The Role of Fecal Microbiota Transplantation in the Treatment of Acute Graft-versus-Host Disease
Abstract
:1. Introduction
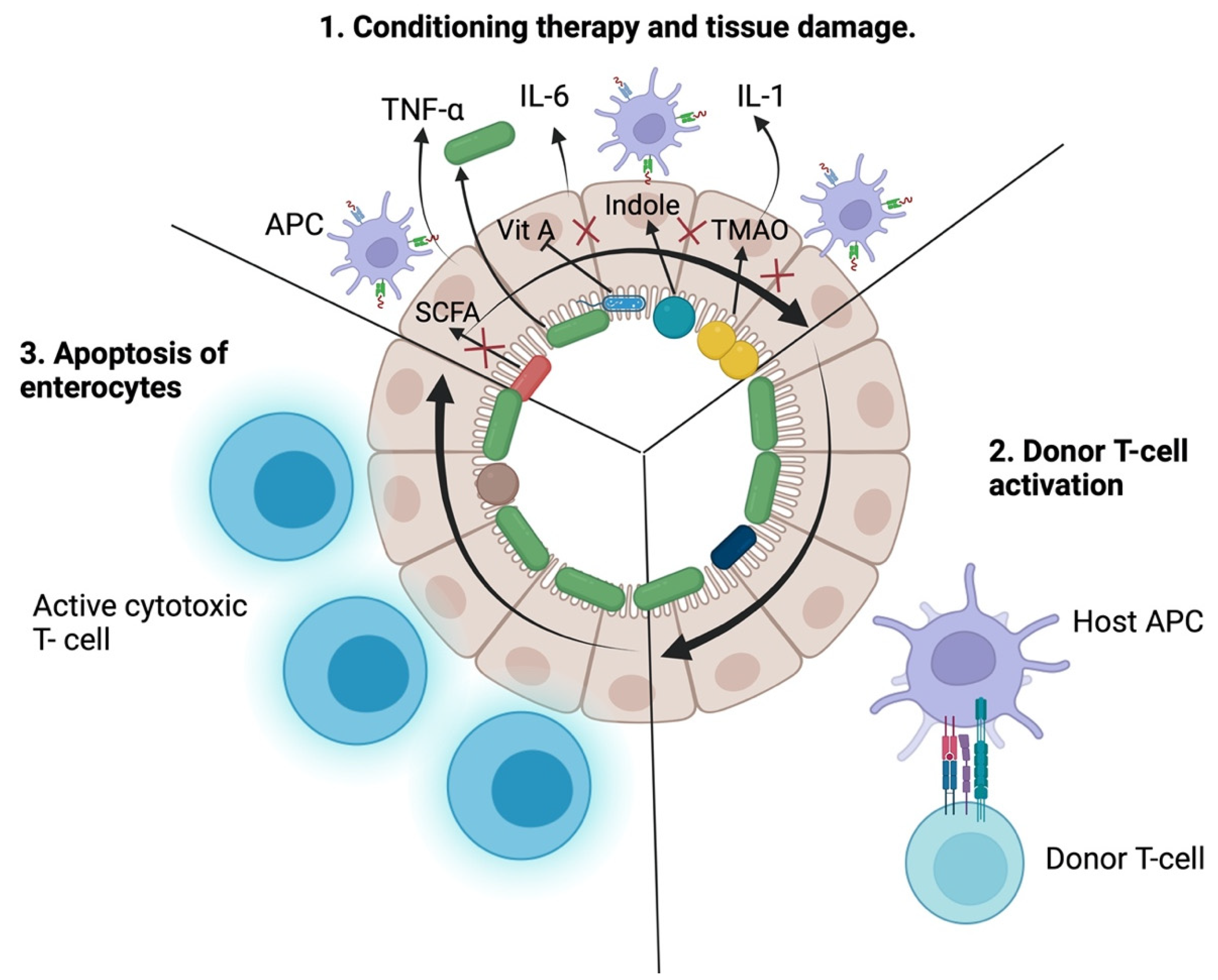
2. Pathogenesis of Acute Graft-versus-Host Disease
3. Crosstalk between the Intestinal Immune System and the Gut Microbiota
3.1. Intestinal Epithelial Cells
3.2. Antigen-Presenting Cells
3.3. T Cells
4. The Role of Gut Microbiota in the Pathogenesis of Acute Graft-Versus-Host Disease
5. Current Guidelines and Potential for the Future
6. Fecal Microbiota Transplantation for the Treatment of Acute Graft-versus-Host Disease: What Do We Know Now?
7. Safety of FMT
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Greinix, H.T.; Eikema, D.-J.; Koster, L.; Penack, O.; Yakoub-Agha, I.; Montoto, S.; Chabannon, C.; Styczynski, J.; Nagler, A.; Robin, M.; et al. Improved outcome of patients with graft-versus-host disease after allogeneic hematopoietic cell transplantation for hematologic malignancies over time: An EBMT mega-file study. Haematologica 2021. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, M.; Wang, Z.; Horowitz, M.M.; Gale, R.P. 2013 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): Current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin. Transpl. 2013, 187–197. Available online: https://pubmed.ncbi.nlm.nih.gov/25095508/ (accessed on 20 February 2022).
