Reward System Dysfunction and the Motoric-Cognitive Risk Syndrome in Older Persons
Abstract
:1. Introduction
2. Reward System in Humans and in Older Persons
3. The Contribution of Reward System to the Motoric Cognitive
3.1. Risk Syndrome in Older Persons
3.2. Reward System and Depressions in Older Persons
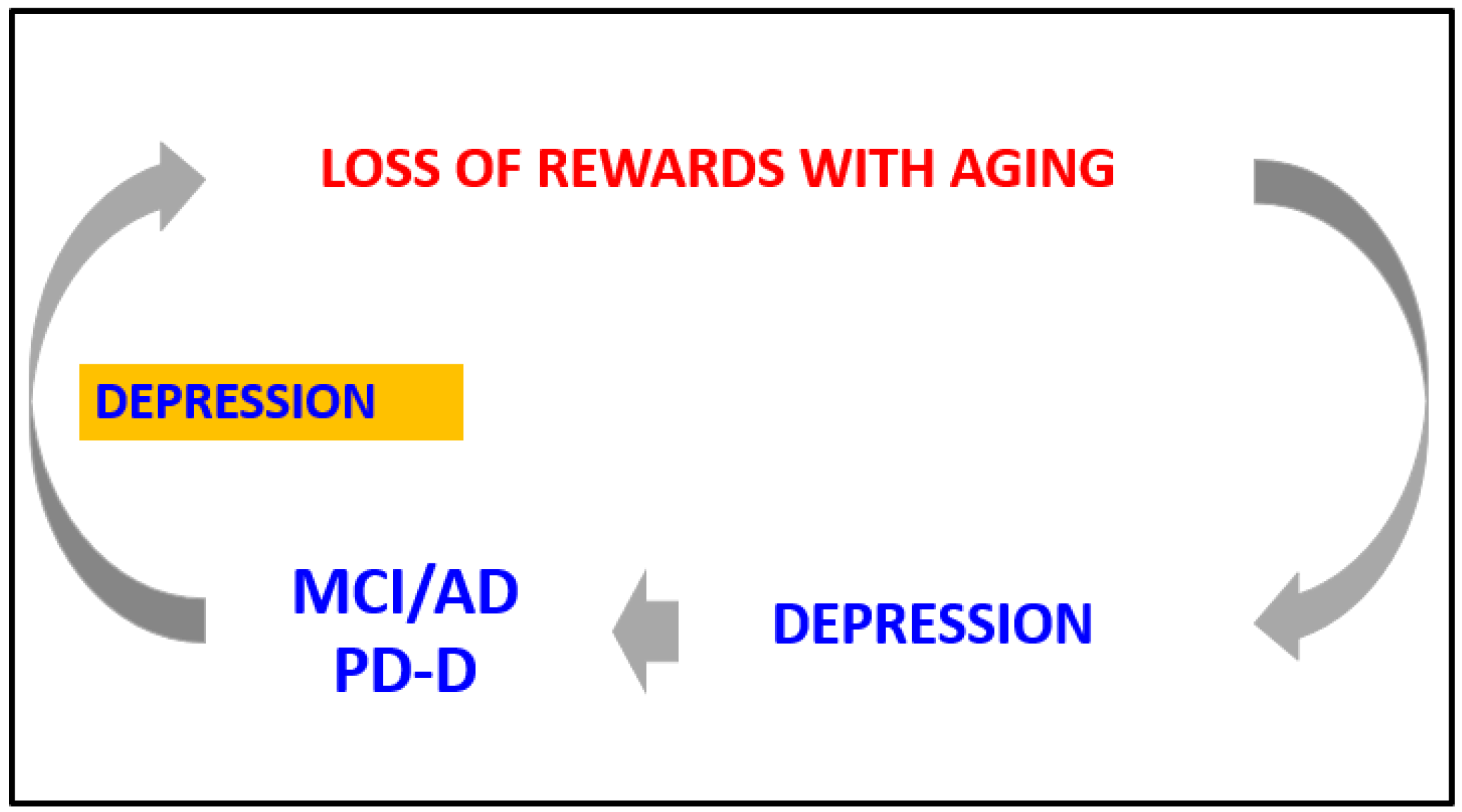
3.3. The Loop between the Reward System, Depression, and Mild Cognitive Impairment in Older Persons
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verghese, J.; Annweiler, C.; Ayers, E.; Barzilai, N.; Beauchet, O.; Bennett, D.A.; Bridenbaugh, S.A.; Buchman, A.S.; Callisaya, M.L.; Camicioli, R.; et al. Motoric cognitive risk syndrome: Multicountry prevalence and dementia risk. Neurology 2014, 83, 718–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prev-alence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Verghese, J.; Wang, C.; Lipton, R.B.; Holtzer, R. Motoric Cognitive Risk Syndrome and the Risk of Dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 68, 412–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semba, R.D.; Tian, Q.; Carlson, M.C.; Xue, Q.-L.; Ferrucci, L. Motoric cognitive risk syndrome: Integration of two early harbingers of dementia in older adults. Ageing Res. Rev. 2020, 58, 101022. [Google Scholar] [CrossRef] [PubMed]
- Meiner, Z.; Ayers, E.; Verghese, J. Motoric Cognitive Risk Syndrome: A Risk Factor for Cognitive Impairment and Dementia in Different Populations. Ann. Geriatr. Med. Res. 2020, 24, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Quan, M.; Xun, P.; Chen, C.; Wen, J.; Wang, Y.; Wang, R.; Chen, P.; He, K. Walking pace and the risk of cognitive decline and dementia in elderly populations: A meta-analysis of prospective cohort studies. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 266–270. [Google Scholar] [CrossRef] [Green Version]
- WHO Team. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines; World Health Organization: Geneva, Switzerland, 2019.
