, 587. [Google Scholar] [CrossRef] [PubMed]
- Dejakaisaya, H.; Kwan, P.; Jones, N.C. Astrocyte and glutamate involvement in the pathogenesis of epilepsy in Alzheimer’s disease. Epilepsia 2021, 62, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.H.; Perucca, P.; O’Brien, T.J.; Kwan, P.; Monif, M. Inflammation, ictogenesis, and epileptogenesis: An exploration through human disease. Epilepsia 2021, 62, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.Y.; Li, J.; Woodbury, D.M. Water and electrolyte contents, cell pH, and membrane potential of primary cultures of astrocytes from DBA, C57, and SW mice. Epilepsia 1992, 33, 393–401. [Google Scholar] [CrossRef]
- Xiong, Z.Q.; Saggau, P.; Stringer, J.L. Activity-dependent intracellular acidification correlates with the duration of seizure activity. J. Neurosci. 2000, 20, 1290–1296. [Google Scholar] [CrossRef]
- Xiong, Z.Q.; Stringer, J.L. Extracellular pH responses in CA1 and the dentate gyrus during electrical stimulation, seizure discharges, and spreading depression. J. Neurophysiol. 2000, 83, 3519–3524. [Google Scholar] [CrossRef]
- Pasternack, M.; Bountra, C.; Voipio, J.; Kaila, K. Influence of extracellular and intracellular pH on GABA-gated chloride conductance in crayfish muscle fibres. Neuroscience 1992, 47, 921–929. [Google Scholar] [CrossRef]
- Tang, C.M.; Dichter, M.; Morad, M. Modulation of the N-methyl-D-aspartate channel by extracellular H+. Proc. Natl. Acad. Sci. USA 1990, 87, 6445–6449. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Cull-Candy, S.G. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature 1990, 345, 347–350. [Google Scholar] [CrossRef]
- Gabriel, S.; Kivi, A.; Kovacs, R.; Lehmann, T.N.; Lanksch, W.R.; Meencke, H.J.; Heinemann, U. Effects of barium on stimulus-induced changes in [K+]o and field potentials in dentate gyrus and area CA1 of human epileptic hippocampus. Neurosci. Lett. 1998, 249, 91–94. [Google Scholar] [CrossRef]
- Gabriel, S.; Eilers, A.; Kivi, A.; Kovacs, R.; Schulze, K.; Lehmann, T.N.; Heinemann, U. Effects of barium on stimulus induced changes in extracellular potassium concentration in area CA1 of hippocampal slices from normal and pilocarpine-treated epileptic rats. Neurosci. Lett. 1998, 242, 9–12. [Google Scholar] [CrossRef]
- Hinterkeuser, S.; Schröder, W.; Hager, G.; Seifert, G.; Blümcke, I.; Elger, C.E.; Schramm, J.; Steinhäuser, C. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur. J. Neurosci. 2000, 12, 2087–2096. [Google Scholar] [CrossRef]
- Duprat, F.; Lesage, F.; Fink, M.; Reyes, R.; Heurteaux, C.; Lazdunski, M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997, 16, 5464–5471. [Google Scholar] [CrossRef]
- Niemeyer, M.I.; González-Nilo, F.D.; Zúñiga, L.; González, W.; Cid, L.P.; Sepúlveda, F.V. Gating of two-pore domain K+ channels by extracellular pH. Biochem. Soc. Trans. 2006, 34, 899–902. [Google Scholar] [CrossRef][Green Version]
- Kim, J.E.; Kwak, S.E.; Choi, S.Y.; Kang, T.C. Region-specific alterations in astroglial TWIK-related acid-sensitive K+-1 channel immunoreactivity in the rat hippocampal complex following pilocarpine-induced status epilepticus. J. Comp. Neurol. 2008, 510, 463–474. [Google Scholar] [CrossRef]
- Kim, J.E.; Yeo, S.I.; Ryu, H.J.; Chung, C.K.; Kim, M.J.; Kang, T.C. Changes in TWIK-related acid sensitive K+-1 and -3 channel expressions from neurons to glia in the hippocampus of temporal lobe epilepsy patients and experimental animal model. Neurochem. Res. 2011, 36, 2155–2168. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, J.E.; Kwak, S.E.; Choi, H.C.; Song, H.K.; Kim, Y.I.; Choi, S.Y.; Kang, T.C. Up-regulated astroglial TWIK-related acid-sensitive K+ channel-1 (TASK-1) in the hippocampus of seizure-sensitive gerbils: A target of anti-epileptic drugs. Brain Res. 2007, 1185, 346–358. [Google Scholar] [CrossRef]
