Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Ethics Statement
2.2. Surgery
2.3. Materials
2.4. Experimental Groups and Treatments
2.5. Behavioral Test: Passive Avoidance
2.6. Statistical Analysis
3. Results
3.1. Passive Avoidance Tests
3.1.1. Pilot Study
3.1.2. Dose–Effect Examination
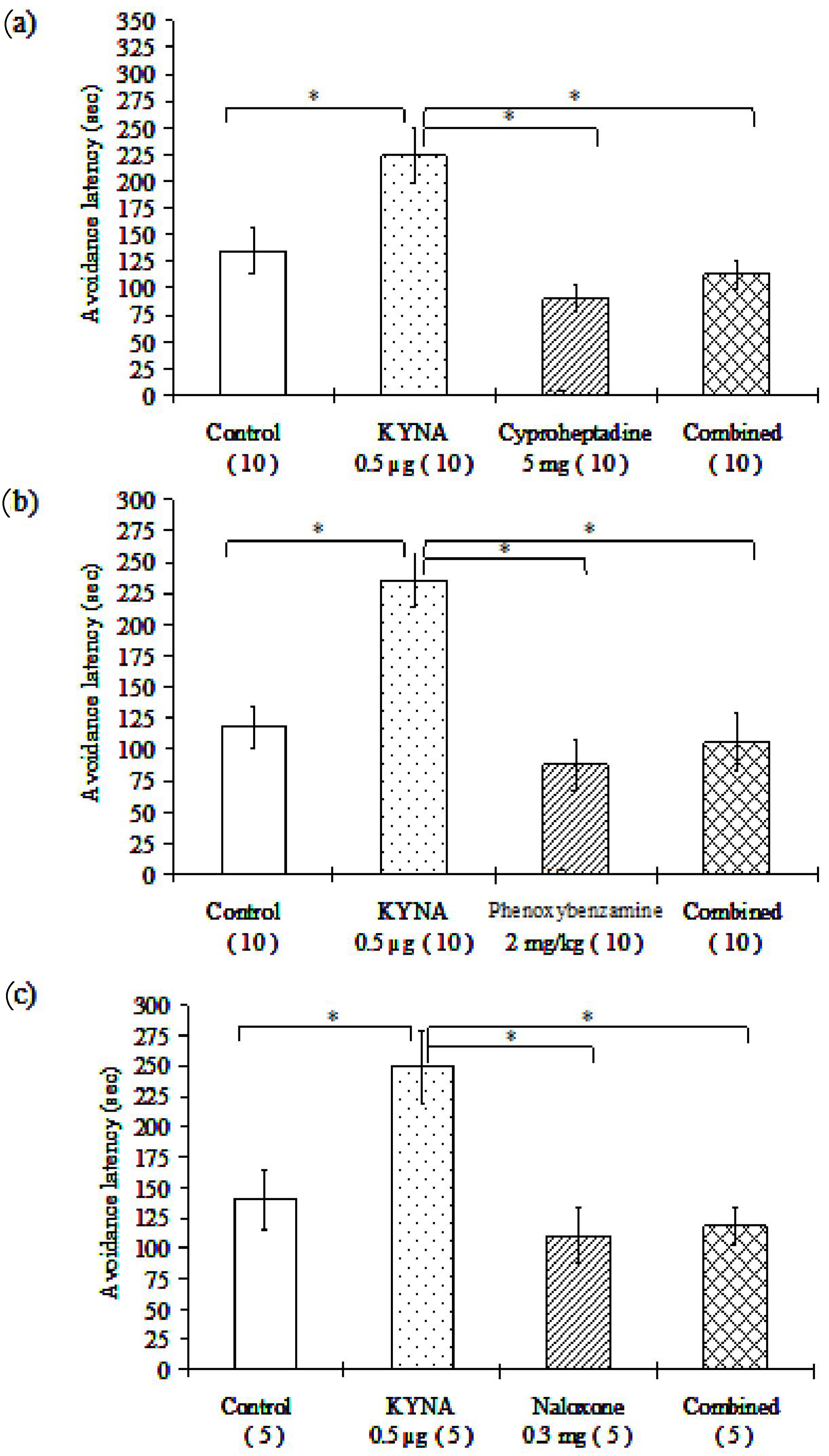
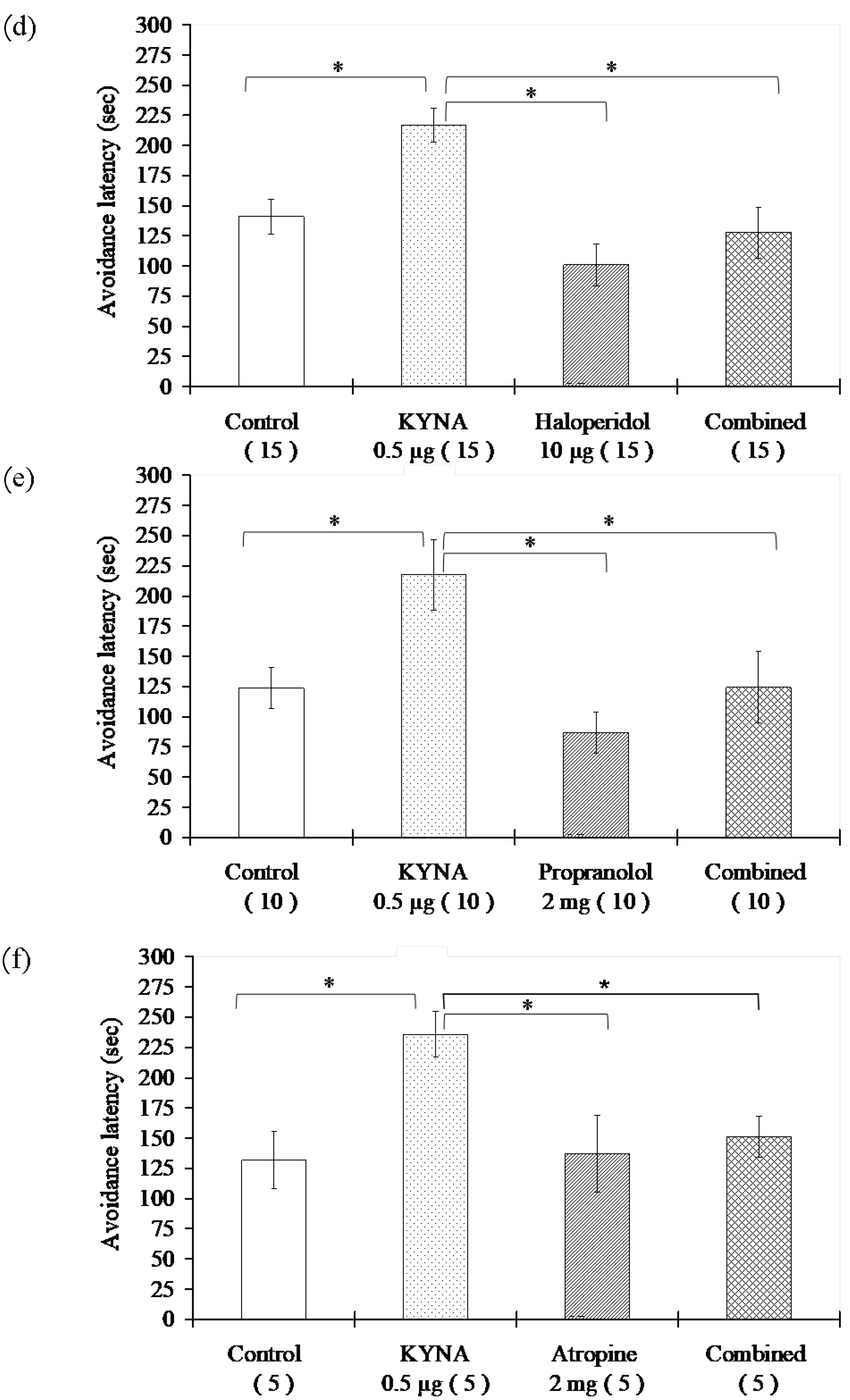
3.1.3. Examination of Different Receptor Blockers
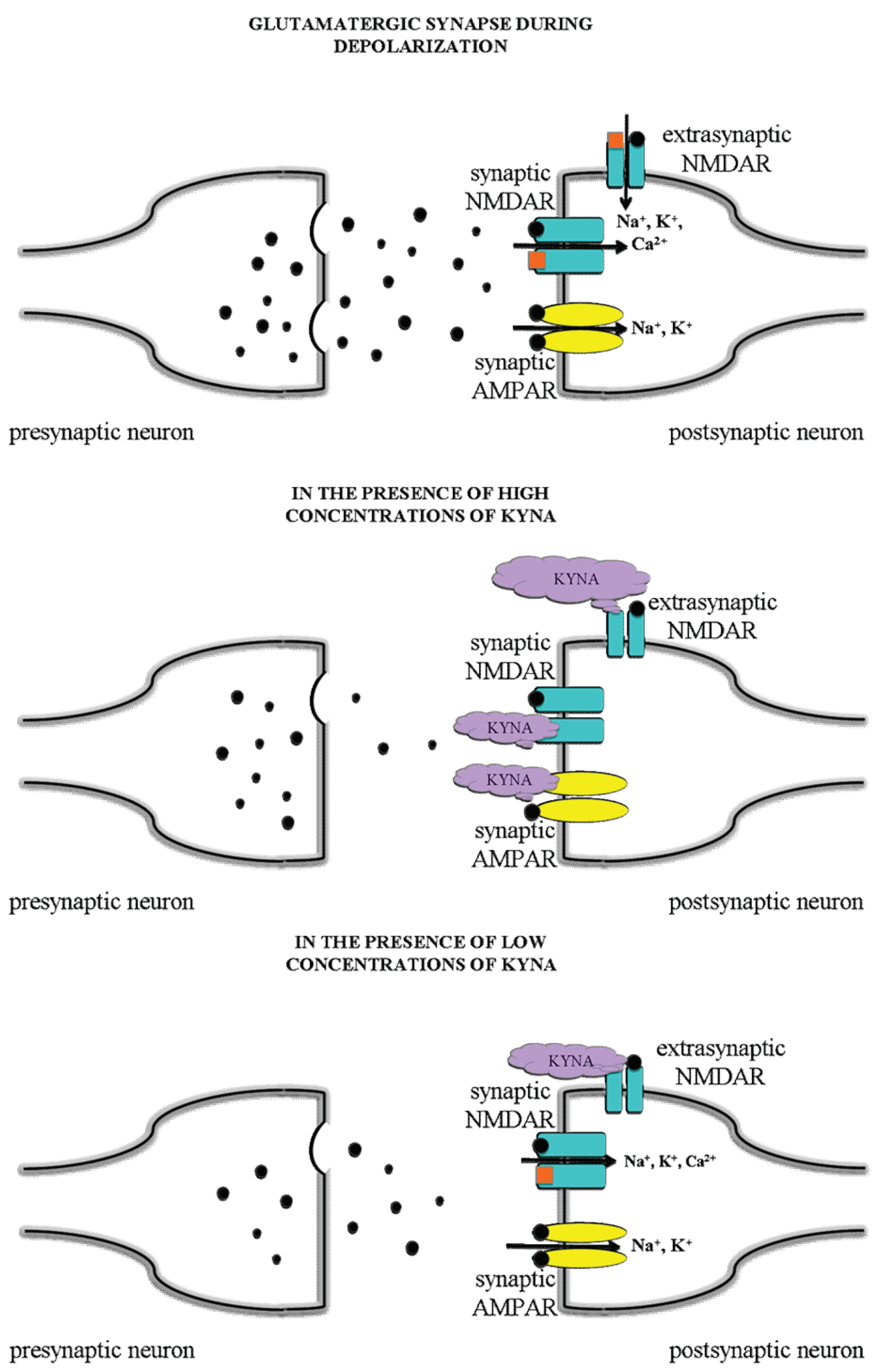
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AHR | aryl hydrocarbon receptor |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid |
| BBB | blood–brain barrier |
| EAARs | excitatory amino acid receptors |
| EPSPs | excitatory postsynaptic potentials |
| GABA | α-aminobutyric acid |
| GPR 35 | G-protein-coupled receptor 35 |
| HD | Huntington’s disease |
| 5-HT2 | 5-hydroxy-triptamin-2 receptor |
| KYNA | kynurenic acid |
| KYN | kynurenine |
| KAT II | kynurenine aminotransferase II enzyme |
| L-KYN | L-kynurenine |
| LTP | long-term potentiation |
| NMDA | N-methyl-D-aspartate receptor |
| PD | Parkinson’s disease |
| PFC | prefrontal cortex |
| QUIN | quinolinic acid |
| Trp | Tryptophan |
References
- World Health Organization. Dementia, World Health Organization. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 18 March 2021).