- Schultz, W. Neuronal Reward and Decision Signals: From Theories to Data. Physiol. Rev. 2015, 95, 853–951. [Google Scholar] [CrossRef]
- Schultz, W. Predictive Reward Signal of Dopamine Neurons. J. Neurophysiol. 1998, 80, 1–27. [Google Scholar] [CrossRef]
- Schultz, W. Behavioral dopamine signals. Trends Neurosci. 2007, 30, 203–210. [Google Scholar] [CrossRef]
- Christoffel, D.J.; Walsh, J.J.; Heifets, B.D.; Hoerbelt, P.; Neuner, S.; Sun, G.; Ravikumar, V.K.; Wu, H.; Halpern, C.H.; Malenka, R.C. In-put-specific modulation of murine nucleus accumbens differentially regulates hedonic feeding. Nat. Commun. 2021, 12, 2135. [Google Scholar] [CrossRef]
- Chinta, S.J.; Andersen, J.K. Dopaminergic neurons. Int. J. Biochem. Cell Biol. 2005, 37, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Prensa, L.; Giménez-Amaya, J.M.; Parent, A.; Bernacer, J.; Cebrián, C. The nigrostriatal pathway: Axonal collateralization and compartmental specificity. J. Neural. Transm. Suppl. 2009, 73, 49–58. [Google Scholar] [CrossRef]
- Baik, J.H. Dopamine signaling in reward-related behaviors. Front. Neural. Circuits 2013, 7, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorelova, N.; Mulholland, P.J.; Chandler, L.J.; Seamans, J.K. The Glutamatergic Component of the Mesocortical Pathway Emanating from Different Subregions of the Ventral Midbrain. Cereb. Cortex 2011, 22, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Korf, H.-W.; Møller, M. Arcuate nucleus, median eminence, and hypophysial pars tuberalis. Handb. Clin. Neurol. 2021, 180, 227–251. [Google Scholar] [CrossRef]
- Gutnick, A.; Levkowitz, G. The Neurohypophysis: Fishing for New Insights. J. Neuroendocr. 2012, 24, 973–974. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors—IUPHAR Review 13. Br. J. Pharmacol. 2014, 172, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, J.-M.; Gainetdinov, R.R. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [Green Version]
- Neve, K.A.; Seamans, J.K.; Trantham-Davidson, H. Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 2004, 24, 165–205. [Google Scholar] [CrossRef]
- Dreher, J.-C.; Kohn, P.; Kolachana, B.; Weinberger, D.R.; Berman, K.F. Variation in dopamine genes influences responsivity of the human reward system. Proc. Natl. Acad. Sci. USA 2009, 106, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Richfield, E.; Penney, J.; Young, A. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience 1989, 30, 767–777. [Google Scholar] [CrossRef]
- Chesselet, M.-F. Presynaptic regulation of neurotransmitter release in the brain: Facts and hypothesis. Neuroscience 1984, 12, 347–375. [Google Scholar] [CrossRef]
- Zhang, L.; Doyon, W.M.; Clark, J.J.; Phillips, P.; Dani, J.A. Controls of Tonic and Phasic Dopamine Transmission in the Dorsal and Ventral Striatum. Mol. Pharmacol. 2009, 76, 396–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, W. Dopamine reward prediction error coding. Dialog. Clin. Neurosci. 2016, 18, 23–32. [Google Scholar]
- Kaasinen, V.; Vilkman, H.; Hietala, J.; Någren, K.; Helenius, H.; Olsson, H.; Farde, L.; Rinne, J. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000, 21, 683–688. [Google Scholar] [CrossRef]
- Volkow, N.D.; Logan, J.; Fowler, J.S.; Wang, G.J.; Gur, R.C.; Wong, C.; Felder, C.; Gatley, S.J.; Ding, Y.S.; Hitzemann, R.; et al. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am. J. Psychiatry 2000, 157, 75–80. [Google Scholar] [CrossRef]
- Reeves, S.; Bench, C.; Howard, R. Ageing and the nigrostriatal dopaminergic system. Int. J. Geriatr. Psychiatry 2002, 17, 359–370. [Google Scholar] [CrossRef]
