Targeted Osmotic Lysis: A Novel Approach to Targeted Cancer Therapies
Abstract
:1. Perspective on the Disease
2. Early Approaches to Treatment
3. Immune Therapies
4. Targeted Therapies
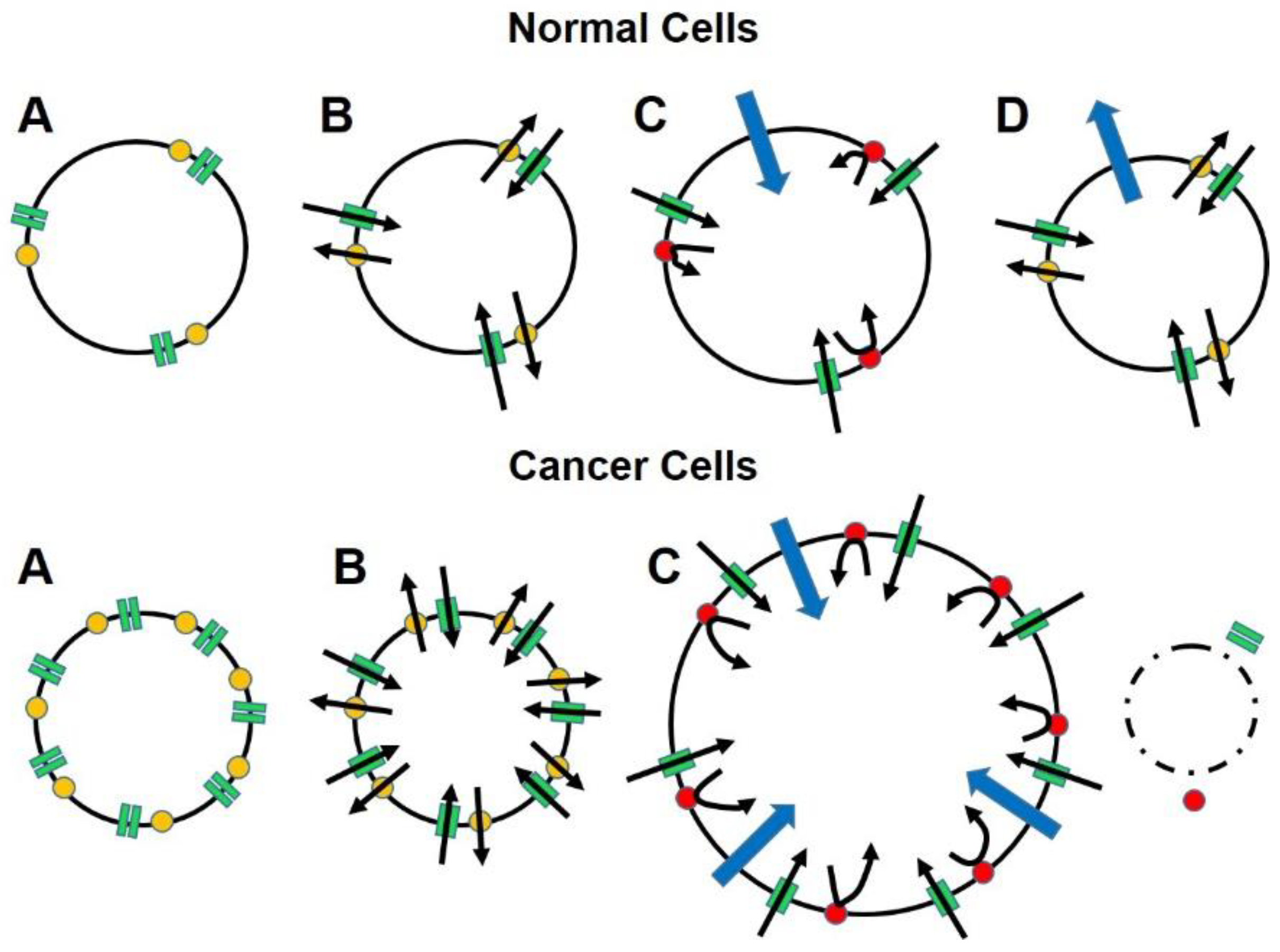
5. An Alternate Approach for Targeting Therapy
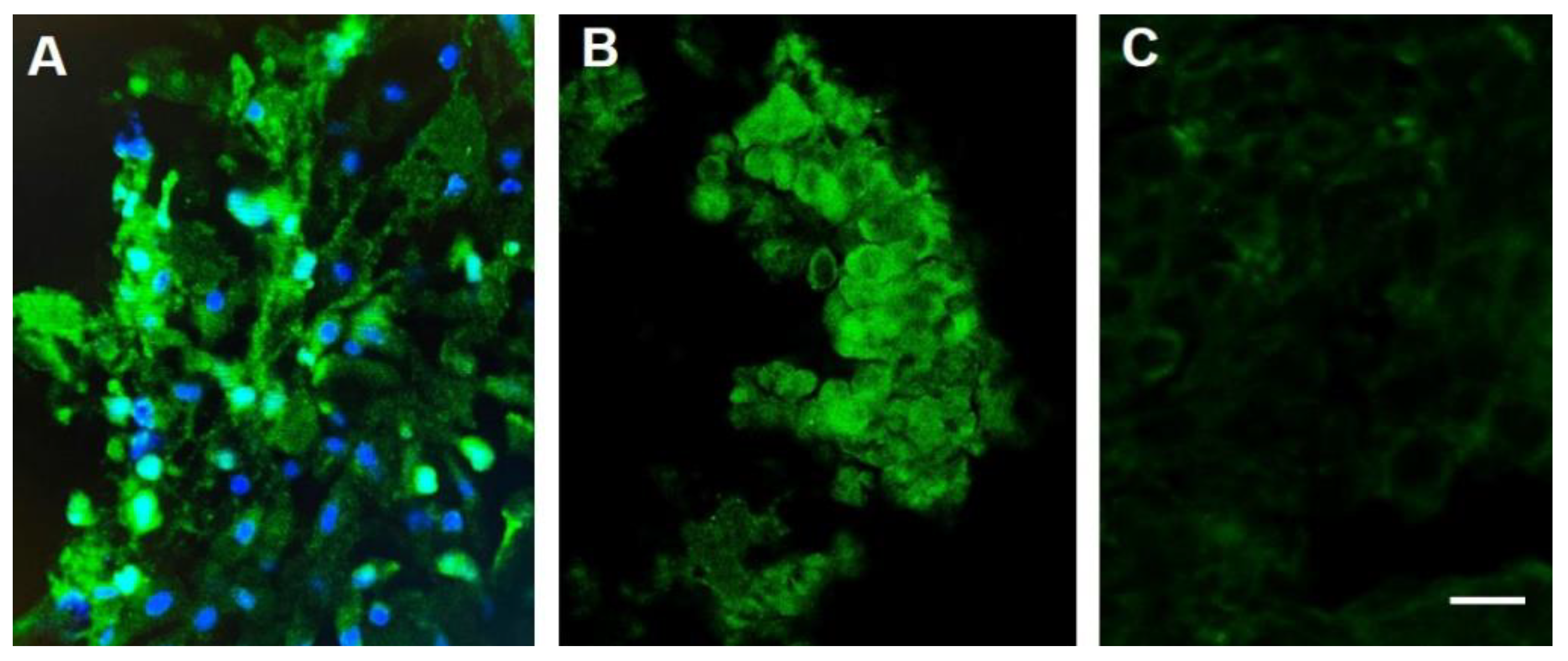
6. Proof-of-Concept Validation
7. Conclusions
8. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Sudhakar, A. History of Cancer, Ancient and Modern Treatment Methods. J. Cancer Sci. Ther. 2009, 1, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwin, C. On the Origin of Species, by Means of Natural Selection or the Preservation of Favoured Races in the Struggle for Life; John Murray: London, UK, 1859. [Google Scholar]
- Branzei, D.; Foiani, M. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 2008, 9, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Branzei, D.; Foiani, M. The checkpoint response to replication stress. DNA Repair 2009, 8, 1038–1046. [Google Scholar] [CrossRef]
- Loeb Classical Library. Hippocrates Volume VII. Epidemics 2, 4–7; Wesley, D.S., Ed.; Harvard University Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Brainy Quote. Desiderius Erasmus Quotes. Available online: http://www.brainyquote.com/quotes/quotes/d/desiderius148997.html (accessed on 20 July 2021).