- Battaglia, S.; Garofalo, S.; di Pellegrino, G. Context-dependent extinction of threat memories: Influences of healthy aging. Sci. Rep. 2018, 8, 12592. [Google Scholar] [CrossRef]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sini, P.; Dang, T.B.C.; Fais, M.; Galioto, M.; Padedda, B.M.; Lugliè, A.; Iaccarino, C.; Crosio, C. Cyanobacteria, Cyanotoxins, and Neurodegenerative Diseases: Dangerous Liaisons. Int. J. Mol. Sci. 2021, 22, 8726. [Google Scholar] [CrossRef]
- Tanaka, M.; Török, N.; Vécsei, L. Novel Pharmaceutical Approaches in Dementia. In NeuroPsychopharmacotherapy; Riederer, P., Laux, G., Nagatsu, T., Le, W., Riederer, C., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Encyclopedia. The Tryptophan-Kynurenine Metabolic Pathway. Available online: https://encyclopedia.pub/8633 (accessed on 15 March 2022).
- Kessler, M.; Terramani, T.; Lynch, G.; Baudry, M. A glycine site associated with N-methyl-D-aspartic acid receptors: Characterization and identification of a new class of antagonists. J. Neurochem. 1989, 52, 1319–1328. [Google Scholar] [CrossRef]
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. 6,7-Dinitro-quinoxaline-2,3-dion and 6-nitro,7-cyano-quinoxaline-2,3-dion antagonise responses to NMDA in the rat spinal cord via an action at the strychnine-insensitive glycine receptor. Eur. J. Parmacol. 1988, 156, 177–180. [Google Scholar] [CrossRef]
- Perkins, M.N.; Stone, T.W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982, 247, 184–187. [Google Scholar] [CrossRef]
- Alkondon, M.; Pereira, E.F.; Albuquerque, E.X. Endogenous activation of nAChRs and NMDA receptors contributes to the excitability of CA1 stratum radiatum interneurons in rat hippocampal slices: Effects of kynurenic acid. Biochem. Pharmacol. 2011, 82, 842–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosi, C.; Mannaioni, G.; Cozzi, A.; Carla, V.; Sili, M.; Cavone, L.; Maratea, D.; Moroni, F. G-protein coupled receptor 35 (GPR35) activation and inflammatory pain: Studies on the antinociceptive effects of kynurenic acid and zaprinast. Neuropharmacology 2011, 60, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic acid: A metabolite with multiple actions and multiple targets in brain and periphery. J. Neural Transm. 2012, 119, 133–139. [Google Scholar] [CrossRef]
- Stone, T.W.; Stoy, N.; Darlington, L.G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol. Sci. 2013, 34, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, G.; Kekesi, G.; Tuboly, G.; Benedek, G. Antinociceptive interactions of triple and quadruple combinations of endogenous ligands at the spinal level. Brain Res. 2007, 1155, 42–48. [Google Scholar] [CrossRef]
- Zador, F.; Samavati, R.; Szlavicz, E.; Tuka, B.; Bojnik, E.; Fulop, F.; Toldi, J.; Vecsei, L.; Borsodi, A. Inhibition of opioid receptor mediated G-protein activity after chronic administration of kynurenic acid and its derivative without direct binding to opioid receptors. CNS Neurol. Dis. Drug Targets 2014, 13, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Hilmas, C.; Pereira, E.F.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef] [PubMed]
- Zadori, D.; Veres, G.; Szalardy, L.; Klivenyi, P.; Fulop, F.; Toldi, J.; Vecsei, L. Inhibitors of the kynurenine pathway as neurotherapeutics: A patent review (2012–2015). Expert Opin. Ther. Pat. 2016, 26, 815–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, T.W. Kynurenic acid blocks nicotinic synaptic transmission to hippocampal interneurons in young rats. Eur. J. Nneurosci. 2007, 25, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- Arnaiz-Cot, J.J.; Gonzalez, J.C.; Sobrado, M.; Baldelli, P.; Carbone, E.; Gandia, L.; Garcia, A.G.; Hernandez-Guijo, J.M. Allosteric modulation of alpha 7 nicotinic receptors selectively depolarizes hippocampal interneurons, enhancing spontaneous GABAergic transmission. Eur. J. Neurosci. 2008, 27, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Mok, M.H.; Fricker, A.C.; Weil, A.; Kew, J.N. Electrophysiological characterisation of the actions of kynurenic acid at ligand-gated ion channels. Neuropharmacology 2009, 57, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Dobelis, P.; Staley, K.J.; Cooper, D.C. Lack of modulation of nicotinic acetylcholine alpha-7 receptor currents by kynurenic acid in adult hippocampal interneurons. PLoS ONE 2012, 7, e41108. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Plangar, I.; Tuka, B.; Gellert, L.; Varga, D.; Demeter, I.; Farkas, T.; Kis, Z.; Marosi, M.; Zadori, D.; et al. Synthesis and biological effects of some kynurenic acid analogs. Bioorgan. Med. Chem. 2011, 19, 7590–7596. [Google Scholar] [CrossRef]
- Vamos, E.; Pardutz, A.; Varga, H.; Bohar, Z.; Tajti, J.; Fulop, F.; Toldi, J. Vecsei L: L-kynurenine combined with probenecid and the novel synthetic kynurenic acid derivative attenuate nitroglycerin-induced nNOS in the rat caudal trigeminal nucleus. Neuropharmacology 2009, 57, 425–429. [Google Scholar] [CrossRef]