- Branch, S.Y.; Chen, C.; Sharma, R.; Lechleiter, J.D.; Li, S.; Beckstead, M.J. Dopaminergic Neurons Exhibit an Age-Dependent Decline in Electrophysiological Parameters in the MitoPark Mouse Model of Parkinson’s Disease. J. Neurosci. 2016, 36, 4026–4037. [Google Scholar] [CrossRef] [Green Version]
- Berry, A.S.; Shah, V.D.; Baker, S.L.; Vogel, J.W.; O’Neil, J.P.; Janabi, M.; Schwimmer, H.D.; Marks, S.M.; Jagust, W.J. Aging Affects Dopaminergic Neural Mechanisms of Cognitive Flexibility. J. Neurosci. 2016, 36, 12559–12569. [Google Scholar] [CrossRef]
- Ma, S.Y.; Röytt, M.; Collan, Y.; Rinne, J.O. Unbiased morphometrical measurements show loss of pigmented nigral neurones with ageing. Neuropathol. Appl. Neurobiol. 1999, 25, 394–399. [Google Scholar] [CrossRef]
- Buchman, A.S.; Shulman, J.M.; Nag, S.; Leurgans, S.E.; Arnold, S.E.; Morris, M.C.; Schneider, J.A.; Bennett, D.A. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann. Neurol. 2011, 71, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, J.; Christian, B.T.; Dunigan, K.A.; Shi, B.; Narayanan, T.K.; Satter, M.; Mantil, J. Brain imaging of 18F-fallypride in normal volunteers: Blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopa-mine D-2/D-3 receptors. Synapse 2002, 46, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Bäckman, L.; Lindenberger, U.; Li, S.C.; Nyberg, L. Linking cognitive aging to alterations in dopamine neurotransmitter function-ing: Recent data and future avenues. Neurosci. Biobehav. Rev. 2010, 34, 670–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Ding, Y.S.; Fowler, J.S.; Wang, G.J.; Logan, J.; Gatley, S.J.; Hitzemann, R.; Smith, G.; Fields, S.D.; Gur, R. Dopamine trans-porters decrease with age. J. Nucl. Med. 1996, 37, 554–559. [Google Scholar] [PubMed]
- Ishibashi, K.; Ishii, K.; Oda, K.; Kawasaki, K.; Mizusawa, H.; Ishiwata, K. Regional analysis of age-related decline in dopamine transporters and dopamine D2-like receptors in human striatum. Synapse 2008, 63, 282–290. [Google Scholar] [CrossRef]
- Karrer, T.M.; Josef, A.K.; Mata, R.; Morris, E.D.; Samanez-Larkin, G.R. Reduced dopamine receptors and transporters but not syn-thesis capacity in normal aging adults: A meta-analysis. Neurobiol. Aging 2017, 57, 36–46. [Google Scholar] [CrossRef]
- Dreher, J.C.; Meyer-Lindenberg, A.; Kohn, P.; Berman, K.F. Age-related changes in midbrain dopaminergic regulation of the hu-man reward system. Proc. Natl. Acad. Sci. USA 2008, 105, 15106–15111. [Google Scholar] [CrossRef] [Green Version]
- Shtilerman, M.D.; Ding, T.T.; Lansbury, P.T., Jr. Molecular crowding accelerates fibrillization of alpha-synuclein: Could an increase in the cytoplasmic protein concentration induce Parkinson’s disease? Biochemistry 2002, 41, 3855–3860. [Google Scholar] [CrossRef]
- Uversky, V.N.; Cooper, M.E.; Bower, K.S.; Li, J.; Fink, A.L. Accelerated alpha-synuclein fibrillation in crowded milieu. FEBS Lett. 2002, 515, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Zucca, F.A.; Vanna, R.; Cupaioli, F.A.; Bellei, C.; De Palma, A.; Di Silvestre, D.; Mauri, P.; Grassi, S.; Prinetti, A.; Casella, L.; et al. Neuromelanin organelles are specialized autolysosomes that accumulate undegraded proteins and lipids in aging human brain and are likely involved in Parkinson’s disease. Npj Park. Dis. 2018, 4, 1–23. [Google Scholar] [CrossRef]
- Vallone, D.; Picetti, R.; Borrelli, E. Structure and function of dopamine receptors. Neurosci. Biobehav. Rev. 2000, 24, 125–132. [Google Scholar] [CrossRef]
- Cooper, S.; Robison, A.; Mazei-Robison, M.S. Reward Circuitry in Addiction. Neurotherapeutics 2017, 14, 687–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavăl, D. A Dopamine Hypothesis of Autism Spectrum Disorder. Dev. Neurosci. 2017, 39, 355–360. [Google Scholar] [CrossRef]