- Khoury, H.J.; Wang, T.; Hemmer, M.T.; Couriel, D.; Alousi, A.; Cutler, C.; Aljurf, M.; Antin, J.H.; Ayas, M.; Battiwalla, M.; et al. Improved survival after acute graft-versus-host disease diagnosis in the modern era. Haematologica 2017, 102, 958–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, G.B. How I treat acute graft-versus-host disease of the gastrointestinal tract and the liver. Blood 2016, 127, 1544–1550. [Google Scholar] [CrossRef] [Green Version]
- Kuykendall, T.D.; Smoller, B.R. Lack of specificity in skin biopsy specimens to assess for acute graft-versus-host disease in initial 3 weeks after bone-marrow transplantation. J. Am. Acad. Dermatol. 2003, 49, 1081–1085. [Google Scholar] [CrossRef]
- Glucksberg, H.; Storb, R.; Fefer, A.; Buckner, C.D.; Neiman, P.E.; Clift, R.A.; Lerner, K.G.; Thomas, E.D. Clinical manifestations of graft-versus-host disease in human recipients of marrow from hl-a-matched sibling donors. Transplantation 1974, 18, 295–304. [Google Scholar] [CrossRef]
- Harris, A.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016, 22, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Nash, R.A.; Pepe, M.S.; Storb, R.; Longton, G.; Pettinger, M.; Anasetti, C.; Appelbaum, R.A.; Bowden, R.; Deeg, H.J.; Doney, K. Acute graft-versus-host disease: Analysis of risk factors after allogeneic marrow transplantation and prophylaxis with cyclosporine and methotrexate. Blood 1992, 80, 1838–1845. [Google Scholar] [CrossRef] [Green Version]
- Jagasia, M.; Arora, M.; Flowers, M.E.D.; Chao, N.J.; McCarthy, P.L.; Cutler, C.S.; Urbano-Ispizua, A.; Pavletic, S.Z.; Haagenson, M.D.; Zhang, M.-J.; et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 2012, 119, 296–307. [Google Scholar] [CrossRef] [Green Version]
- Flowers, M.E.D.; Inamoto, Y.; Carpenter, P.A.; Lee, S.J.; Kiem, H.-P.; Petersdorf, E.W.; Pereira, S.E.; Nash, R.A.; Mielcarek, M.; Fero, M.; et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 2011, 117, 3214–3219. [Google Scholar] [CrossRef]
- Storb, R.; Deeg, H.J.; Whitehead, J.; Appelbaum, F.; Beatty, P.; Bensinger, W.; Buckner, C.D.; Clift, R.; Doney, K.; Farewell, V.; et al. Methotrexate and Cyclosporine Compared with Cyclosporine Alone for Prophylaxis of Acute Graft versus Host Disease after Marrow Transplantation for Leukemia. N. Engl. J. Med. 1986, 314, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Storb, R.; Antin, J.H.; Cutler, C. Should Methotrexate plus Calcineurin Inhibitors Be Considered Standard of Care for Prophylaxis of acute Graft-versus-Host Disease? Biol. Blood Marrow Transplant. 2010, 16, S18–S27. [Google Scholar] [CrossRef] [Green Version]
- Westin, J.R.; Saliba, R.M.; De Lima, M.; Alousi, A.; Hosing, C.; Qazilbash, M.H.; Khouri, I.F.; Shpall, E.J.; Anderlini, P.; Rondon, G.; et al. Steroid-Refractory Acute GVHD: Predictors and Outcomes. Adv. Hematol. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.J.; Rizzo, J.D.; Wingard, J.R.; Ballen, K.; Curtin, P.T.; Cutler, C.; Litzow, M.R.; Nieto, Y.; Savani, B.N.; Schriber, J.R.; et al. First- and Second-Line Systemic Treatment of Acute Graft-versus-Host Disease: Recommendations of the American Society of Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2012, 18, 1150–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xhaard, A.; Rocha, V.; Bueno, B.; de Latour, R.P.; Lenglet, J.; Petropoulou, A.; Rodriguez-Otero, P.; Ribaud, P.; Porcher, R.; Socié, G.; et al. Steroid-Refractory Acute GVHD: Lack of Long-Term Improved Survival Using New Generation Anticytokine Treatment. Biol. Blood Marrow Transplant. 2012, 18, 406–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeiser, R.; Von Bubnoff, N.; Butler, J.; Mohty, M.; Niederwieser, D.; Or, R.; Szer, J.; Wagner, E.M.; Zuckerman, T.; Mahuzier, B.; et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N. Engl. J. Med. 2020, 382, 1800–1810. [Google Scholar] [CrossRef]
- Walker, I.; Panzarella, T.; Couban, S.; Couture, F.; Devins, G.; Elemary, M.; Gallagher, G.; Kerr, H.; Kuruvilla, J.; Lee, S.J.; et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: A randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016, 17, 164–173. [Google Scholar] [CrossRef]
- Luznik, L.; O’Donnell, P.V.; Fuchs, E.J. Post-Transplantation Cyclophosphamide for Tolerance Induction in HLA-Haploidentical Bone Marrow Transplantation. Semin. Oncol. 2012, 39, 683–693. [Google Scholar] [CrossRef] [Green Version]
- Busca, A.; Locatelli, F.; Marmont, F.; Ceretto, C.; Falda, M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am. J. Hematol. 2007, 82, 45–52. [Google Scholar] [CrossRef]
- Ishiwata, K.; Nishida, A.; Ota, H.; Ikebe, T.; Tsuji, M.; Yamamoto, H.; Asano-Mori, Y.; Uchida, N.; Izutsu, K.; Taniguchi, S. Infliximab Treatment for Steroid-Refractory Acute Graft-Versus-Host Disease After Reduced-Intensity Cord Blood Transplantation in Adults. Blood 2011, 118, 4553. [Google Scholar] [CrossRef]
