Intermittent Exposure of Hypercapnia Suppresses Allograft Rejection via Induction of Treg Differentiation and Inhibition of Neutrophil Accumulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Skin Graft Experiments
2.3. Experimental Protocols
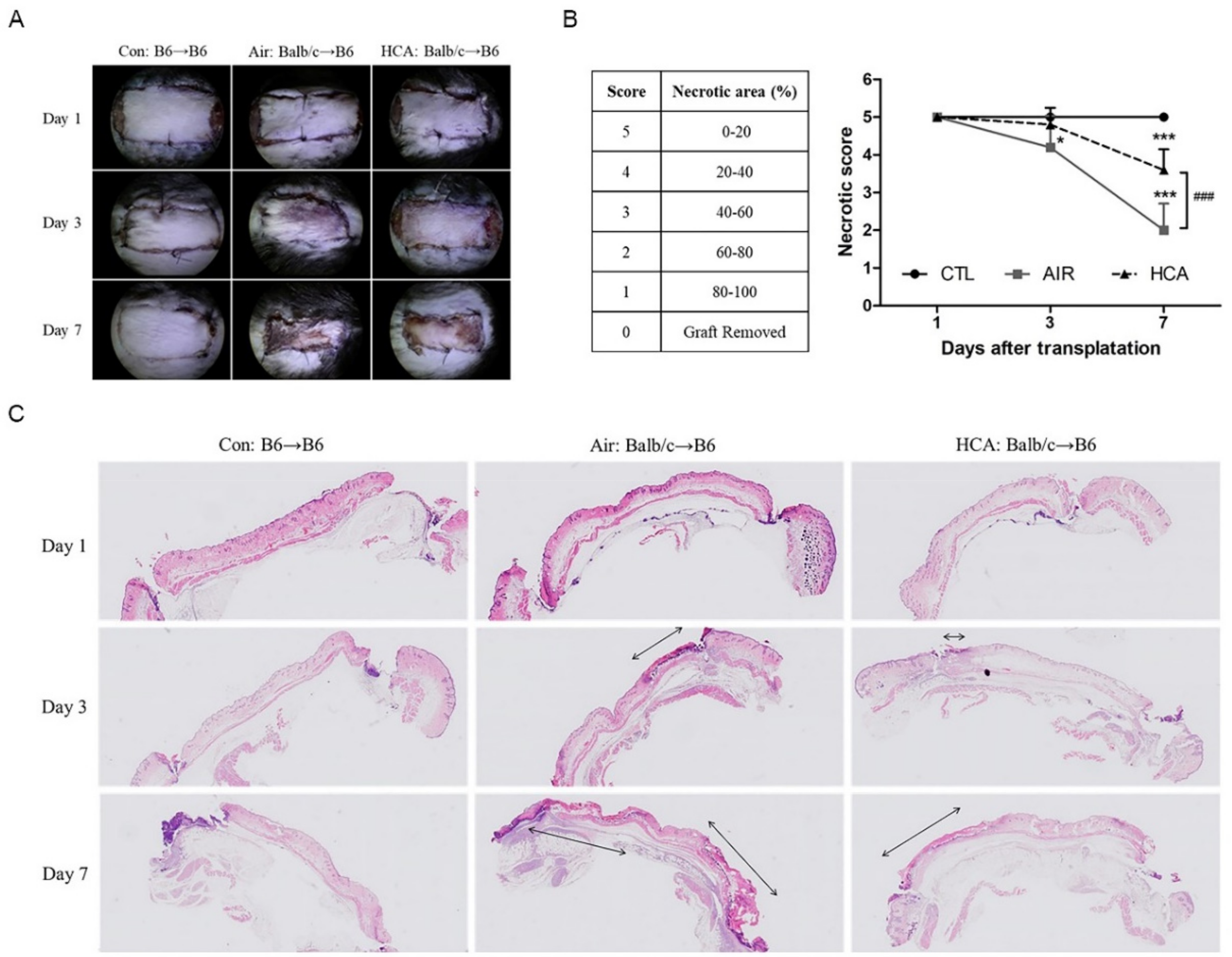
2.4. Evaluation of Skin Rejection
2.5. Measurement of Cytokines in Serum
2.6. Flow Cytometry Analysis
2.7. Western Blot Analysis
2.8. Naïve T Cell Isolation and Treg Differentiation
2.9. Histological Assessment
2.10. Immunohistochemical Analysis
2.11. Neutrophil Isolation and Migration Assay
2.12. Statistical Analysis
3. Results
3.1. Effects of HCA on Necrotic Levels in Skin Graft Mice
3.2. Effects of HCA on Serum Cytokine Expression in Skin Graft Mice
3.3. Effects of HCA on T Cell Population in Skin Graft Mice
3.4. Effects of HCA on Differentiation of Regulatory T Cells from Splenic-Isolated Naïve CD4+ T Cells
3.5. Effects of HCA on Neutrophil Infiltration in Skin Graft Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declaration of Transparency and Scientific Rigour
References
- Cope, O.; Langohr, J.L.; Moore, F.D.; Webster, R.C. Expeditious care of full-thickness burn wounds by surgical excision and grafting. Ann. Surg. 1947, 125, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Janzekovic, Z. A new concept in the early excision and immediate grafting of burns. J. Trauma Acute Care Surg. 1970, 10, 1103–1108. [Google Scholar] [CrossRef]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Daniel, N.; Mori, D.K.; Fullerton, J.N.; Gilroy, D.W.; Goldstein, D.R. Inflammatory triggers of acute rejection of organ allografts. Immunol. Rev. 2014, 258, 132–144. [Google Scholar]
- Burd, A.; Chiu, T. Allogenic skin in the treatment of burns. Clin. Dermatol. 2005, 23, 376. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Gunn, S.W.; Hayek, S.N. State of the art in burn treatment. World J. Surg. 2005, 29, 131. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.-S.; Chen, S.-G.; Dai, N.-T.; Fu, J.-P.; Chang, S.-C.; Deng, S.-C.; Lin, F.-H.; Chen, T.-M. Clinical Experience Using Cadaveric Skin for Wound Closure in Taiwan. Wounds 2012, 24, 293–298. [Google Scholar]
- Snyder, R.J.; Simonson, D.A. Cadaveric allograft as adjunct therapy for nonhealing ulcers. J. Foot Ankle Surg. 1999, 38, 93–101. [Google Scholar] [CrossRef]
- Bale, J.F., Jr.; Kealey, G.P.; Ebelhack, C.L.; Platz, C.E.; Goeken, J.A. Cytomegalovirus infection in a cyclosporine-treated burn patient: Case report. J. Trauma. 1992, 32, 263–267. [Google Scholar] [CrossRef]
- Gao, W.; Liu, D.; Di, L.; Che, X.; Cui, G. Effects of hypercapnia on T cells in lung ischemia/reperfusion injury after lung transplantation. Exp. Biol. Med. 2014, 239, 1597–1605. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, X.; Gaowa, S.; Meng, Q.; Zhan, Z.; Liu, J.; Li, J.; Fan, H.; Liu, Z. The critical role of induced CD4+ FoxP3+ regulatory cells in suppression of interleukin-17 production and attenuation of mouse orthotopic lung allograft rejection. Transplantation 2015, 99, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, L.G.; Halloran, P.F. Role of IFN-gamma in allograft rejection. Crit. Rev. Immunol. 2002, 22, 317–349. [Google Scholar] [CrossRef] [PubMed]