- Soares-Cunha, C.; de Vasconcelos, N.A.P.; Coimbra, B.; Domingues, A.V.; Silva, J.M.; Loureiro-Campos, E.; Gaspar, R.; Sotiropoulos, I.; Sousa, N.; Rodrigues, A.J. Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol. Psychiatry 2020, 25, 3241–3255. [Google Scholar] [CrossRef] [Green Version]
- Chau, B.K.H.; Jarvis, H.; Law, C.-K.; Chong, T. Dopamine and reward: A view from the prefrontal cortex. Behav. Pharmacol. 2018, 29, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.S.; Muhammed, K.; Baig, F.; Kelly, M.; Saleh, Y.; Sarangmat, N.; Okai, D.; Hu, M.; Manohar, S.; Husain, M. Dopamine and re-ward hypersensitivity in Parkinson’s disease with impulse control disorder. Brain 2020, 143, 2502–2518. [Google Scholar] [CrossRef]
- Borra, E.; Luppino, G. Large-scale temporo–parieto–frontal networks for motor and cognitive motor functions in the primate brain. Cortex 2018, 118, 19–37. [Google Scholar] [CrossRef]
- Mendoza, G.; Merchant, H. Motor system evolution and the emergence of high cognitive functions. Prog. Neurobiol. 2014, 122, 73–93. [Google Scholar] [CrossRef]
- Ramsey, R.; Kaplan, D.M.; Cross, E.S. Watch and Learn: The Cognitive Neuroscience of Learning from Others’ Actions. Trends Neurosci. 2021, 44, 478–491. [Google Scholar] [CrossRef]
- Hosp, J.A.; Pekanovic, A.; Rioult-Pedotti, M.S.; Luft, A.R. Dopaminergic projections from midbrain to primary motor cortex medi-ate motor skill learning. J. Neurosci. 2011, 31, 2481–2487. [Google Scholar] [CrossRef] [Green Version]
- Wickens, J.R.; Reynolds, J.N.; Hyland, B.I. Neural mechanisms of reward-related motor learning. Curr. Opin. Neurobiol. 2003, 13, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.K.; Tanaka, S.; Okazaki, S.; Watanabe, K.; Sadato, N. Social Rewards Enhance Offline Improvements in Motor Skill. PLoS ONE 2012, 7, e48174. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.; Nemeroff, C.B. The Role of Dopamine in the Pathophysiology of Depression. Arch. Gen. Psychiatry 2007, 64, 327–337. [Google Scholar] [CrossRef]
- Ferrari, P.; Gerbella, M.; Coudé, G.; Rozzi, S. Two different mirror neuron networks: The sensorimotor (hand) and limbic (face) pathways. Neuroscience 2017, 358, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, M.C.; Greuel, A.; Tahmasian, M.; Schwartz, F.; Stürmer, S.; Maier, F.; Hammes, J.; Tittgemeyer, M.; Timmermann, L.; Van Eimeren, T.; et al. Network degeneration in Parkinson’s disease: Multimodal imaging of nigrostriato-cortical dysfunction. Brain 2020, 143, 944–959. [Google Scholar] [CrossRef] [PubMed]
- Tsvetanov, K.A.; Henson, R.N.; Tyler, L.K.; Razi, A.; Geerligs, L.; Ham, T.E.; Rowe, J.B.; Cambridge Centre for Ageing and Neuroscience. Extrinsic and Intrinsic Brain Network Connectivity Maintains Cognition across the Lifespan Despite Accelerated Decay of Regional Brain Activation. J. Neurosci. 2016, 36, 3115–3126. [Google Scholar] [CrossRef] [Green Version]
- Tsvetanov, K.A.; Ye, Z.; Hughes, L.; Samu, D.; Treder, M.S.; Wolpe, N.; Tyler, L.K.; Rowe, J.B. Cambridge Centre for Ageing and Neuroscience. Activity and Connectivity Differences Underlying Inhibitory Control across the Adult Life Span. J. Neurosci. 2018, 38, 7887–7900. [Google Scholar] [CrossRef] [Green Version]
- Fischer, F.U.; Wolf, D.; Tüscher, O.; Fellgiebel, A. Structural Network Efficiency Predicts Resilience to Cognitive Decline in Elderly at Risk for Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 637002. [Google Scholar] [CrossRef]
- Lauretani, F.; Ruffini, L.; Scarlattei, M.; Maggio, M. Relationship between comprehensive geriatric assessment and amyloid PET in older persons with MCI. BMC Geriatr. 2020, 20, 337. [Google Scholar] [CrossRef]