- BiliŃski, J.; Jasiński, M.; Tomaszewska, A.; Lis, K.; Kacprzyk, P.; Chmielewska, L.; Karakulska-Prystupiuk, E.; Mullish, B.H.; Basak, G.W. Fecal microbiota transplantation with ruxolitinib as a treatment modality for steroid-refractory/dependent acute, gastrointestinal graft-versus-host disease: A case series. Am. J. Hematol. 2021, 96, 461. [Google Scholar] [CrossRef] [PubMed]
- Biliński, J.; Winter, K.; Jasiński, M.; Szczęś, A.; Bilinska, N.; Mullish, B.H.; Małecka-Panas, E.; Basak, G.W. Rapid resolution of COVID-19 after faecal microbiota transplantation. Gut 2021, 71, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Bilinski, J.; Lis, K.; Tomaszewska, A.; Grzesiowski, P.; Dzieciatkowski, T.; Tyszka, M.; Karakulska-Prystupiuk, E.; Boguradzki, P.; Tormanowska, M.; Halaburda, K.; et al. Fecal microbiota transplantation in patients with acute and chronic graft-versus-host disease—spectrum of responses and safety profile. Results from a prospective, multicenter study. Am. J. Hematol. 2021, 96. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Loschi, M.; Cluzeau, T.; Huynh, A.; Guenounou, S.; Borel, C.; Legrand, F.; Granata, A.; Devillier, R.; Maisano, V.; et al. Pooled Allogenic Fecal Microbiotherapy MaaT013 for the Treatment of Steroid-Refractory Gastrointestinal Acute Graft-Versus-Host Disease: Results from the Phase IIa Heracles Study and Expanded Access Program. Blood 2021, 138, 262. [Google Scholar] [CrossRef]
- Shouval, R.; Geva, M.; Nagler, A.; Youngster, I. Fecal Microbiota Transplantation for Treatment of Acute Graft-versus-Host Disease. Clin. Hematol. Int. 2019, 1, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef]
- Ferrara, J.L.M.; Yanik, G. Acute graft versus host disease: Pathophysiology, risk factors, and prevention strategies. Clin. Adv. Hematol. Oncol. H&O 2005, 3, 415. [Google Scholar]
- Shlomchik, W.D.; Couzens, M.S.; Tang, C.B.; McNiff, J.; Robert, M.E.; Liu, J.; Shlomchik, M.J.; Emerson, S.G. Prevention of Graft Versus Host Disease by Inactivation of Host Antigen-Presenting Cells. Science 1999, 285, 412–415. [Google Scholar] [CrossRef]
- Hanash, A.M.; Dudakov, J.; Hua, G.; O’Connor, M.H.; Young, L.F.; Singer, N.V.; West, M.L.; Jenq, R.R.; Holland, A.M.; Kappel, L.W.; et al. Interleukin-22 Protects Intestinal Stem Cells from Immune-Mediated Tissue Damage and Regulates Sensitivity to Graft versus Host Disease. Immunity 2012, 37, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Ayabe, T.; Satchell, D.P.; Wilson, C.L.; Parks, W.C.; Selsted, M.E.; Ouellette, A.J. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 2000, 1, 113–118. [Google Scholar] [CrossRef]
- Eriguchi, Y.; Nakamura, K.; Hashimoto, D.; Shimoda, S.; Shimono, N.; Akashi, K.; Ayabe, T.; Teshima, T. Decreased secretion of Paneth cell α-defensins in graft-versus-host disease. Transpl. Infect. Dis. 2015, 17, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penack, O.; Holler, E.; Brink, M.R.M.V.D. Graft-versus-host disease: Regulation by microbe-associated molecules and innate immune receptors. Blood 2010, 115, 1865–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysocki, C.A.; Panoskaltsis-Mortari, C.A.W.A.; Blazar, B.R.; Serody, J.S. Leukocyte migration and graft-versus-host disease. Blood 2005, 105, 4191–4199. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.; Kuns, R.D.; Olver, S.D.; Raffelt, N.C.; Wilson, Y.A.; Don, A.L.; Lineburg, K.E.; Cheong, M.; Robb, R.J.; Markey, K.A.; et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat. Med. 2011, 18, 135–142. [Google Scholar] [CrossRef]
- Theobald, M.; Nierle, T.; Bunjes, D.; Arnold, R.; Heimpel, H. Host-Specific Interleukin-2–Secreting Donor T-Cell Precursors as Predictors of Acute Graft-versus-Host Disease in Bone Marrow Transplantation between HLA-Identical Siblings. N. Engl. J. Med. 1992, 327, 1613–1617. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef]
- Bilinski, J.; Robak, K.; Peric, Z.; Marchel, H.; Karakulska-Prystupiuk, E.; Halaburda, K.; Rusicka, P.; Swoboda-Kopec, E.; Wroblewska, M.; Wiktor-Jedrzejczak, W.; et al. Impact of Gut Colonization by Antibiotic-Resistant Bacteria on the Outcomes of Allogeneic Hematopoietic Stem Cell Transplantation: A Retrospective, Single-Center Study. Biol. Blood Marrow Transplant. 2016, 22, 1087–1093. [Google Scholar] [CrossRef] [Green Version]
- Peled, J.U.; Gomes, A.L.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef]