- Avni, B.; Grisariu, S.; Shapira, M.Y. Interleukin-2: A double-edge sword in allogeneic stem cell transplantation. Immunotherapy 2016, 8, 241–243. [Google Scholar] [CrossRef] [PubMed]
- LaRosa, D.F.; Rahman, A.; Turka, L.A. The innate immune system in allograft rejection and tolerance. J. Immunol. 2007, 178, 7503–7509. [Google Scholar] [CrossRef] [Green Version]
- Benichou, G.; Yamada, Y.; Yun, S.-H.; Lin, C.; Fray, M.; Tocco, G. Immune recognition and rejection of allogeneic skin grafts. Immunotherapy 2011, 3, 757–770. [Google Scholar] [CrossRef] [Green Version]
- Ingulli, E. Mechanism of cellular rejection in transplantation. Pediatr. Nephrol. 2010, 25, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.-P.; Lee, J.-J.; Chi, C.-W.; Chang, K.-M.; Chen, Y.-J. Platonin improves survival of skin allografts. J. Surg. Res. 2010, 164, 146–154. [Google Scholar] [CrossRef]
- Kao, T.-K.; Ou, Y.-C.; Lin, S.-Y.; Pan, H.-C.; Song, P.-J.; Raung, S.-L.; Lai, C.-Y.; Liao, S.-L.; Lu, H.-C.; Chen, C.-J. Luteolin inhibits cytokine expression in endotoxin/cytokine-stimulated microglia. J. Nutr. Biochem. 2011, 22, 612–624. [Google Scholar] [CrossRef]
- Kang, O.-H.; Choi, J.-G.; Lee, J.-H.; Kwon, D.-Y. Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-kappaB and MAPKs activation pathways in HMC-1 cells. Molecules 2010, 15, 385–398. [Google Scholar] [CrossRef] [Green Version]
- Burke, J.F.; May, J.W.; Albright, N.; Quinby, W.C.; Russell, P.S. Temporary skin transplantation and immunosuppression for extensive burns. N. Engl. J. Med. 1974, 290, 269–271. [Google Scholar] [CrossRef]
- Muscedere, J.G.; Mullen, J.B.; Gan, K.; Slutsky, A.S. Tidal ventilation at low airway pressures can augment lung injury. Am. J. Respir. Crit. Care Med. 1994, 149, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Thome, U.H.; Ambalavana, N. Permissive hypercapnia to decrease lung injury in ventilated preterm neonates. Semin. Fetal. Neonatal. Med. 2009, 14, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-E.; Wu, S.-Y.; Chu, S.-J.; Tzeng, Y.-S.; Peng, C.-K.; Lan, C.-C.; Perng, W.-C.; Wu, C.-P.; Huang, K.-L. Pre-Treatment with Ten-Minute Carbon Dioxide Inhalation Prevents Lipopolysaccharide-Induced Lung Injury in Mice via Down-Regulation of Toll-Like Receptor 4 Expression. Int. J. Mol. Sci. 2019, 20, 6293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laffey, J.G.; Honan, D.; Hopkins, N.; Hyvelin, J.M.; Boylan, J.F.; McLoughlin, P. Hypercapnic acidosis attenuates endotoxin-induced acute lung injury. Am. J. Respir. Crit. Care Med. 2004, 169, 46–56. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wu, C.P.; Kang, B.H.; Li, M.H.; Chu, S.J.; Huang, K.L. Hypercapnic acidosis attenuates reperfusion injury in isolated and perfused rat lungs. Crit. Care Med. 2012, 40, 553–559. [Google Scholar] [CrossRef]
- Jing Tan, Y.L.; Jiang, T.; Wang, L.; Zhao, C.; Shen, D.; Cui, X. Effects of Hypercapnia on Acute Cellular Rejection after Lung Transplantation in Rats. Anesthesiology 2018, 128, 130–139. [Google Scholar]