- Lopes Alves, I.; Collij, L.E.; Altomare, D.; Frisoni, G.B.; Saint-Aubert, L.; Payoux, P.; Kivipelto, M.; Jessen, F.; Drzezga, A.; Leeuwis, A.; et al. AMYPAD Consortium. Quantitative amyloid PET in Alz-heimer’s disease: The AMYPAD prognostic and natural history study. Alzheimers Dement. 2020, 16, 750–758. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Hachinski, V. Preludes to brain failure: Executive dysfunction and gait disturbances. Neurol. Sci. 2013, 35, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Allali, G.; Annweiler, C.; Verghese, J. Association of Motoric Cognitive Risk Syndrome with Brain Volumes: Results from the GAIT Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1081–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumen, H.M.; Allali, G.; Beauchet, O.; Lipton, R.B.; Verghese, J. A Gray Matter Volume Covariance Network Associated with the Motoric Cognitive Risk Syndrome: A Multicohort MRI Study. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 74, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, H.; Allali, G.; Launay, C.P.; Barden, J.; Szturm, T.; Liu-Ambrose, T.; Chester, V.L.; Wong, C.H.; Beauchet, O. Canadian Gait Consortium. Motoric cognitive risk syndrome, incident cognitive impairment and morphological brain abnormalities: Sys-tematic review and meta-analysis. Maturitas 2019, 123, 45–54. [Google Scholar] [CrossRef]
- Dohrn, I.-M.; Papenberg, G.; Winkler, E.; Welmer, A.-K. Impact of dopamine-related genetic variants on physical activity in old age—A cohort study. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Bzdok, D.; Dunbar, R.I. The Neurobiology of Social Distance. Trends Cogn. Sci. 2020, 24, 717–733. [Google Scholar] [CrossRef]
- Caggiano, V.; Fogassi, L.; Rizzolatti, G.; Casile, A.; Giese, M.A.; Thier, P. Mirror neurons encode the subjective value of an observed action. Proc. Natl. Acad. Sci. USA 2012, 109, 11848–11853. [Google Scholar] [CrossRef] [Green Version]
- Pizzagalli, D.A.; Iosifescu, D.; Hallett, L.A.; Ratner, K.G.; Fava, M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. J. Psychiatr. Res. 2008, 43, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Manera, V.; Fabre, R.; Stella, F.; Loureiro, J.C.; Agüera-Ortiz, L.; López-Álvarez, J.; Hanon, C.; Hoertel, N.; Aalten, P.; Ramakers, I.; et al. A survey on the prevalence of apathy in elderly people referred to specialized memory centers. Int. J. Geriatr. Psychiatry 2019, 34, 1369–1377. [Google Scholar] [CrossRef]
- Groeneweg-Koolhoven, I.; Ploeg, M.; Comijs, H.C.; Wjh Penninx, B.; van der Mast, R.C.; Schoevers, R.A.; Rhebergen, D.; Exel, E.V. Apathy in early and late-life depression. J. Affect Disord. 2017, 223, 76–81. [Google Scholar] [CrossRef]
- Brevers, D.; Bechara, A.; Cleeremans, A.; Noël, X. Iowa Gambling Task (IGT): Twenty years after—gambling disorder and IGT. Front. Psychol. 2013, 30, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGovern, A.R.; Alexopoulos, G.S.; Yuen, G.S.; Morimoto, S.S.; Gunning-Dixon, F.M. Reward-related decision making in older adults: Relationship to clinical presentation of depression. Int. J. Geriatr. Psychiatry 2014, 29, 1125–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexopoulos, G.S.; Hoptman, M.J.; Yuen, G.; Kanellopoulos, D.; Seirup, J.K.; Lim, K.O.; Gunning, F.M. Functional connectivity in apathy of late-life depression: A preliminary study. J. Affect. Disord. 2012, 149, 398–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, S.J.; Nestler, E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013, 14, 609–625, Erratum in Nat. Rev. Neurosci. 2013, 14, 736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, M.; Eatmon, C.V.; Slevin, J.T. Drug treatment strategies for depression in Parkinson disease. Expert Opin. Pharmacother. 2019, 20, 1351–1363. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Fowler, J.S.; Tomasi, D.; Telang, F. Addiction: Beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. USA 2011, 108, 15037–15042. [Google Scholar] [CrossRef] [Green Version]