- Weber, D.; Jenq, R.R.; Peled, J.U.; Taur, Y.; Hiergeist, A.; Koestler, J.; Dettmer, K.; Weber, M.; Wolff, D.; Hahn, J.; et al. Microbiota Disruption Induced by Early Use of Broad-Spectrum Antibiotics Is an Independent Risk Factor of Outcome after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Physicians Abstracts. Bone Marrow Transplant. 2016, 51, S1–S102. [CrossRef] [PubMed] [Green Version]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coombes, J.; Siddiqui, K.R.; Arancibia-Cárcamo, C.V.; Hall, J.; Sun, C.-M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid– dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Uhr, T. Induction of Protective IgA by Intestinal Dendritic Cells Carrying Commensal Bacteria. Science 2004, 303, 1662–1665. [Google Scholar] [CrossRef] [Green Version]
- Smythies, L.E.; Sellers, M.; Clements, R.H.; Mosteller-Barnum, M.; Meng, G.; Benjamin, W.H.; Orenstein, J.M.; Smith, P.D. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Investig. 2005, 115, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Denning, T.L.; Wang, Y.-C.; Patel, S.R.; Williams, I.R.; Pulendran, B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17–producing T cell responses. Nat. Immunol. 2007, 8, 1086–1094. [Google Scholar] [CrossRef]
- Bain, C.C.; Scott, C.L.; Uronen-Hansson, H.; Gudjonsson, S.; Jansson, O.; Grip, O.; Guilliams, M.; Malissen, B.; Agace, W.W.; Mowat, A.M. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013, 6, 498–510. [Google Scholar] [CrossRef]
- Calcinotto, A.; Brevi, A.; Chesi, M.; Ferrarese, R.; Perez, L.G.; Grioni, M.; Kumar, S.; Garbitt, V.M.; Sharik, M.E.; Henderson, K.J.; et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Proietti, M.; Cornacchione, V.; Jost, T.R.; Romagnani, A.; Faliti, C.E.; Perruzza, L.; Rigoni, R.; Radaelli, E.; Caprioli, F.; Preziuso, S.; et al. ATP-Gated Ionotropic P2X7 Receptor Controls Follicular T Helper Cell Numbers in Peyer’s Patches to Promote Host-Microbiota Mutualism. Immunity 2014, 41, 789–801. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.A.; Lees, C.J.; Blazar, B.R. The infusion of ex vivo activated and expanded CD4+CD25+ immune regulatory cells inhibits graft-versus-host disease lethality. Blood 2002, 99, 3493–3499. [Google Scholar] [CrossRef] [PubMed]
- Sawitzki, B.; Brunstein, C.; Meisel, C.; Schumann, J.; Vogt, K.; Appelt, C.; Curtsinger, J.M.; Verneris, M.R.; Miller, J.S.; Wagner, J.E.; et al. Prevention of Graft-versus-Host Disease by Adoptive T Regulatory Therapy Is Associated with Active Repression of Peripheral Blood Toll-Like Receptor 5 mRNA Expression. Biol. Blood Marrow Transplant. 2014, 20, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skert, C.; Fogli, M.; Perucca, S.; Garrafa, E.; Fiorentini, S.; Filì, C.; Bergonzi, C.; Malagola, M.; Turra, A.; Colombi, C.; et al. Profile of Toll-Like Receptors on Peripheral Blood Cells in Relation to Acute Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2013, 19, 227–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangus, C.W.; Massey, P.R.; Fowler, D.H.; Amarnath, S. Rapamycin Resistant Murine Th9 Cells Have a Stable In Vivo Phenotype and Inhibit Graft-Versus-Host Reactivity. PLoS ONE 2013, 8, e72305. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.M.; Wilson, R.; Bealmear, P.M. Mortality and Gross Pathology of Secondary Disease in Germfree Mouse Radiation Chimeras. Radiat. Res. 1971, 45, 577. [Google Scholar] [CrossRef]
- van Bekkum, D.W.; Roodenburg, J.; Heidt, P.J.; van der Waaij, D. Mitigation of Secondary Disease of Allogeneic Mouse Radiation Chimeras by Modification of the Intestinal Microflora. JNCI J. Natl. Cancer Inst. 1974, 52, 401–404. [Google Scholar] [CrossRef]
- Beelen, D.W.; Elmaagacli, A.; Müller, K.-D.; Hirche, H.; Schaefer, U.W. Influence of Intestinal Bacterial Decontamination Using Metronidazole and Ciprofloxacin or Ciprofloxacin Alone on the Development of Acute Graft-Versus-Host Disease After Marrow Transplantation in Patients with Hematologic Malignancies: Final Results and Long-Term Follow-Up of an Open-Label Prospective Randomized Trial. Blood 1999, 93, 3267–3275. [Google Scholar] [CrossRef]
- Guthery, S.L.; Heubi, J.E.; Filipovich, A. Enteral metronidazole for the prevention of graft versus host disease in pediatric marrow transplant recipients: Results of a pilot study. Bone Marrow Transplant. 2004, 33, 1235–1239. [Google Scholar] [CrossRef] [Green Version]
- Shono, Y.; Docampo, M.D.; Peled, J.U.; Perobelli, S.M.; Velardi, E.; Tsai, J.J.; Slingerland, A.E.; Smith, O.M.; Young, L.F.; Gupta, J.; et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci. Transl. Med. 2016, 8, 339ra71. [Google Scholar] [CrossRef] [Green Version]