- Wyld, L.; Audisio, R.A.; Poston, G.J. The evolution of cancer surgery and future perspectives. Nat. Rev. Clin. Oncol. 2015, 12, 115–124. [Google Scholar] [CrossRef]
- Plesca, M.; Bordea, C.; El Houcheimi, B.; Ichim, E.; Blidaru, A. Evolution of radical mastectomy for breast cancer. J. Med. Life 2016, 9, 183–186. [Google Scholar] [PubMed]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the Evolution of Cancer Treatment Therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, D.J.; Goel, A. Wilhelm Roentgen. Available online: https://radiopaedia.org/articles/wilhelm-roentgen-1?lang=us (accessed on 23 July 2021).
- Berkey, F.J. Managing the adverse effects of radiation therapy. Am. Fam. Physician 2010, 82, 381–388. [Google Scholar]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Hall, E.J. Cancer caused by X-rays—A random event? Lancet Oncol. 2007, 8, 369–370. [Google Scholar] [CrossRef]
- Goodman, M. Managing the side effects of chemotherapy. Semin. Oncol. Nurs. 1989, 5 (Suppl. 1), 29–52. [Google Scholar] [CrossRef]
- Livshits, Z.; Rao, R.B.; Smith, S.W. An Approach to Chemotherapy-Associated Toxicity. Emerg. Med. Clin. N. Am. 2014, 32, 167–203. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Day 2019, Emphasis on Early Detection. The ASCO Post. 2019. Available online: https://ascopost.com/News/59711 (accessed on 23 July 2021).
- Osborne, C.K. Tamoxifen in the Treatment of Breast Cancer. N. Engl. J. Med. 1998, 339, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, M.A. The global eradication of smallpox. Am. J. Infect. Control 1982, 10, 53–59. [Google Scholar] [CrossRef]
- Bahl, S.; Bhatnagar, P.; Sutter, R.W.; Roesel, S.; Zaffran, M. Global Polio Eradication–Way Ahead. Indian J. Pediatr. 2018, 85, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haanen, J.B.A.G.; Robert, C. Immune Checkpoint Inhibitors. Prog. Tumor. Res. 2015, 42, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Rora’, A.G.L.; Iacobucci, I.; Martinelli, G. The cell cycle checkpoint inhibitors in the treatment of leukemias. J. Hematol. Oncol. 2017, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Chan, H.L.; Chen, P. Immune Checkpoint Inhibitors: Basics and Challenges. Curr. Med. Chem. 2019, 26, 3009–3025. [Google Scholar] [CrossRef]
- Garber, J.E.; Offit, K. Hereditary Cancer Predisposition Syndromes. J. Clin. Oncol. 2005, 23, 276–292. [Google Scholar] [CrossRef]
- Ormiston, W. Hereditary breast cancer. Eur. J. Cancer Care 1996, 5, 13–20. [Google Scholar] [CrossRef]
- Scalia-Wilbur, J.; Colins, B.L.; Penson, R.T.; Dizon, D.S. Breast Cancer Risk Assessment: Moving Beyond BRCA 1 and 2. Semin. Radiat. Oncol. 2016, 26, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Pandupuspitasari, N.S.; Chun-Jie, H.; Ao, Z.; Jamal, M.; Zohaib, A.; Khan, F.A.; Hakim, M.R.; ShuJun, Z. CRISPR/Cas9 therapeutics: A cure for cancer and other genetic diseases. Oncotarget 2016, 7, 52541–52552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, T.; Rindtorff, N.; Betge, J.; Ebert, M.P.; Boutros, M. CRISPR/Cas9 for cancer research and therapy. Semin. Cancer Biol. 2019, 55, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Fang, T.; Yao, L.; Zheng, Y.; Zhang, L.; Zhu, K. The efficacy and adverse effects of PARP inhibitor combined with chemotherapy compared with chemotherapy alone in the treatment of cancer patient: A protocol for systematic review. Medicine 2020, 99, e23040. [Google Scholar] [CrossRef] [PubMed]