- Chauvel, V.; Vamos, E.; Pardutz, A.; Vecsei, L.; Schoenen, J.; Multon, S. Effect of systemic kynurenine on cortical spreading depression and its modulation by sex hormones in rat. Exp. Neurol. 2012, 236, 207–214. [Google Scholar] [CrossRef]
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, Á.; Vécsei, L. Co-Players in Chronic Pain: Neuroinflammation and the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef]
- Ciapała, K.; Mika, J.; Rojewska, E. The Kynurenine Pathway as a Potential Target for Neuropathic Pain Therapy Design: From Basic Research to Clinical Perspectives. Int. J. Mol. Sci. 2021, 22, 11055. [Google Scholar] [CrossRef]
- Jovanovic, F.; Candido, K.D.; Knezevic, N.N. The Role of the Kynurenine Signaling Pathway in Different Chronic Pain Conditions and Potential Use of Therapeutic Agents. Int. J. Mol. Sci. 2020, 21, 6045. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Fila, M.; Chojnacki, J.; Pawlowska, E.; Szczepanska, J.; Chojnacki, C.; Blasiak, J. Kynurenine Pathway of Tryptophan Metabolism in Migraine and Functional Gastrointestinal Disorders. Int. J. Mol. Sci. 2021, 22, 10134. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Laborc, K.F.; Bohár, Z.; Nagy-Grócz, G.; Fejes-Szabó, A.; Szűcs, M.; Vécsei, L.; Párdutz, Á. Effect of dural inflammatory soup application on activation and sensitization markers in the caudal trigeminal nucleus of the rat and the modulatory effects of sumatriptan and kynurenic acid. J. Headache Pain 2021, 22, 17. [Google Scholar] [CrossRef]
- Sas, K.; Robotka, H.; Rozsa, E.; Agoston, M.; Szenasi, G.; Gigler, G.; Marosi, M.; Kis, Z.; Farkas, T.; Vecsei, L.; et al. Kynurenine diminishes the ischemia-induced histological and electrophysiological deficits in the rat hippocampus. Neurobiol. Dis. 2008, 32, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Gellert, L.; Fuzik, J.; Goblos, A.; Sarkozi, K.; Marosi, M.; Kis, Z.; Farkas, T.; Szatmari, I.; Fulop, F.; Vecsei, L.; et al. Neuroprotection with a new kynurenic acid analog in the four-vessel occlusion model of ischemia. Eur. J. Pharmacol. 2001, 667, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Gellert, L.; Knapp, L.; Nemeth, K.; Heredi, J.; Varga, D.; Olah, G.; Kocsis, K.; Menyhart, A.; Kis, Z.; Farkas, T.; et al. Post-ischemic treatment with L-kynurenine sulfate exacerbates neuronal damage after transient middle cerebral artery occlusion. Neuroscience 2013, 247, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Demeter, I.; Nagy, K.; Gellert, L.; Vecsei, L.; Fulop, F.; Toldi, J. A novel kynurenic acid analog (SZR104) inhibits pentylenetetrazole-induced epileptiform seizures. An electrophysiological study: Special issue related to kynurenine. J. Neural Transm. 2012, 119, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Zadori, D.; Nyiri, G.; Szonyi, A.; Szatmari, I.; Fulop, F.; Toldi, J.; Freund, T.F.; Vecsei, L.; Klivenyi, P. Neuroprotective effects of a novel kynurenic acid analogue in a transgenic mouse model of Huntington’s disease. J. Neural Transm. 2011, 118, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Hartai, Z.; Klivenyi, P.; Janaky, T.; Penke, B.; Dux, L.; Vecsei, L. Kynurenine metabolism in plasma and in red blood cells in Parkinson’s disease. J. Neurol. Sci. 2005, 239, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Puerto, N.; Giménez-Gómez, P.; Pérez-Hernández, M.; Abuin-Martínez, C.; Leticia, G.B.; Vidal, R.; Gutiérrez-López, M.D.; O’Shea, E.; Colado, M.I. Addiction and the kynurenine pathway: A new dancing couple? Pharmacol. Ther. 2021, 223, 107807. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Török, N.; Vécsei, L. Are 5-HT1 receptor agonists effective anti-migraine drugs? Expert Opin Pharmacother. 2021, 22, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Erabi, H.; Okada, G.; Shibasaki, C.; Setoyama, D.; Kang, D.; Takamura, M.; Yoshino, A.; Fuchikami, M.; Kurata, A.; Kato, T.A.; et al. Kynurenic acid is a potential overlapped biomarker between diagnosis and treatment response for depression from metabolome analysis. Sci. Rep. 2020, 10, 16822. [Google Scholar] [CrossRef]
- Balogh, L.; Tanaka, M.; Török, N.; Vécsei, L.; Taguchi, S. Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders. Biomedicines 2021, 9, 340. [Google Scholar] [CrossRef]
- Hunt, C.; Macedo e Cordeiro, T.; Suchting, R.; de Dios, C.; Cuellar Leal, V.A.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of immune activation on the kynurenine pathway and depression symptoms—A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 514. [Google Scholar] [CrossRef]