- Mathewson, N.D.; Jenq, R.; Mathew, A.V.; Koenigsknecht, M.; Hanash, A.; Toubai, T.; Oravecz-Wilson, K.; Wu, S.-R.; Sun, Y.; Rossi, C.; et al. Gut microbiome–derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 2016, 17, 505–513. [Google Scholar] [CrossRef]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2015, 21, 1373–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Zhang, H.; Chen, S.; Zhou, L.; Li, Y.; Zhao, K.; Huang, F.; Fan, Z.; Xuan, L.; Zhang, X.; et al. Intestinal Microbiota Can Predict Acute Graft-versus-Host Disease Following Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- Eriguchi, Y.; Takashima, S.; Oka, H.; Shimoji, S.; Nakamura, K.; Uryu, H.; Shimoda, S.; Iwasaki, H.; Shimono, N.; Ayabe, T.; et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of α-defensins. Blood 2012, 120, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, J.E.; Huber, E.; Hammer, S.T.G.; Harris, A.; Greenson, J.K.; Braun, T.M.; Ferrara, J.L.M.; Holler, E. Low Paneth cell numbers at onset of gastrointestinal graft-versus-host disease identify patients at high risk for nonrelapse mortality. Blood 2013, 122, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.; Ferrara, J.L.M. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: Rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 2000, 95, 2754–2759. [Google Scholar] [CrossRef]
- Cook, D.; Pisetsky, D.S.; Schwartz, D.A. Toll-like receptors in the pathogenesis of human disease. Nat. Immunol. 2004, 5, 975–979. [Google Scholar] [CrossRef]
- Shaw, M.H.; Reimer, T.; Kim, Y.-G.; Nuñez, G. NOD-like receptors (NLRs): Bona fide intracellular microbial sensors. Curr. Opin. Immunol. 2008, 20, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Pillai, S.; Netravali, I.A.; Cariappa, A.; Mattoo, H. Siglecs and Immune Regulation. Annu. Rev. Immunol. 2012, 30, 357–392. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-E.; Lim, J.-Y.; Ryu, D.-B.; Kim, T.W.; Park, S.S.; Jeon, Y.-W.; Yoon, J.-H.; Cho, B.-S.; Eom, K.-S.; Kim, Y.-J.; et al. Alteration of the Intestinal Microbiota by Broad-Spectrum Antibiotic Use Correlates with the Occurrence of Intestinal Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2019, 25, 1933–1943. [Google Scholar] [CrossRef]
- Payen, M.; Nicolis, I.; Robin, M.; Michonneau, D.; Delannoye, J.; Mayeur, C.; Kapel, N.; Berçot, B.; Butel, M.-J.; Le Goff, J.; et al. Functional and phylogenetic alterations in gut microbiome are linked to graft-versus-host disease severity. Blood Adv. 2020, 4, 1824–1832. [Google Scholar] [CrossRef]
- Holler, E.; Butzhammer, P.; Schmid, K.; Hundsrucker, C.; Koestler, J.; Peter, K.; Zhu, W.; Sporrer, D.; Hehlgans, T.; Kreutz, M.; et al. Metagenomic Analysis of the Stool Microbiome in Patients Receiving Allogeneic Stem Cell Transplantation: Loss of Diversity Is Associated with Use of Systemic Antibiotics and More Pronounced in Gastrointestinal Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2014, 20, 640–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein-Thoeringer, C.K.; Nichols, K.B.; Lazrak, A.; Docampo, M.D.; Slingerland, A.E.; Slingerland, J.B.; Clurman, A.G.; Armijo, G.; Gomes, A.L.C.; Shono, Y.; et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Sci. 2019, 366, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Peled, J.U.; Devlin, S.M.; Staffas, A.; Lumish, M.; Khanin, R.; Littmann, E.R.; Ling, L.; Kosuri, S.; Maloy, M.; Slingerland, J.B.; et al. Intestinal Microbiota and Relapse After Hematopoietic-Cell Transplantation. J. Clin. Oncol. 2017, 35, 1650–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canani, R.B.; Di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Romick-Rosendale, L.E.; Haslam, D.B.; Lane, A.; Denson, L.; Lake, K.; Wilkey, A.; Watanabe, M.; Bauer, S.; Litts, B.; Luebbering, N.; et al. Antibiotic Exposure and Reduced Short Chain Fatty Acid Production after Hematopoietic Stem Cell Transplant. Biol. Blood Marrow Transplant. 2018, 24, 2418–2424. [Google Scholar] [CrossRef] [Green Version]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450, Erratum in Nature 2014, 506, 254. [Google Scholar] [CrossRef]
- Edinger, M.; Powrie, F.; Chakraverty, R. Regulatory Mechanisms in Graft-versus-Host Responses. Biol. Blood Marrow Transplant. 2009, 15, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.W.; Gatza, E.; Hou, G.; Sun, Y.; Whitfield, J.; Song, Y.; Oravecz-Wilson, K.; Tawara, I.; Dinarello, C.A.; Reddy, P. Histone deacetylase inhibition regulates inflammation and enhances Tregs after allogeneic hematopoietic cell transplantation in humans. Blood 2015, 125, 815–819. [Google Scholar] [CrossRef] [Green Version]
- Swimm, A.; Giver, C.R.; DeFilipp, Z.; Rangaraju, S.; Sharma, A.; Antonova, A.U.; Sonowal, R.; Capaldo, C.; Powell, D.; Qayed, M.; et al. Indoles derived from intestinal microbiota act via type I interferon signaling to limit graft-versus-host disease. Blood 2018, 132, 2506–2519. [Google Scholar] [CrossRef] [Green Version]
- Lounder, D.T.; Khandelwal, P.; Dandoy, C.E.; Jodele, S.; Grimley, M.S.; Wallace, G.; Lane, A.; Taggart, C.; Teusink-Cross, A.C.; Lake, K.E.; et al. Lower levels of vitamin A are associated with increased gastrointestinal graft-versus-host disease in children. Blood 2017, 129, 2801–2807. [Google Scholar] [CrossRef] [Green Version]