- Gleave, M.E.; Monia, B.P. Antisense therapy for cancer. Nat. Rev. Cancer 2005, 5, 468–479. [Google Scholar] [CrossRef]
- Bradshaw, M.J.; Linnoila, J.J. An Overview of Autoimmune and Paraneoplastic Encephalitides. Semin. Neurol. 2018, 38, 330–343. [Google Scholar] [CrossRef]
- Chen, M.; Mao, A.; Xu, M.; Weng, Q.; Mao, J.; Ji, J. CRISPR-Cas9 for cancer therapy: Opportunities and challenges. Cancer Lett. 2019, 447, 48–55. [Google Scholar] [CrossRef]
- Malhotra, M.K.; Emens, L.A. The evolving management of metastatic triple negative breast cancer. Semin. Oncol. 2020, 47, 229–237. [Google Scholar] [CrossRef]
- Diaby, V.; Tawk, R.; Sanogo, V.; Xiao, H.; Montero, A.J. A review of systematic reviews of the cost-effectiveness of hormone therapy, chemotherapy, and targeted therapy for breast cancer. Breast Cancer Res. Treat. 2015, 151, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Nichols, H. The Top 10 Leading Causes of Death in the US, Medical News Today. 2015. Available online: http://www.medicalnewstoday.com/articles/282929.php (accessed on 23 July 2021).
- Giddings, M.C. On the Process of Becoming a Great Scientist. PLoS Comput. Biol. 2008, 4, e33. [Google Scholar] [CrossRef]
- De Lille, A. All Roads Lead to Rome: New Acquisitions Relating to the Eternal City. Available online: https://italianstudies.nd.edu/news-events/news/all-roads-lead-to-rome-new-acquisitions-relating-to-the-eternal-city/ (accessed on 23 July 2021).
- Gould, H.J., III; Norleans, J.; Ward, T.D.; Reid, C.; Paul, D. Selective lysis of breast carcinomas by simultaneous stimulation of sodium channels and blockade of sodium pumps. Oncotarget 2018, 9, 15606–15615. [Google Scholar] [CrossRef] [Green Version]
- Paul, D.; Maggi, P.; Piero, F.D.; Scahill, S.D.; Sherman, K.J.; Edenfield, S.; Gould, H.J., III. Targeted Osmotic Lysis of Highly Invasive Breast Carcinomas Using Pulsed Magnetic Field Stimulation of Voltage-Gated Sodium Channels and Pharmacological Blockade of Sodium Pumps. Cancers 2020, 12, 1420. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.P.; Diss, J.K.J.; Chioni, A.-M.; Mycielska, M.E.; Pan, H.; Yamaci, R.F.; Pani, F.; Siwy, Z.; Krasowska, M.; Grzywna, Z.; et al. Voltage-Gated Sodium Channel Expression and Potentiation of Human Breast Cancer Metastasis. Clin. Cancer Res. 2005, 11, 5381–5389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onkal, R.; Djamgoz, M.B. Molecular pharmacology of voltage-gated sodium channel expression in metastatic disease: Clinical potential of neonatal Nav1.5 in breast cancer. Eur. J. Pharmacol. 2009, 625, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Djamgoz, M.B.; Onkal, R. Persistent Current Blockers of Voltage-Gated Sodium Channels: A Clinical Opportunity for Controlling Metastatic Disease. Recent Patents Anti-Cancer Drug Discov. 2013, 8, 66–84. [Google Scholar] [CrossRef]