- Bananej, M.; Karimi-Sori, A.; Zarrindast, M.R.; Ahmadi, S. D1 and D2 dopaminergic systems in the rat basolateral amygdala are involved in anxiogenic-like effects induced by histamine. J. Psychopharmacol. 2011, 26, 564–574. [Google Scholar] [CrossRef]
- Hu, X.; Song, X.; Yuan, Y.; Li, E.; Liu, J.; Liu, W.; Liu, Y. Abnormal functional connectivity of the amygdala is associated with depres-sion in Parkinson’s disease. Mov. Disord. 2015, 30, 238–244. [Google Scholar] [CrossRef]
- Huang, P.; Xuan, M.; Gu, Q.; Yu, X.; Xu, X.; Luo, W.; Zhang, M. Abnormal amygdala function in Parkinson’s disease patients and its relationship to depression. J. Affect Disord. 2015, 183, 263–268. [Google Scholar] [CrossRef]
- Francis, T.; Lobo, M.K. Emerging Role for Nucleus Accumbens Medium Spiny Neuron Subtypes in Depression. Biol. Psychiatry 2017, 81, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Costello, H.; Berry, A.J.; Reeves, S.; Weil, R.S.; Joyce, E.M.; Howard, R.; Roiser, J.P. Disrupted reward processing in Parkinson’s disease and its relationship with dopamine state and neuropsychiatric syndromes: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ceyzériat, K.; Gloria, Y.; Tsartsalis, S.; Fossey, C.; Cailly, T.; Fabis, F.; Millet, P.; Tournier, B.B. Alterations in dopamine system and in its connectivity with serotonin in a rat model of Alzheimer’s disease. Brain Commun. 2021, 3, fcab029. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, M.; Puglisi-Allegra, S.; Mercuri, N. The role of dopaminergic midbrain in Alzheimer’s disease: Translating basic sci-ence into clinical practice. Pharmacol. Res. 2018, 130, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.A.; Herrmann, N.; Lanctôt, K.L. The role of dopamine in symptoms and treatment of apathy in Alzheimer’s disease. CNS Neurosci. Ther. 2011, 17, 411–427. [Google Scholar] [CrossRef]
- Hackett, M.L.; Pickles, K. Part I: Frequency of depression after stroke: An updated systematic review and meta-analysis of ob-servational studies. Int. J. Stroke 2014, 9, 1017–1025. [Google Scholar] [CrossRef]
- Paolucci, S. Epidemiology and treatment of post-stroke depression. Neuropsychiatr. Dis. Treat. 2008, 4, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Oestreich, L.K.L.; Wright, P.; O’Sullivan, M.J. Microstructural changes in the reward system are associated with post-stroke de-pression. Neuroimage Clin. 2020, 28, 102360. [Google Scholar] [CrossRef]
- Doi, T.; Verghese, J.; Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Suzuki, T. Motoric Cognitive Risk Syn-drome: Prevalence and Risk Factors in Japanese Seniors. J. Am. Med. Dir. Assoc. 2015, 16, 1103.e21–1103.e25. [Google Scholar] [CrossRef]
- Doi, T.; Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Verghese, J.; Suzuki, T. Motoric Cognitive Risk Syndrome: Association with Incident Dementia and Disability. J. Alzheimer’s Dis. 2017, 59, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Beauchet, O.; Sekhon, H.; Launay, C.P.; Chabot, J.; Rolland, Y.; Schott, A.-M.; Allali, G. Motoric cognitive risk syndrome and mortality: Results from the EPIDOS cohort. Eur. J. Neurol. 2018, 26, 794-e56. [Google Scholar] [CrossRef]