- Grizotte-Lake, M.; Zhong, G.; Duncan, K.; Kirkwood, J.; Iyer, N.; Smolenski, I.; Isoherranen, N.; Vaishnava, S. Commensals Suppress Intestinal Epithelial Cell Retinoic Acid Synthesis to Regulate Interleukin-22 Activity and Prevent Microbial Dysbiosis. Immunity 2018, 49, 1103–1115.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lounder, D.T.; Khandelwal, P.; Gloude, N.J.; Dandoy, C.E.; Jodele, S.; Medvedovic, M.; Denson, L.A.; Lane, A.; Lake, K.; Litts, B.; et al. Interleukin-22 levels are increased in gastrointestinal graft-versus-host disease in children. Haematologica 2018, 103, e480–e482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Fan, Z.; Jin, H.; Xuan, L.; Dai, M.; Huang, F.; Lin, R.; Sun, J.; Liu, Q. Intestinal Microbiota at Neutrophil Engraftment Can Predict Acute Graft-Versus-Host Disease in the Recipients of Allogeneic Hematopoietic Stem Cell Transplantation. Blood 2018, 132, 2122. [Google Scholar] [CrossRef]
- Golob, J.L.; Pergam, S.A.; Srinivasan, S.; Fiedler, T.L.; Liu, C.; Garcia, K.; Mielcarek, M.; Ko, D.; Aker, S.; Marquis, S.; et al. Stool Microbiota at Neutrophil Recovery Is Predictive for Severe Acute Graft vs Host Disease After Hematopoietic Cell Transplantation. Clin. Infect. Dis. 2017, 65, 1984–1991. [Google Scholar] [CrossRef]
- Ingham, A.C.; Kielsen, K.; Mordhorst, H.; Ifversen, M.; Aarestrup, F.M.; Müller, K.G.; Pamp, S.J. Microbiota long-term dynamics and prediction of acute graft-versus-host disease in pediatric allogeneic stem cell transplantation. Microbiome 2021, 9, 1–28. [Google Scholar] [CrossRef]
- Ruutu, T.; Gratwohl, A.; De Witte, T.; Afanasyev, B.; Apperley, J.F.; Bacigalupo, A.; Dazzi, F.; Dreger, P.; Duarte, R.; Finke, J.; et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transplant. 2014, 49, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Dignan, F.L.; Clark, A.; Amrolia, P.; Cornish, J.; Jackson, G.; Mahendra, P.; Scarisbrick, J.J.; Taylor, P.C.; Hadzic, N.; Shaw, B.E.; et al. Diagnosis and management of acute graft-versus-host disease. Br. J. Haematol. 2012, 158, 30–45. [Google Scholar] [CrossRef]
- Martin, P.J.; Inamoto, Y.; Flowers, M.E.; Carpenter, P.A. Secondary Treatment of Acute Graft-versus-Host Disease: A Critical Review. Biol. Blood Marrow Transplant. 2012, 18, 982–988. [Google Scholar] [CrossRef] [Green Version]
- García-Cadenas, I.; Rivera, I.; Martino, R.; Esquirol, A.; Barba, P.; Novelli, S.; Orti, G.; Briones, J.; Brunet, S.; Valcarcel, D.; et al. Patterns of infection and infection-related mortality in patients with steroid-refractory acute graft versus host disease. Bone Marrow Transplant. 2016, 52, 107–113. [Google Scholar] [CrossRef]
- Schroeder, M.A.; Choi, J.; Staser, K.; DiPersio, J.F. The Role of Janus Kinase Signaling in Graft-Versus-Host Disease and Graft Versus Leukemia. Biol. Blood Marrow Transplant. 2018, 24, 1125–1134. [Google Scholar] [CrossRef] [Green Version]
- Magenau, J.M.; Goldstein, S.C.; Peltier, D.; Soiffer, R.J.; Braun, T.; Pawarode, A.; Riwes, M.M.; Kennel, M.; Antin, J.H.; Cutler, C.S.; et al. α1-Antitrypsin infusion for treatment of steroid-resistant acute graft-versus-host disease. Blood 2018, 131, 1372–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groth, C.; van Groningen, L.F.; Matos, T.R.; Bremmers, M.E.; Preijers, F.W.; Dolstra, H.; Reicherts, C.; Schaap, N.P.; van Hooren, E.H.; IntHout, J.; et al. Phase I/II Trial of a Combination of Anti-CD3/CD7 Immunotoxins for Steroid-Refractory Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2019, 25, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danylesko, I.; KLundin, E.A.; Lazarevic, V.; Kristiansen, J.D.; Osnes, L.T.N.; Tjonnfjord, G.E.; Reims, H.M.; Gedde-Dahl, T. Anti-α4β7 integrin monoclonal antibody (vedolizumab) for the treatment of steroid-resistant severe intestinal acute graft-versus-host disease. Bone Marrow Transplant. 2018, 54, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Fløisand, Y.; Lundin, K.E.; Lazarevic, V.; Kristiansen, J.D.; Osnes, L.T.; Tjønnfjord, G.E.; Reims, H.; Gedde-Dahl, T. Targeting Integrin α4β7 in Steroid-Refractory Intestinal Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2017, 23, 172–175. [Google Scholar] [CrossRef] [Green Version]
- Bukauskas, A.; Griskevicius, L.; Peceliunas, V. Lessons Learned from Early Experiences with Vedolizumab for Steroid-Refractory Acute Graft- versus-Host Disease with Gastrointestinal Involvement. Biol. Blood Marrow Transplant. 2017, 23, 1597. [Google Scholar] [CrossRef] [Green Version]
- Coltoff, A.; Lancman, G.; Kim, S.; Steinberg, A. Vedolizumab for treatment of steroid-refractory lower gastrointestinal acute graft-versus-host disease. Bone Marrow Transplant. 2018, 53, 900–904. [Google Scholar] [CrossRef]