- Bennett, E.S.; Smith, B.A.; Harper, J.M. Voltage-gated Na + channels confer invasive properties on human prostate cancer cells. Pflügers Archiv. 2004, 447, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Brackenbury, W.J.; Chioni, A.-M.; Diss, J.K.J.; Djamgoz, M.B.A. The neonatal splice variant of Nav1.5 potentiates in vitro invasive behaviour of MDA-MB-231 human breast cancer cells. Breast Cancer Res. Treat. 2007, 101, 149–160. [Google Scholar] [CrossRef]
- Brackenbury, W.J.; Isom, L.L. Voltage-gated Na+channels: Potential for β subunits as therapeutic targets. Expert Opin. Ther. Targets 2008, 12, 1191–1203. [Google Scholar] [CrossRef] [Green Version]
- Djamgoz, M.B.A.; Mycielska, M.; Madeia, Z.; Fraser, S.P.; Korohoda, W. Directional movement of rat prostate cancer cells in direct-current electric field: Involvement of voltage gated Na+ channel activity. J. Cell Sci. 2001, 114, 2697–2705. [Google Scholar] [CrossRef]
- Fraser, S.P.; Ozerlat, I.; Diss, J.K.J.; Djamgoz, M.B. Electrophysiological effects of estrogen on voltage-gated Na+ channels in human breast cancer cells. Eur. Biophys. J. 2007, 36, S228. [Google Scholar]
- Wang, Z.; Gao, R.; Shen, Y.; Cai, J.; Lei, M.; Wang, L.-Y. Expression of voltage-gated sodium channel α subunit in human ovarian cancer. Oncol. Rep. 2010, 23, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Gillet, L.; Roger, S.; Besson, P.; Lecaille, F.; Gore, J.; Bougnoux, P.; Lalmanach, G.; Le Guennec, J.-Y. Voltage-gated Sodium Channel Activity Promotes Cysteine Cathepsin-dependent Invasiveness and Colony Growth of Human Cancer Cells. J. Biol. Chem. 2009, 284, 8680–8691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimes, J.A.; Fraser, S.P.; Stephens, G.J.; Downing, J.E.G.; Laniado, M.E.; Foster, C.S.; Abel, P.D.; Djamgoz, M.B.A. Differential expression of voltage-activated Na+ currents in two prostatic tumour cell lines: Contribution to invasiveness in vitro. FEBS Lett. 1995, 369, 290–294. [Google Scholar] [CrossRef] [Green Version]
- House, C.D.; Vaske, C.; Schwartz, A.M.; Obias, V.; Frank, B.; Luu, T.; Sarvazyan, N.; Irby, R.; Strausberg, R.L.; Hales, T.G.; et al. Voltage-Gated Na+ Channel SCN5A Is a Key Regulator of a Gene Transcriptional Network That Controls Colon Cancer Invasion. Cancer Res. 2010, 70, 6957–6967. [Google Scholar] [CrossRef] [Green Version]
- Mechaly, I.; Scamps, F.; Chabbert, C.; Sans, A.; Valmier, J. Molecular diversity of voltage-gated sodium channel alpha subunits expressed in neuronal and non-neuronal excitable cells. Neuroscience 2005, 130, 389–396. [Google Scholar] [CrossRef]
- Onganer, P.U.; Djamgoz, M.B.A. Small-cell Lung Cancer (Human): Potentiation of Endocytic Membrane Activity by Voltage-gated Na+ Channel Expression in Vitro. J. Membr. Biol. 2005, 204, 67–75. [Google Scholar] [CrossRef]
- Roger, S.; Potier, M.; Vandier, C.; Besson, P.; Le Guennec, J.-Y. Voltage-Gated Sodium Channels: New Targets in Cancer Therapy? Curr. Pharm. Des. 2006, 12, 3681–3695. [Google Scholar] [CrossRef] [Green Version]
- Fraser, S.P.; Ozerlat-Gunduz, I.; Brackenbury, W.J.; Fitzgerald, E.M.; Campbell, T.M.; Coombes, R.C.; Djamgoz, M.B.A. Regulation of voltage-gated sodium channel expression in cancer: Hormones, growth factors and auto-regulation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130105. [Google Scholar] [CrossRef] [Green Version]