- Juvale, I.I.A.; Che Has, A.T. Possible interplay between the theories of pharmacoresistant epilepsy. Eur. J. Neurosci. 2021, 53, 1998–2026. [Google Scholar] [CrossRef]
- Lynch, J.M.; Tate, S.K.; Kinirons, P.; Weale, M.E.; Cavalleri, G.L.; Depondt, C.; Murphy, K.; O’Rourke, D.; Doherty, C.P.; Shianna, K.V.; et al. No major role of common SV2A variation for predisposition or levetiracetam response in epilepsy. Epilepsy Res. 2009, 83, 44–51. [Google Scholar] [CrossRef]
- Glien, M.; Brandt, C.; Potschka, H.; Löscher, W. Effects of the novel antiepileptic drug levetiracetam on spontaneous recurrent seizures in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia 2002, 43, 350–357. [Google Scholar] [CrossRef]
- Ko, A.R.; Kang, T.C. Blockade of endothelin B receptor improves the efficacy of levetiracetam in chronic epileptic rats. Seizure 2015, 31, 133–140. [Google Scholar] [CrossRef][Green Version]
- Leniger, T.; Thöne, J.; Bonnet, U.; Hufnagel, A.; Bingmann, D.; Wiemann, M. Levetiracetam inhibits Na+-dependent Cl-/HCO3- exchange of adult hippocampal CA3 neurons from guinea-pigs. Br. J. Pharmacol. 2004, 142, 1073–1080. [Google Scholar] [CrossRef][Green Version]
- Bonnet, U.; Bingmann, D.; Speckmann, E.J.; Wiemann, M. Levetiracetam mediates subtle pH-shifts in adult human neocortical pyramidal cells via an inhibition of the bicarbonate-driven neuronal pH-regulation—Implications for excitability and plasticity modulation. Brain Res. 2019, 1710, 146–156. [Google Scholar] [CrossRef]
- Aribi, A.M.; Stringer, J.L. Effects of antiepileptic drugs on extracellular pH regulation in the hippocampal CA1 region in vivo. Epilepsy Res. 2002, 49, 143–151. [Google Scholar] [CrossRef]
- Koç, E.R.; Erken, G.; Bilen, C.; Sackes, Z.; Gencer, N. The effects of anti-epileptic drugs on human erythrocyte carbonic anhydrase I and II isozymes. Arch. Physiol. Biochem. 2014, 120, 131–135. [Google Scholar] [CrossRef]
- Theparambil, S.M.; Hosford, P.S.; Ruminot, I.; Kopach, O.; Reynolds, J.R.; Sandoval, P.Y.; Rusakov, D.A.; Barros, L.F.; Gourine, A.V. Astrocytes regulate brain extracellular pH via a neuronal activity-dependent bicarbonate shuttle. Nat. Commun. 2020, 11, 5073. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, D.S.; Park, H.; Kang, T.C. Src/CK2/PTEN-Mediated GluN2B and CREB Dephosphorylations Regulate the Responsiveness to AMPA Receptor Antagonists in Chronic Epilepsy Rats. Int. J. Mol. Sci. 2020, 21, 9633. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, D.S.; Park, H.; Kim, T.H.; Kang, T.C. Inhibition of AKT/GSK3β/CREB Pathway Improves the Responsiveness to AMPA Receptor Antagonists by Regulating GRIA1 Surface Expression in Chronic Epilepsy Rats. Biomedicines 2021, 9, 425. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Flaherty, D.P.; Simpson, D.S.; Miller, M.; Maki, B.E.; Zou, B.; Shi, J.; Wu, M.; McManus, O.B.; Aubé, J.; Li, M.; et al. Potent and selective inhibitors of the TASK-1 potassium channel through chemical optimization of a bis-amide scaffold. Bioorg. Med. Chem. Lett. 2014, 24, 3968–3973. [Google Scholar] [CrossRef] [PubMed]
- Kindler, C.H.; Pietruck, C.; Yost, C.S.; Sampson, E.R.; Gray, A.T. Localization of the tandem pore domain K+ channel TASK-1 in the rat central nervous system. Brain Res. Mol. Brain Res. 2000, 80, 99–108. [Google Scholar] [CrossRef]
- Grimminger, T.; Pernhorst, K.; Surges, R.; Niehusmann, P.; Priebe, L.; von Lehe, M.; Hoffmann, P.; Cichon, S.; Schoch, S.; Becker, A.J. Levetiracetam resistance: Synaptic signatures & corresponding promoter SNPs in epileptic hippocampi. Neurobiol. Dis. 2013, 60, 115–125. [Google Scholar] [PubMed]