- Chen, Y.-B.; Perales, M.-A.; Li, S.; Kempner, M.; Reynolds, C.; Brown, J.; Efebera, Y.A.; Devine, S.M.; El-Jawahri, A.; McAfee, S.L.; et al. Phase 1 multicenter trial of brentuximab vedotin for steroid-refractory acute graft-versus-host disease. Blood 2017, 129, 3256–3261. [Google Scholar] [CrossRef] [Green Version]
- Bacigalupo, A.; Angelucci, E.; Raiola, A.M.; Varaldo, R.; Di Grazia, C.; Gualandi, F.; Benedetti, E.; Risitano, A.; Musso, M.; Zallio, F.; et al. Treatment of steroid resistant acute graft versus host disease with an anti-CD26 monoclonal antibody—Begelomab. Bone Marrow Transplant. 2020, 55, 1580–1587. [Google Scholar] [CrossRef]
- Kakihana, K.; Fujioka, Y.; Suda, W.; Najima, Y.; Kuwata, G.; Sasajima, S.; Mimura, I.; Morita, H.; Sugiyama, D.; Nishikawa, H.; et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 2016, 128, 2083–2088. [Google Scholar] [CrossRef]
- Spindelboeck, W.; Schulz, E.; Uhl, B.; Kashofer, K.; Aigelsreiter, A.; Zinke-Cerwenka, W.; Mulabecirovic, A.; Kump, P.K.; Halwachs, B.; Gorkiewicz, G.; et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haematologica 2017, 102, e210–e213. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Li, X.; Zhao, Y.; Wu, X.; Chen, F.; Ma, X.; Zhang, F.; Wu, D. Treating Steroid Refractory Intestinal Acute Graft-vs.-Host Disease with Fecal Microbiota Transplantation: A Pilot Study. Front. Immunol. 2018, 9, 2195. [Google Scholar] [CrossRef] [PubMed]
- Shouval, R.; Youngster, I.; Geva, M.; Eshel, A.; Danylesko, I.; Shimoni, A.; Beider, K.; Fein, J.A.; Sharon, I.; Koren, O.; et al. Repeated Courses of Orally Administered Fecal Microbiota Transplantation for the Treatment of Steroid Resistant and Steroid Dependent Intestinal Acute Graft Vs. Host Disease: A Pilot Study (NCT 03214289). Blood 2018, 132, 2121. [Google Scholar] [CrossRef]
- van Lier, Y.F.; Davids, M.; Haverkate, N.J.E.; de Groot, P.F.; Donker, M.L.; Meijer, E.; Heubel-Moenen, F.C.J.I.; Nur, E.; Zeerleder, S.S.; Nieuwdorp, M.; et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci. Transl. Med. 2020, 12, eaaz8926. [Google Scholar] [CrossRef] [PubMed]
- Kaito, S.; Toya, T.; Yoshifuji, K.; Kurosawa, S.; Inamoto, K.; Takeshita, K.; Suda, W.; Kakihana, K.; Honda, K.; Hattori, M.; et al. Fecal microbiota transplantation with frozen capsules for a patient with refractory acute gut graft-versus-host disease. Blood Adv. 2018, 2, 3097–3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, S.; Zeng, J.; Deng, Z.; Jiang, L.; Zhang, B.; Yang, K.; Wang, W.; Zhang, T. Fecal microbiota transplantation for refractory diarrhea in immunocompromised diseases: A pediatric case report. Ital. J. Pediatr. 2019, 45, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Biernat, M.M.; Urbaniak-Kujda, D.; Dybko, J.; Kapelko-Słowik, K.; Prajs, I.; Wróbel, T. Fecal microbiota transplantation in the treatment of intestinal steroid-resistant graft-versus-host disease: Two case reports and a review of the literature. J. Int. Med Res. 2020, 48, 300060520925693. [Google Scholar] [CrossRef]
- Mao, D.; Jiang, Q.; Sun, Y.; Mao, Y.; Guo, L.; Zhang, Y.; Man, M.; Ouyang, G.; Sheng, L. Treatment of intestinal graft-versus-host disease with unrelated donor fecal microbiota transplantation capsules. Medicine 2020, 99, e22129. [Google Scholar] [CrossRef]
- Goloshchapov, O.V.; Chukhlovin, A.B.; Bakin, E.A.; Stanevich, O.V.; Klementeva, R.V.; Shcherbakov, A.A.; Shvetsov, A.N.; Suvorova, M.A.; Bondarenko, S.N.; Kucher, M.A.; et al. Fecal microbiota transplantation for graft-versus-host disease in children and adults: Methods, clinical effects, safety. Ter. Arkhiv 2020, 92, 43–54. [Google Scholar] [CrossRef]
- Goloshchapov, O.; Bakin, E.; Stanevich, O.; Klementeva, R.; Shcherbakov, A.; Shvetsov, A.; Paina, O.; Kozhokar, P.; Gorchakova, M.; Babenko, E.; et al. Clinical and immune effects of fecal microbiota transplantation in children with acute graft-versus-host disease. Cell. Ther. Transplant. 2021, 10, 69–78. [Google Scholar] [CrossRef]
- Goeser, F.; Sifft, B.; Stein-Thoeringer, C.; Farowski, F.; Strassburg, C.P.; Brossart, P.; Higgins, P.G.; Scheid, C.; Wolf, D.; Holderried, T.A.W.; et al. Fecal microbiota transfer for refractory intestinal graft-versus-host disease—experience from two German tertiary centers. Eur. J. Haematol. 2021, 107, 229–245. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Zhou, Y.; Gao, J.; Jiao, Y.; Zhu, B.; Wu, D.; Qi, X. Safety and Efficacy of Fecal Microbiota Transplantation for Grade IV Steroid Refractory GI-GvHD Patients: Interim Results from FMT2017002 Trial. Front. Immunol. 2021, 12, 678476. [Google Scholar] [CrossRef] [PubMed]