- Carrillo-Mora, P.; Pérez-De la Cruz, V.; Estrada-Cortés, B.; Toussaint-González, P.; Martínez-Cortéz, J.A.; Rodríguez-Barragán, M.; Quinzaños-Fresnedo, J.; Rangel-Caballero, F.; Gamboa-Coria, G.; Sánchez-Vázquez, I.; et al. Serum Kynurenines Correlate With Depressive Symptoms and Disability in Poststroke Patients: A Cross-sectional Study. Neurorehabil. Neural Repair 2020, 34, 936–944. [Google Scholar] [CrossRef]
- Simonato, M.; Dall’Acqua, S.; Zilli, C.; Sut, S.; Tenconi, R.; Gallo, N.; Sfriso, P.; Sartori, L.; Cavallin, F.; Fiocco, U.; et al. Tryptophan Metabolites, Cytokines, and Fatty Acid Binding Protein 2 in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines 2021, 9, 1724. [Google Scholar] [CrossRef]
- Ramírez Ortega, D.; Ugalde Muñiz, P.E.; Blanco Ayala, T.; Vázquez Cervantes, G.I.; Lugo Huitrón, R.; Pineda, B.; González Esquivel, D.F.; Pérez de la Cruz, G.; Pedraza Chaverrí, J.; Sánchez Chapul, L.; et al. On the Antioxidant Properties of L-Kynurenine: An Efficient ROS Scavenger and Enhancer of Rat Brain Antioxidant Defense. Antioxidants 2022, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Ramírez, J.; Rodríguez-Núñez, M.; Marco, M.-P.; Mir, M.; Samitier, J. Kynurenic Acid Electrochemical Immunosensor: Blood-Based Diagnosis of Alzheimer’s Disease. Biosensors 2021, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Fabius, J.H.; Moravkova, K.; Fracasso, A.; Borgomaneri, S. The Neurobiological Correlates of Gaze Perception in Healthy Individuals and Neurologic Patients. Biomedicines 2022, 10, 627. [Google Scholar] [CrossRef]
- Baran, H.; Jellinger, K.; Deecke, L. Kynurenine metabolism in Alzheimer’s disease. J. Neural Transm. 1999, 106, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Pershing, M.L.; Bortz, D.M.; Pocivavsek, A.; Fredericks, P.J.; Jorgensen, C.V.; Vunck, S.A.; Leuner, B.; Schwarcz, R.; Bruno, J.P. Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: Implications for schizophrenia. Neuropharmacology 2015, 90, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocivavsek, A.; Thomas, M.A.; Elmer, G.I.; Bruno, J.P.; Schwarcz, R. Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology 2014, 231, 2799–2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Battaglia, S.; Avenanti, A.; Pellegrino, G.D. Don’t Hurt Me No More: State-dependent Transcranial Magnetic Stimulation for the treatment of specific phobia. J. Affect. Disord. 2021, 286, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Battaglia, S.; Garofalo, S.; Tortora, F.; Avenanti, A.; di Pellegrino, G. State-Dependent TMS over Prefrontal Cortex Disrupts Fear-Memory Reconsolidation and Prevents the Return of Fear. Curr. Biol. 2020, 30, 3672–3679.e4. [Google Scholar] [CrossRef]
- Gellert, L.; Varga, D.; Ruszka, M.; Toldi, J.; Farkas, T.; Szatmari, I.; Fulop, F.; Vecsei, L.; Kis, Z. Behavioural studies with a newly developed neuroprotective KYNA-amide. J. Neural Transm. 2012, 119, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Demeter, I.; Nagy, K.; Farkas, T.; Kis, Z.; Kocsis, K.; Knapp, L.; Gellert, L.; Fulop, F.; Vecsei, L.; Toldi, J. Paradox effects of kynurenines on LTP induction in the Wistar rat. An in vivo study. Neurosci. Let. 2013, 553, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.; Weeks, A.M.; Staley, K.J.; Partin, K.M. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci. Lett. 2006, 402, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Rozsa, E.; Robotka, H.; Vecsei, L.; Toldi, J. The Janus-face kynurenic acid. J. Neural Transm. 2008, 115, 1087–1091. [Google Scholar] [CrossRef]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol. Psychiatry 2021. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S. Neurobiological advances of learned fear in humans. Adv. Clin. Exp. Med. 2022, 31, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, S.; Timmermann, C.; Battaglia, S.; Maier, M.E.; di Pellegrino, G. Mediofrontal Negativity Signals Unexpected Timing of Salient Outcomes. J. Cogn. Neurosci. 2017, 29, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Garofalo, S.; di Pellegrino, G.; Starita, F. Revaluing the Role of vmPFC in the Acquisition of Pavlovian Threat Conditioning in Humans. J. Neurosci. 2020, 40, 8491–8500. [Google Scholar] [CrossRef] [PubMed]