- Osimo, E.F.; Baxter, L.J.; Lewis, G.; Jones, P.B.; Khandaker, G.M. Prevalence of low-grade inflammation in depression: A systematic review and meta-analysis of CRP levels. Psychol. Med. 2019, 49, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nielsen, S.F.; Nordestgaard, B.G. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry 2013, 70, 176–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Vorburger, R.; Scarmeas, N.; Luchsinger, J.A.; Manly, J.J.; Schupf, N.; Mayeux, R.; Brickman, A.M. Circulating inflammatory biomarkers in relation to brain structural measurements in a non-demented elderly population. Brain Behav. Immun. 2017, 65, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Satizabal, C.L.; Zhu, Y.C.; Mazoyer, B.; Dufouil, C.; Tzourio, C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: The 3C-Dijon Study. Neurology 2012, 78, 720–727. [Google Scholar] [CrossRef]
- Roubenoff, R.; Harris, T.B.; Abad, L.W.; Wilson, P.W.; Dallal, G.E.; Dinarello, C.A. Monocyte cytokine production in an elderly population: Effect of age and inflammation. J. Gerontol. A Biol. Sci. Med. Sci. 1998, 53, M20–M26. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Taylor, W.D.; Aizenstein, H.; Alexopoulos, G.S. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Mol. Psychiatry 2013, 18, 963–974. [Google Scholar] [CrossRef] [Green Version]
- Alexopoulos, G.S.; Morimoto, S.S. The inflammation hypothesis in geriatric depression. Int. J. Geriatr. Psychiatry 2011, 26, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Taylor, W.D.; Zald, D.H.; Felger, J.C.; Christman, S.; Claassen, D.O.; Horga, G.; Miller, J.M.; Gifford, K.; Rogers, B.; Szymkowicz, S.M.; et al. Influences of dopaminergic system dysfunction on late-life depression. Mol. Psychiatry 2022, 27, 180–191. [Google Scholar] [CrossRef]
- Sekhon, H.; Allali, G.; Beauchet, O. The association of anxio-depressive disorders and depression with motoric cognitive risk syndrome: Results from the baseline assessment of the Canadian longitudinal study on aging. GeroScience 2019, 41, 409–418. [Google Scholar] [CrossRef]
- Roberto, N.; Portella, M.J.; Marquié, M.; Alegret, M.; Hernández, I.; Mauleón, A.; Rosende-Roca, M.; Abdelnour, C.; de Antonio, E.E.; Gil, S.; et al. Neuropsychiatric profiles and conversion to dementia in mild cognitive impairment, a latent class analysis. Sci. Rep. 2021, 11, 6448. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, J.W.; van Wanrooij, L.L.; Moll van Charante, E.P.; Brayne, C.; van Gool, W.A.; Richard, E. Association of Apathy With Risk of Incident Dementia: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, J.W.; Van Wanrooij, L.L.; Moll van Charante, E.P.; Richard, E.; van Gool, W.A. Apathy is associated with incident de-mentia in community-dwelling older people. Neurology 2018, 90, e82–e89. [Google Scholar] [CrossRef] [Green Version]
- Ayers, E.; Shapiro, M.; Holtzer, R.; Barzilai, N.; Milman, S.; Verghese, J. Symptoms of Apathy Independently Predict Incident Frailty and Disability in Community-Dwelling Older Adults. J. Clin. Psychiatry 2017, 78, e529–e536. [Google Scholar] [CrossRef] [Green Version]
- Forrester, S.N.; Gallo, J.J.; Smith, G.S.; Leoutsakos, J.-M.S. Patterns of Neuropsychiatric Symptoms in Mild Cognitive Impairment and Risk of Dementia. Am. J. Geriatr. Psychiatry 2015, 24, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceïde, M.E.; Warhit, A.; Ayers, E.I.; Kennedy, G.; Verghese, J. Apathy and the Risk of Predementia Syndromes in Communi-ty-Dwelling Older Adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2020, 75, 1443–1450. [Google Scholar] [CrossRef]
- Pillai, J.A.; Verghese, J. Social networks and their role in preventing dementia. Indian J. Psychiatry 2009, 51, S22–S28. [Google Scholar]