- Marcella, C.; Cui, B.; Kelly, C.R.; Ianiro, G.; Cammarota, G.; Zhang, F. Systematic review: The global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment. Pharmacol. Ther. 2020, 53, 33–42. [Google Scholar] [PubMed]
- Wu, X.; Cui, B.-T.; Zhang, F.-M. Washed microbiota transplantation for the treatment of recurrent fungal infection in a patient with ulcerative colitis. Chin. Med. J. 2021, 134, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, G.; Zhao, Z.; Liu, Y.; Shen, Q.; Li, P.; Chen, Y.; Yin, H.; Wang, H.; Marcella, C.; et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: Clinical findings, animal studies and in vitro screening. Protein Cell 2020, 11, 251–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study | Indication/Population | Number of Patients | Administration Route | Study Type | Donor Relation | Total Number of FMTs | Serious Adverse Events | Response |
|---|---|---|---|---|---|---|---|---|
| Kakihana et al. [99] | Steroid-resistant/dependent gut GvHD | 4 | Nasogastric tube | Prospective | Spouse/relative | 7 | 1 lower GI bleeding, hypoxemia (probably not related) | n = 3, CR; n = 1, PR |
| Spindelboeck et al. [100] | Steroid-resistant grade IV gut GvHD | 3 | Colonoscopy | Retrospective, case series | Unrelated/sibling | 9 | No serious AEs | n = 2, CR; n = 1, PR |
| Qi et al. [101] | Steroid-resistant GvHD | 8 | Nasoduodenal tube | Prospective | Unrelated | 12 | No serious AEs | n = 5, CR; n = 1, PR |
| Shouval et al. [102] | Steroid-resistant/dependent GvHD | 7 | Oral capsules | Prospective | Unrelated | 15 | 2 bacteremia (deemed unrelated) | n = 2, CR |
| van Lier et al. [103] | Steroid-resistant/dependent GvHD | 15 | Nasoduodenal tube | Prospective | Unrelated | 15 | No serious AEs | n = 11, CR |
| Kaito et al. [104] | Steroid-resistant grade IV gut GvHD | 1 | Oral capsules | Prospective | Unrelated | 2 | No serious AEs | n = 1, PR |
| Zhong et al. [105] | Steroid-resistant grade III gut GvHD | 1 | Nasoduodenal tube | Retrospective | Unrelated | 2 | No serious AEs | n = 1, CR |
| Biernat et al. [106] | Steroid-resistant grade IV gut GvHD | 2 | Nasoduodenal tube | Retrospective | Unrelated | 7 | No serious AEs | n = 1, CR |
| Mao et al. [107] | Steroid-resistant grade IV gut GvHD | 1 | Oral capsules | Retrospective, case report | Unrelated | 2 | No serious AEs | n = 1, CR |
| Goloshchapov et al. [108] | Steroid-resistant GvHD/4-overlap GvHD | 19 | 3 gastroscopy, 3 nasointestinal tube, 13 oral capsules | Prospective | 15 unrelated, 4 related | 19 | No data | n = 8, CR; n = 8, PR |
| Goloshchapov et al. [109] | Steroid-resistant GvHD/2-overlap GvHD | 7 | 2 gastroscopy, 2 nasoduodenal tube, 3 oral capsules | Prospective pediatric | 4 unrelated, 3 related, All were also HSC donors | 7 | No serious AEs | n = 5, CR; n = 1, PR |
| Goeser et al. [110] | Steroid-resistant GvHD | 11 | 9 oral capsules, 2 nasojejunal tube | Retrospective, case series | Unrelated | 11 | No serious AEs | n = 9, CR; n = 2, PR |
| Zhao et al. [111] | Steroid-resistant GvHD | 23 | Nasoduodenal/nasogastric tube | Prospective | Unrelated | 43 | 2 thrombocytopenia and cardiac events | n = 13, CR; n = 3, PR |
| Biliński et al. [23] | Steroid-resistant GvHD | 11 | Nasoduodenal tube | Prospective | Unrelated | 14 | 2 sepsis and septic shock | n = 5, CR; n = 1, PR |
| Biliński et al. [21] | Steroid-resistant GvHD | 4 | Nasoduodenal tube | Prospective | Unrelated | 15 | No serious AEs | n = 3, CR |
| Malard et al. [24] | Steroid-resistant grade III–IV gut aGvHD n = 24, Steroid-dependent or Steroid-resistant gut aGvHD (classical n = 41, late onset n = 3, overlap syndrome n = 8) for Expanded Access Program | 76 | 2 nasogastric tube, 74 enema | Prospective | Pooled unrelated | 192 | 5 serious AEs in 2 patients | n =29, CR; n = 14, VGPR n = 5, PR |
| TOTAL | 193 | 372 | 12 (4.8%) | ORR (CR + VGPR + PR) = 74% CR = 50% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biliński, J.; Jasiński, M.; Basak, G.W. The Role of Fecal Microbiota Transplantation in the Treatment of Acute Graft-versus-Host Disease. Biomedicines 2022, 10, 837. https://doi.org/10.3390/biomedicines10040837
Biliński J, Jasiński M, Basak GW. The Role of Fecal Microbiota Transplantation in the Treatment of Acute Graft-versus-Host Disease. Biomedicines. 2022; 10(4):837. https://doi.org/10.3390/biomedicines10040837
Chicago/Turabian StyleBiliński, Jarosław, Marcin Jasiński, and Grzegorz W. Basak. 2022. "The Role of Fecal Microbiota Transplantation in the Treatment of Acute Graft-versus-Host Disease" Biomedicines 10, no. 4: 837. https://doi.org/10.3390/biomedicines10040837
APA StyleBiliński, J., Jasiński, M., & Basak, G. W. (2022). The Role of Fecal Microbiota Transplantation in the Treatment of Acute Graft-versus-Host Disease. Biomedicines, 10(4), 837. https://doi.org/10.3390/biomedicines10040837