- Tzeng, Y.-S.; Wu, S.-Y.; Peng, Y.-J.; Cheng, C.-P.; Tang, S.-E.; Huang, K.-L.; Chu, S.-J. Hypercapnic acidosis prolongs survival of skin allografts. J. Surg. Res. 2015, 195, 351–359. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, K.; Daniel, P.; Wisbrun, N.; Fuchs, H.; Fan, H. Delayed allogeneic skin graft rejection in CD26-deficient mice. Cell Mol. Immunol. 2019, 16, 557–567. [Google Scholar] [CrossRef] [Green Version]
- Laffey, J.G.; Tanaka, M.; Engelberts, D.; Luo, X.; Yuan, S.; Tanswell, A.K.; Post, M.; Lindsay, T.; Kavanagh, B.P. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am. J. Respir. Crit. Care Med. 2000, 162, 2287–2294. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Liu, D.-D.; Di, L.; Cui, G.-X. Effect of Therapeutic Hypercapnia on Inflammatory Responses to One-lung Ventilation in Lobectomy Patients. Anesthesiology 2015, 122, 1235–1252. [Google Scholar] [CrossRef]
- O’Croinin, D.F.; Nichol, A.D.; Hopkins, N.; Boylan, J.; O’Brien, S.; O’Connor, C.; Laffey, J.G.; McLoughlin, P. Sustained hypercapnic acidosis during pulmonary infection increases bacterial load and worsens lung injury. Crit. Care Med. 2008, 36, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Chonghaile, M.N.; Higgins, B.D.; Costello, J.; Laffey, J.G. Hypercapnic acidosis attenuates lung injury induced by established bacterial pneumonia. Anesthesiology 2008, 109, 837–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni Chonghaile, M.; Higgins, B.D.; Costello, J.F.; Laffey, J.G. Hypercapnic acidosis attenuates severe acute bacterial pneumonia-induced lung injury by a neutrophil-independent mechanism. Crit. Care Med. 2008, 36, 3135–3144. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fueyo, A.; Markmann, J.F. Immune Exhaustion and Transplantation. Am. J. Transplant. 2016, 16, 1953–1957. [Google Scholar] [CrossRef]
- Smith, K.M.; Pottage, L.; Thomas, E.R.; Leishman, A.J.; Doig, T.N.; Xu, D.; Liew, F.Y.; Garside, P. Th1 and Th2 CD4+ T cells provide help for B cell clonal expansion and antibody synthesis in a similar manner in vivo. J. Immunol. 2000, 165, 3136–3144. [Google Scholar] [CrossRef] [Green Version]
- Walsh, P.T.; Strom, T.B.; Turka, L.A. Routes to transplant tolerance versus rejection; the role of cytokines. Immunity 2004, 20, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Shenoy, K.V.; Solomides, C.; Cordova, F.; Rogers, T.J.; Ciccolella, D.; Criner, G.J. Low CD4/CD8 ratio in bronchus-associated lymphoid tissue is associated with lung allograft rejection. J. Transplant. 2012, 2012, 928081. [Google Scholar] [CrossRef]
- Vignali, D.A.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Bushell, A.; Wood, K. GITR ligation blocks allograft protection by induced CD25+CD4+ regulatory T cells without enhancing effector T-cell function. Am. J. Transplant. 2007, 7, 759. [Google Scholar] [CrossRef]
- Issa, F.; Hester, J.; Goto, R.; Nadig, S.N.; Goodacre, T.E.; Wood, K. Ex vivo–expanded human regulatory T cells prevent the rejection of skin allografts in a humanised mouse model. Transplantation 2010, 90, 1321. [Google Scholar] [CrossRef] [Green Version]