- Tomioka, K.; Kurumatani, N.; Hosoi, H. Social Participation and the Prevention of Decline in Effectance among Communi-ty-Dwelling Elderly: A Population-Based Cohort Study. PLoS ONE 2015, 10, e0139065, Erratum in PLoS ONE 2016, 11, e0164925. [Google Scholar]
- Ellwardt, L.; Van Tilburg, T.G.; Aartsen, M.J. The mix matters: Complex personal networks relate to higher cognitive functioning in old age. Soc. Sci. Med. 2015, 125, 107–115. [Google Scholar] [CrossRef]
- Tomioka, K.; Kurumatani, N.; Hosoi, H. Association between Social Participation and 3-Year Change in Instrumental Activities of Daily Living in Community-Dwelling Elderly Adults. J. Am. Geriatr. Soc. 2016, 65, 107–113. [Google Scholar] [CrossRef]
- Tomioka, K.; Kurumatani, N.; Saeki, K. The differential effects of type and frequency of social participation on IADL declines of older people. PLoS ONE 2018, 13, e0207426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, C.J.; Verghese, J. Motoric Cognitive Risk Syndrome in Polypharmacy. J. Am. Geriatr. Soc. 2020, 68, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Jayakody, O.; Blumen, H.M.; Breslin, M.; Ayers, E.; Lipton, R.B.; Verghese, J.; Callisaya, M.L. Longitudinal associations between falls and future risk of cognitive decline, the Motoric Cognitive Risk syndrome and dementia: The Einstein Ageing Study. Age Ageing 2022, 51, afac058. [Google Scholar] [CrossRef] [PubMed]
- Raji, A.M.; Padubidri, A.; Samper-Ternent, R.; Al Snih, S.; Markides, K.S.; Ottenbacher, K.J. Falls and cognitive decline in Mexican Americans 75 years and older. Clin. Interv. Aging 2014, 9, 719–726. [Google Scholar] [CrossRef] [Green Version]
- Belagaje, S.R. Stroke Rehabilitation. Contin. Minneap Minn 2017, 23, 238–253. [Google Scholar] [CrossRef]
- Stahl, S.T.; Altmann, H.M.; Dew, M.A.; Albert, S.M.; Butters, M.; Gildengers, A.; Reynolds, C.F.; Karp, J.F., III. The Effects of Gait Speed and Psychomotor Speed on Risk for Depression and Anxiety in Older Adults with Medical Comorbidities. J. Am. Geriatr. Soc. 2021, 69, 1265–1271. [Google Scholar] [CrossRef]
- Rutherford, B.R.; Slifstein, M.; Chen, C.; Abi-Dargham, A.; Brown, P.J.; Wall, M.W.; Vanegas-Arroyave, N.; Stern, Y.; Bailey, V.; Valente, E.; et al. Effects of L-DOPA Monotherapy on Psychomotor Speed and [11C]Raclopride Binding in High-Risk Older Adults With Depression. Biol. Psychiatry 2019, 86, 221–229. [Google Scholar] [CrossRef]
- Becker, J.B.; Chartoff, E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 2018, 44, 166–183. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauretani, F.; Testa, C.; Salvi, M.; Zucchini, I.; Lorenzi, B.; Tagliaferri, S.; Cattabiani, C.; Maggio, M. Reward System Dysfunction and the Motoric-Cognitive Risk Syndrome in Older Persons. Biomedicines 2022, 10, 808. https://doi.org/10.3390/biomedicines10040808
Lauretani F, Testa C, Salvi M, Zucchini I, Lorenzi B, Tagliaferri S, Cattabiani C, Maggio M. Reward System Dysfunction and the Motoric-Cognitive Risk Syndrome in Older Persons. Biomedicines. 2022; 10(4):808. https://doi.org/10.3390/biomedicines10040808
Chicago/Turabian StyleLauretani, Fulvio, Crescenzo Testa, Marco Salvi, Irene Zucchini, Beatrice Lorenzi, Sara Tagliaferri, Chiara Cattabiani, and Marcello Maggio. 2022. "Reward System Dysfunction and the Motoric-Cognitive Risk Syndrome in Older Persons" Biomedicines 10, no. 4: 808. https://doi.org/10.3390/biomedicines10040808
APA StyleLauretani, F., Testa, C., Salvi, M., Zucchini, I., Lorenzi, B., Tagliaferri, S., Cattabiani, C., & Maggio, M. (2022). Reward System Dysfunction and the Motoric-Cognitive Risk Syndrome in Older Persons. Biomedicines, 10(4), 808. https://doi.org/10.3390/biomedicines10040808