- Koshy Cherian, A.; Gritton, H.; Johnson, D.E.; Young, D.; Kozak, R.; Sarter, M. A systemically-available kynurenine aminotransferase II (KAT II) inhibitor restores nicotine-evoked glutamatergic activity in the cortex of rats. Neuropharmacology 2014, 82, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozak, R.; Campbell, B.M.; Strick, C.A.; Horner, W.; Hoffmann, W.E.; Kiss, T.; Chapin, D.S.; McGinnis, D.; Abbott, A.L.; Roberts, B.M.; et al. Reduction of brain kynurenic acid improves cognitive function. J. Neurosci. 2014, 34, 10592–10602. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.C.; Elmer, G.I.; Bergeron, R.; Albuquerque, E.X.; Guidetti, P.; Wu, H.Q.; Schwarcz, R. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology 2010, 35, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Schally, A.V.; Telegdy, G. Neurotransmission of the antidepressant-like effects of the growth hormone-releasing hormone antagonist MZ-4-71. Behav. Brain Res. 2012, 228, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Telegdy, G.; Schally, A.V. Involvement of neurotransmitters in the action of growth hormone-releasing hormone antagonist on passive avoidance learning. Behav. Brain. Res. 2012, 233, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Telegdy, G.; Tiricz, H.; Adamik, A. Involvement of neurotransmitters in urocortin-induced passive avoidance learning in mice. Behav. Brain. Bull. 2005, 67, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Palotai, M.; Telegdy, G.; Bagosi, Z.; Jaszberenyi, M. The action of neuropeptide AF on passive avoidance learning. Involvement of neurotransmitters. Neurobiol. Learn Mem. 2016, 127, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Palotai, M.; Telegdy, G.; Tanaka, M.; Bagosi, Z.; Jászberényi, M. Neuropeptide AF induces anxiety-like and antidepressant-like behavior in mice. Behav. Brain Res. 2014, 274, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Telegdy, G.; Tanaka, M.; Schally, A.V. Effects of the growth hormone-releasing hormone (GH-RH) antagonist on brain functions in mice. Behav. Brain Res. 2011, 224, 155–158. [Google Scholar] [CrossRef]
- Telegdy, G.; Adamik, A.; Tanaka, M.; Schally, A.V. Effects of the LHRH antagonist Cetrorelix on affective and cognitive functions in rats. Regul. Pept. 2010, 159, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Petroianu, G.A. Hyperthermia and Serotonin: The Quest for a “Better Cyproheptadine”. Int. J. Mol. Sci. 2022, 23, 3365. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.W.; DeMarinis, R.M.; Caron, M.G.; Lefkowitz, R.J. Identification of the subunit-binding site of alpha 2-adrenergic receptors using [3H]phenoxybenzamine. J. Biol. Chem. 1984, 259, 7864–7869. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Bioassay Record for Bioactivity AID 1135637—SID 103170037, Bioactivity for AID 1135637—SID 103170037, Source: ChEMBL. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/1135637#sid=103170037 (accessed on 25 March 2022).
- National Center for Biotechnology Information. PubChem Bioassay Record for Bioactivity AID 65111—SID 103167216, Bioactivity for AID 65111—SID 103167216, Source: ChEMBL. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/65111#sid=103167216 (accessed on 25 March 2022).
- Fan, L.; Tan, L.; Chen, Z.; Qi, J.; Nie, F.; Luo, Z.; Cheng, J.; Wang, S. Haloperidol bound D2 dopamine receptor structure inspired the discovery of subtype selective ligands. Nat. Commun. 2020, 11, 1074. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Bioassay Record for Bioactivity AID 42040—SID 103164951, Bioactivity for AID 42040—SID 103164951, Source: ChEMBL. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/42040#sid=103164951 (accessed on 25 March 2022).
- Xu, J.; Chuang, D.M. Muscarinic acetylcholine receptor-mediated phosphoinositide turnover in cultured cerebellar granule cells: Desensitization by receptor agonists. J. Pharmacol. Exp. Ther. 1987, 242, 238–244. [Google Scholar]
- Tanaka, M.; Telegdy, G. Neurotransmissions of antidepressant-like effects of neuromedin U-23 in mice. Behav. Brain Res. 2014, 259, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Thabault, M.; Turpin, V.; Maisterrena, A.; Jaber, M.; Egloff, M.; Galvan, L. Cerebellar and Striatal Implications in Autism Spectrum Disorders: From Clinical Observations to Animal Models. Int. J. Mol. Sci. 2022, 23, 2294. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Csabafi, K.; Telegdy, G. Neurotransmissions of antidepressant-like effects of kisspeptin-13. Regul. Pept. 2013, 180, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Correia, B.S.B.; Nani, J.V.; Waladares Ricardo, R.; Stanisic, D.; Costa, T.B.B.C.; Hayashi, M.A.F.; Tasic, L. Effects of Psychostimulants and Antipsychotics on Serum Lipids in an Animal Model for Schizophrenia. Biomedicines 2021, 9, 235. [Google Scholar] [CrossRef]
- Swingler, T.E.; Niu, L.; Pontifex, M.G.; Vauzour, D.; Clark, I.M. The microRNA-455 Null Mouse Has Memory Deficit and Increased Anxiety, Targeting Key Genes Involved in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 554. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kádár, K.; Tóth, G.; Telegdy, G. Antidepressant-like effects of urocortin 3 fragments. Brain Res. Bull. 2011, 84, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Llort, L.; Marin-Pardo, D.; Marazuela, P.; Hernández-Guillamón, M. Survival Bias and Crosstalk between Chronological and Behavioral Age: Age- and Genotype-Sensitivity Tests Define Behavioral Signatures in Middle-Aged, Old, and Long-Lived Mice with Normal and AD-Associated Aging. Biomedicines 2021, 9, 636. [Google Scholar] [CrossRef]