- Qiu, F.; Liu, H.; Liang, C.L.; Nie, G.D.; Dai, Z. A new immunosuppressive molecule emodin induces both CD4+FoxP3+ and CD8+CD122+ regulatory T cells and suppresses murine allograft rejection. Front. Immunol. 2017, 8, 1519. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Liu, H.; Chen, Y.; Qiu, F.; Liang, C.-L.; Zhang, Q.; Huang, H.; Wang, S.; Zhang, Z.-D.; Lu, W.; et al. A Novel Immunosuppressant, Luteolin, Modulates Alloimmunity and Suppresses Murine Allograft Rejection. J. Immunol. 2019, 203, 3436–3446. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.D.; Balmert, S.C.; Zhang, W.; Schweizer, R.; Schnider, J.T.; Komatsu, C.; Dong, L.; Erbas, V.E.; Unadkat, J.V.; Aral, A.M.; et al. Treg-inducing microparticles promote donor-specific tolerance in experimental vascularized composite allotransplantation. Proc. Natl. Acad. Sci. USA 2019, 116, 25784–25789. [Google Scholar] [CrossRef]
- Braza, F.; Brouard, S.; Chadban, S.; Goldstein, D.R. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat. Rev. Nephrol. 2016, 12, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Song, Y.; Colangelo, C.M.; Wu, T.; Bruce, C.; Scabia, G.; Galan, A.; Maffei, M.; Goldstein, D.R. Haptoglobin activates innate immunity to enhance acute transplant rejection in mice. J. Clin. Investig. 2012, 122, 383–387. [Google Scholar] [CrossRef] [Green Version]
- Whitson, B.A.; Prekker, M.E.; Herrington, C.S.; Whelan, T.P.; Radosevich, D.M.; Hertz, M.I.; Dahlberg, P.S. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J. Heart Lung. Transplant. 2007, 26, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.-M.; Scozzi, D.; Gauthier, J.M.; Kreisel, D. Mechanisms of graft rejection after lung transplantation. Curr. Opin. Organ. Transplant. 2017, 22, 29–35. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzeng, Y.-S.; Peng, Y.-J.; Tang, S.-E.; Huang, K.-L.; Chu, S.-J.; Wu, S.-Y.; Cheng, C.-P. Intermittent Exposure of Hypercapnia Suppresses Allograft Rejection via Induction of Treg Differentiation and Inhibition of Neutrophil Accumulation. Biomedicines 2022, 10, 836. https://doi.org/10.3390/biomedicines10040836
Tzeng Y-S, Peng Y-J, Tang S-E, Huang K-L, Chu S-J, Wu S-Y, Cheng C-P. Intermittent Exposure of Hypercapnia Suppresses Allograft Rejection via Induction of Treg Differentiation and Inhibition of Neutrophil Accumulation. Biomedicines. 2022; 10(4):836. https://doi.org/10.3390/biomedicines10040836
Chicago/Turabian StyleTzeng, Yuan-Sheng, Yi-Jen Peng, Shih-En Tang, Kun-Lun Huang, Shi-Jye Chu, Shu-Yu Wu, and Chia-Pi Cheng. 2022. "Intermittent Exposure of Hypercapnia Suppresses Allograft Rejection via Induction of Treg Differentiation and Inhibition of Neutrophil Accumulation" Biomedicines 10, no. 4: 836. https://doi.org/10.3390/biomedicines10040836
APA StyleTzeng, Y.-S., Peng, Y.-J., Tang, S.-E., Huang, K.-L., Chu, S.-J., Wu, S.-Y., & Cheng, C.-P. (2022). Intermittent Exposure of Hypercapnia Suppresses Allograft Rejection via Induction of Treg Differentiation and Inhibition of Neutrophil Accumulation. Biomedicines, 10(4), 836. https://doi.org/10.3390/biomedicines10040836