- Chesler, M. The regulation and modulation of pH in the nervous system. Prog. Neurobiol. 1990, 34, 401–427. [Google Scholar] [CrossRef]
- Xiong, Z.Q.; Stringer, J.L. Regulation of extracellular pH in the developing hippocampus. Brain Res. Dev. Brain Res. 2000, 122, 113–117. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, X.; Zhou, M.; Chen, H. Acid-sensitive TWIK and TASK two-pore domain potassium channels change ion selectivity and become permeable to sodium in extracellular acidification. J. Biol. Chem. 2012, 287, 37145–37153. [Google Scholar] [CrossRef]
- Kim, J.E.; Kang, T.C. CDDO-Me Attenuates Astroglial Autophagy via Nrf2-, ERK1/2-SP1- and Src-CK2-PTEN-PI3K/AKT-Mediated Signaling Pathways in the Hippocampus of Chronic Epilepsy Rats. Antioxidants 2021, 10, 655. [Google Scholar] [CrossRef]
- Binder, D.K.; Yao, X.; Zador, Z.; Sick, T.J.; Verkman, A.S.; Manley, G.T. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia 2006, 53, 631–636. [Google Scholar] [CrossRef]
- Madeja, M.; Margineanu, D.G.; Gorji, A.; Siep, E.; Boerrigter, P.; Klitgaard, H.; Speckmann, E.J. Reduction of voltage-operated potassium currents by levetiracetam: A novel antiepileptic mechanism of action? Neuropharmacology 2003, 45, 661–671. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, C.Y.; Tsai, T.S.; Liou, H.H. PKA-mediated phosphorylation is a novel mechanism for levetiracetam, an antiepileptic drug, activating ROMK1 channels. Biochem. Pharmacol. 2008, 76, 225–235. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, G.; Xie, M.; Zhang, X.; Schools, G.P.; Ma, L.; Kimelberg, H.K.; Chen, H. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J. Neurosci. 2009, 29, 8551–8564. [Google Scholar] [CrossRef]
- Aller, M.I.; Wisden, W. Changes in expression of some two-pore domain potassium channel genes (KCNK) in selected brain regions of developing mice. Neuroscience 2008, 151, 1154–1172. [Google Scholar] [CrossRef]
- Cammarota, M.; Losi, G.; Chiavegato, A.; Zonta, M.; Carmignoto, G. Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. J. Physiol. 2013, 591, 807–822. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, D.S.; Park, H.; Kim, T.H.; Kang, T.C. AMPA receptor antagonists facilitate NEDD4-2-mediated GRIA1 ubiquitination by regulating PP2B-ERK1/2-SGK1 pathway in chronic epilepsy rats. Biomedicines 2021, 9, 1069. [Google Scholar] [CrossRef]
- Rinné, S.; Kiper, A.K.; Schmidt, C.; Ortiz-Bonnin, B.; Zwiener, S.; Seebohm, G.; Decher, N. Stress-Kinase Regulation of TASK-1 and TASK-3. Cell Physiol. Biochem. 2017, 44, 1024–1037. [Google Scholar] [CrossRef]




| Antigen | Host | Manufacturer (Catalog Number) | Dilution |
|---|---|---|---|
| Glia fibrillary acidic protein (GFAP) | Mouse | Millipore (MAB3402) | 1:4000 (IH) |
| Neuronal nuclear antigen (NeuN) | Guinea pig | Millipore (ABN90P) | 1:2000 (IH) |
| TASK-1 | Rabbit | Millipore (AB5250) | 1:50 (IH) 1:200 (WB) |
| β-actin | Mouse | Sigma (A5316) | 1:5000 (WB) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-E.; Kang, T.-C. Blockade of TASK-1 Channel Improves the Efficacy of Levetiracetam in Chronically Epileptic Rats. Biomedicines 2022, 10, 787. https://doi.org/10.3390/biomedicines10040787
Kim J-E, Kang T-C. Blockade of TASK-1 Channel Improves the Efficacy of Levetiracetam in Chronically Epileptic Rats. Biomedicines. 2022; 10(4):787. https://doi.org/10.3390/biomedicines10040787
Chicago/Turabian StyleKim, Ji-Eun, and Tae-Cheon Kang. 2022. "Blockade of TASK-1 Channel Improves the Efficacy of Levetiracetam in Chronically Epileptic Rats" Biomedicines 10, no. 4: 787. https://doi.org/10.3390/biomedicines10040787
APA StyleKim, J.-E., & Kang, T.-C. (2022). Blockade of TASK-1 Channel Improves the Efficacy of Levetiracetam in Chronically Epileptic Rats. Biomedicines, 10(4), 787. https://doi.org/10.3390/biomedicines10040787