- Muntsant, A.; Giménez-Llort, L. Genotype Load Modulates Amyloid Burden and Anxiety-like Patterns in Male 3xTg-AD Survivors despite Similar Neuro-Immunoendocrine, Synaptic and Cognitive Impairments. Biomedicines 2021, 9, 715. [Google Scholar] [CrossRef]
- Santana-Santana, M.; Bayascas, J.-R.; Giménez-Llort, L. Fine-Tuning the PI3K/Akt Signaling Pathway Intensity by Sex and Genotype-Load: Sex-Dependent Homozygotic Threshold for Somatic Growth but Feminization of Anxious Phenotype in Middle-Aged PDK1 K465E Knock-In and Heterozygous Mice. Biomedicines 2021, 9, 747. [Google Scholar] [CrossRef]
- Vila-Merkle, H.; González-Martínez, A.; Campos-Jiménez, R.; Martínez-Ricós, J.; Teruel-Martí, V.; Blasco-Serra, A.; Lloret, A.; Celada, P.; Cervera-Ferri, A. The Oscillatory Profile Induced by the Anxiogenic Drug FG-7142 in the Amygdala–Hippocampal Network Is Reversed by Infralimbic Deep Brain Stimulation: Relevance for Mood Disorders. Biomedicines 2021, 9, 783. [Google Scholar] [CrossRef]
- Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Belozertseva, I.V.; Tamkovich, N.V.; Baranov, K.O.; Kudryavtseva, N.N. Chronic Lithium Treatment Affects Anxious Behaviors and theExpression of Serotonergic Genes in Midbrain Raphe Nuclei of Defeated Male Mice. Biomedicines 2021, 9, 1293. [Google Scholar] [CrossRef]
- Lee, E.C.; Hong, D.-Y.; Lee, D.-H.; Park, S.-W.; Lee, J.Y.; Jeong, J.H.; Kim, E.-Y.; Chung, H.-M.; Hong, K.-S.; Park, S.-P.; et al. Inflammation and Rho-Associated Protein Kinase-Induced Brain Changes in Vascular Dementia. Biomedicines 2022, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Mariqueo, L.; Pérez-García, M.J.; Giménez-Llort, L. Modeling Functional Limitations, Gait Impairments, and Muscle Pathology in Alzheimer’s Disease: Studies in the 3xTg-AD Mice. Biomedicines 2021, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Lamoine, S.; Cumenal, M.; Barriere, D.A.; Pereira, V.; Fereyrolles, M.; Prival, L.; Barbier, J.; Boudieu, L.; Brasset, E.; Bertin, B.; et al. The Class I HDAC Inhibitor, MS-275, Prevents Oxaliplatin-Induced Chronic Neuropathy and Potentiates Its Antiproliferative Activity in Mice. Int. J. Mol. Sci. 2022, 23, 98. [Google Scholar] [CrossRef]
- Quirant-Sánchez, B.; Mansilla, M.J.; Navarro-Barriuso, J.; Presas-Rodríguez, S.; Teniente-Serra, A.; Fondelli, F.; Ramo-Tello, C.; Martínez-Cáceres, E. Combined Therapy of Vitamin D3-Tolerogenic Dendritic Cells and Interferon-β in a Preclinical Model of Multiple Sclerosis. Biomedicines 2021, 9, 1758. [Google Scholar] [CrossRef]
- Jeong, W.-H.; Kim, W.-I.; Lee, J.-W.; Park, H.-K.; Song, M.-K.; Choi, I.-S.; Han, J.-Y. Modulation of Long-Term Potentiation by Gamma Frequency Transcranial Alternating Current Stimulation in Transgenic Mouse Models of Alzheimer’s Disease. Brain Sci. 2021, 11, 1532. [Google Scholar] [CrossRef] [PubMed]
- Chiamulera, C.; Costa, S.; Reggiani, A. Effect of NMDA- and strychnine-insensitive glycine site antagonists on NMDA-mediated convulsions and learning. Psychopharmacology 1990, 102, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Tocco, G.; Maren, S.; Shors, T.J.; Baudry, M.; Thompson, R.F. Long-term potentiation is associated with increased [3H]AMPA binding in rat hippocampus. Brain Res. 1992, 573, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.M.; Guevremont, D.; Mason-Parker, S.E.; Luxmanan, C.; Tate, W.P.; Abraham, W.C. Differential trafficking of AMPA and NMDA receptors during long-term potentiation in awake adult animals. J. Neurosci. 2007, 27, 14171–14178. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, N.; Ghelardini, C.; Pittaluga, A.; Pugliese, A.M.; Bartolini, A.; Manetti, D.; Romanelli, M.N.; Gualtieri, F. AMPA-receptor activation is involved in the antiamnesic effect of DM 232 (unifiram) and DM 235 (sunifiram). Naunyn-Schmiedeberg’s Arch. Pharmacol. 2003, 368, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Vecsei, L.; Szalardy, L.; Fulop, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci. Ther. 2022, 28, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szalardy, L.; Zadori, D.; Toldi, J.; Fulop, F.; Klivenyi, P.; Vecsei, L. Manipulating kynurenic acid levels in the brain—On the edge between neuroprotection and cognitive dysfunction. Curr. Top. Med. Chem. 2012, 12, 1797–1806. [Google Scholar] [CrossRef]
- Dezsi, L.; Tuka, B.; Martos, D.; Vecsei, L. Alzheimer’s disease, astrocytes and kynurenines. Cur. Alzheimer Res. 2015, 12, 462–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecsei, L. (Ed.) Kynurenines in the Brain: From Experiments to Clinics; Nova Science Publishers Inc.: New York, NY, USA, 2005; Available online: https://www.abebooks.com/9781594543654/Kynurenines-Brain-Experiments-Clinics-Vecsei-1594543658/plp (accessed on 18 March 2022).