- Roger, S.; Rollin, J.; Barascu, A.; Besson, P.; Raynal, P.-I.; Iochmann, S.; Lei, M.; Bougnoux, P.; Gruel, Y.; Le Guennec, J.-Y. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int. J. Biochem. Cell Biol. 2007, 39, 774–786. [Google Scholar] [CrossRef] [Green Version]
- Mao, W.; Zhang, J.; Körner, H.; Jiang, Y.; Ying, S. The emerging role of voltage-gated sodium channels in tumor biology. Front Oncol. 2019, 9, 124. [Google Scholar] [CrossRef] [Green Version]
- Wuethrich, P.Y.; Schmitz, S.-F.H.; Kessler, T.M.; Thalmann, G.N.; Studer, U.E.; Stueber, F.; Burkhard, F.C. Potential Influence of the Anesthetic Technique Used during Open Radical Prostatectomy on Prostate Cancer-related Outcome: A retrospective study. Anesthesiology 2010, 113, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, W. Molecular Biology of Aquaporins. Adv. Exp. Med. Biol. 2017, 969, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Gould, H.J., III; Miller, P.R.; Edenfield, S.; Sherman, K.J.; Brady, C.K.; Paul, D. Emergency Use of Targeted Osmotic Lysis for the Treatment of a Patient with Aggressive Late-Stage Squamous Cell Carcinoma of the Cervix. Curr. Oncol. 2021, 28, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Gould, H.J., 3rd; England, J.D.; Liu, Z.P.; Levinson, S.R. Rapid sodium channel augmentation in response to inflammation induced by complete Freund’s adjuvant. Brain Res. 1998, 802, 69–74. [Google Scholar] [CrossRef]
- Gould, H.J.; England, J.D.; Soignier, R.D.; Nolan, P.; Minor, L.D.; Liu, Z.P.; Levinson, S.R.; Paul, D. Ibuprofen blocks changes in nav 1.7 and 1.8 sodium channels associated with complete freund’s adjuvant–induced inflammation in rat. J. Pain 2004, 5, 270–280. [Google Scholar] [CrossRef]
- Casey, G.P.; Roberts, J.S.; Paul, D.; Diamond, I.; Gould, H.J., 3rd. Ranolazine attenuation of CFA-induced mechanical hyper-algesia. Pain Med. 2010, 11, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Casey, G.; Paul, D.; Gould, H.J.; Gould, I. Insulin Is Essential for the Recovery from Allodynia Induced by Complete Freund’s Adjuvant. Pain Med. 2010, 11, 1401–1410. [Google Scholar] [CrossRef]
- Gould, H.J., 3rd; Casey, G.P.; Paul, D. Painful diabetic neuropathy: Current perspective on development and management from bench to bedside–A Review. In Analgesics: New Research; Nova Science Publishers: Hauppauge, NY, USA, 2012. [Google Scholar]
- Paul, D.; Soignier, R.D.; Minor, L.; Tau, H.; Songu-Mize, E.; Gould, H.J., III. Regulation and pharmacological blockade of sodium-potassium ATPase: A novel pathway to neuropath. J. Neurol. Sci. 2014, 340, 139–143. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gould, H.J., III; Paul, D. Targeted Osmotic Lysis: A Novel Approach to Targeted Cancer Therapies. Biomedicines 2022, 10, 838. https://doi.org/10.3390/biomedicines10040838
Gould HJ III, Paul D. Targeted Osmotic Lysis: A Novel Approach to Targeted Cancer Therapies. Biomedicines. 2022; 10(4):838. https://doi.org/10.3390/biomedicines10040838
Chicago/Turabian StyleGould, Harry J., III, and Dennis Paul. 2022. "Targeted Osmotic Lysis: A Novel Approach to Targeted Cancer Therapies" Biomedicines 10, no. 4: 838. https://doi.org/10.3390/biomedicines10040838
APA StyleGould, H. J., III, & Paul, D. (2022). Targeted Osmotic Lysis: A Novel Approach to Targeted Cancer Therapies. Biomedicines, 10(4), 838. https://doi.org/10.3390/biomedicines10040838