- Vecsei, L.; Beal, M.F. Influence of kynurenine treatment on open-field activity, elevated plus-maze, avoidance behaviors and seizures in rats. Pharmacol. Biochem. Behav. 1990, 37, 71–76. [Google Scholar] [CrossRef]
- Swartz, K.J.; During, M.J.; Freese, A.; Beal, M.F. Cerebral synthesis and release of kynurenic acid: An endogenous antagonist of excitatory amino acid receptors. J. Neurosci. 1990, 10, 2965–2973. [Google Scholar] [CrossRef] [PubMed]
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988, 154, 85–87. [Google Scholar] [CrossRef]
- Csapo, E.; Majlath, Z.; Juhasz, A.; Roosz, B.; Hetenyi, A.; Toth, G.K.; Tajti, J.; Vecsei, L.; Dekany, I. Determination of binding capacity and adsorption enthalpy between Human Glutamate Receptor (GluR1) peptide fragments and kynurenic acid by surface plasmon resonance experiments. Colloids and surfaces B. Biointerfaces 2014, 123, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D. A review of the effects of memantine on clinical progression in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2012, 27, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, N.; Li, A.; Lanctot, K. Memantine in dementia: A review of the current evidence. Expert Opin. Parmacother. 2011, 12, 787–800. [Google Scholar] [CrossRef]
- Majlath, Z.; Torok, N.; Toldi, J.; Vecsei, L. Memantine and Kynurenic Acid: Current Neuropharmacological Aspects. Curr. Neuropharmacol. 2016, 14, 200–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood-brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Varga, N.; Csapo, E.; Majlath, Z.; Ilisz, I.; Krizbai, I.A.; Wilhelm, I.; Knapp, L.; Toldi, J.; Vecsei, L.; Dekany, I. Targeting of the kynurenic acid across the blood-brain barrier by core-shell nanoparticles. Eur. J. Pharm. Sci. 2016, 86, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Vecsei, L.; Beal, M.F. Intracerebroventricular injection of kynurenic acid, but not kynurenine, induces ataxia and stereotyped behavior in rats. Brain Res. Bull. 1990, 25, 623–627. [Google Scholar] [CrossRef]
- Carrillo-Mora, P.; Mendez-Cuesta, L.A.; Perez-De La Cruz, V.; Fortoul-van Der Goes, T.I.; Santamaria, A. Protective effect of systemic L-kynurenine and probenecid administration on behavioural and morphological alterations induced by toxic soluble amyloid beta (25–35) in rat hippocampus. Behav. Brain Res. 2010, 210, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Silva-Adaya, D.; Perez-De La Cruz, V.; Villeda-Hernandez, J.; Carrillo-Mora, P.; Gonzalez-Herrera, I.G.; Garcia, E.; Colin-Barenque, L.; Pedraza-Chaverri, J.; Santamaria, A. Protective effect of L-kynurenine and probenecid on 6-hydroxydopamine-induced striatal toxicity in rats: Implications of modulating kynurenate as a protective strategy. Neurotoxicol. Teratol. 2011, 33, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Justinova, Z.; Mascia, P.; Wu, H.Q.; Secci, M.E.; Redhi, G.H.; Panlilio, L.V.; Scherma, M.; Barnes, C.; Parashos, A.; Zara, T.; et al. Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nat. Neurosci. 2013, 16, 1652–1661. [Google Scholar] [CrossRef] [Green Version]
- Luchowska, E.; Kloc, R.; Olajossy, B.; Wnuk, S.; Wielosz, M.; Owe-Larsson, B.; Urbanska, E.M. beta-adrenergic enhancement of brain kynurenic acid production mediated via cAMP-related protein kinase A signaling. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Linderholm, K.R.; Alm, M.T.; Larsson, M.K.; Olsson, S.K.; Goiny, M.; Hajos, M.; Erhardt, S.; Engberg, G. Inhibition of kynurenine aminotransferase II reduces activity of midbrain dopamine neurons. Neuropharmacology 2016, 102, 42–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajti, J.; Csati, A.; Vecsei, L. Novel strategies for the treatment of migraine attacks via the CGRP, serotonin, dopamine, PAC1, and NMDA receptors. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1509–1520. [Google Scholar] [CrossRef] [Green Version]


| 1th Day | 2nd Day | 3rd Day | ||||
|---|---|---|---|---|---|---|
| Groups | Trials | Trial | Post-Trial Treatments | Measure | ||
| Control | 3 × 2 min | Footshock in the dark part | i.p. saline | 30 min later | i.c.v. saline | 300 s |
| KYNA | 3 × 2 min | Footshock in the dark part | i.p. saline | i.c.v. KYNA | 300 s | |
| Receptor blockers | 3 × 2 min | Footshock in the dark part | i.p. receptor blocker | i.c.v. saline | 300 s | |
| Combined | 3 × 2 min | Footshock in the dark part | i.p. receptor blocker | i.c.v. KYNA | 300 s | |
| Receptor Blockers (Doses) | Binding Affinity (Ki) | Control vs. Receptor Blocker | Control vs. KYNA | KYNA vs. Receptor Blocker | KYNA vs. Receptor Blocker Combined |
|---|---|---|---|---|---|
| Cyproheptadine (5 mg/kg) | 1–9 nM [75] | p < 0.384 | p < 0.013 | p < 0.001 | p < 0.002 |
| Phenoxybenzamine (2 mg/kg) | 108 nM [76] | p < 0.739 | p < 0.002 | p < 0.001 | p < 0.001 |
| Naloxone (0.3 mg/kg) | 1 nM [77] | p < 0.814 | p < 0.022 | p < 0.004 | p < 0.006 |
| Haloperidol (10 μg/kg) | 1.1 nM [78,79] | p < 0.351 | p < 0.014 | p < 0.001 | p < 0.003 |
| Propranolol (2 mg/kg) | 8.7 nM [80] | p < 0.711 | p < 0.043 | p < 0.003 | p < 0.046 |
| Atropine (2 mg/kg) | 0.5 nM [81] | p < 0.998 | p < 0.030 | p < 0.041 | p < 0.092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10, 849. https://doi.org/10.3390/biomedicines10040849
Martos D, Tuka B, Tanaka M, Vécsei L, Telegdy G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines. 2022; 10(4):849. https://doi.org/10.3390/biomedicines10040849
Chicago/Turabian StyleMartos, Diána, Bernadett Tuka, Masaru Tanaka, László Vécsei, and Gyula Telegdy. 2022. "Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission" Biomedicines 10, no. 4: 849. https://doi.org/10.3390/biomedicines10040849
APA StyleMartos, D., Tuka, B., Tanaka, M., Vécsei, L., & Telegdy, G. (2022). Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines, 10(4), 849. https://doi.org/10.3390/biomedicines10040849










