The Impact of Prolonged Inflammation on Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Resiquimod R848 Solution
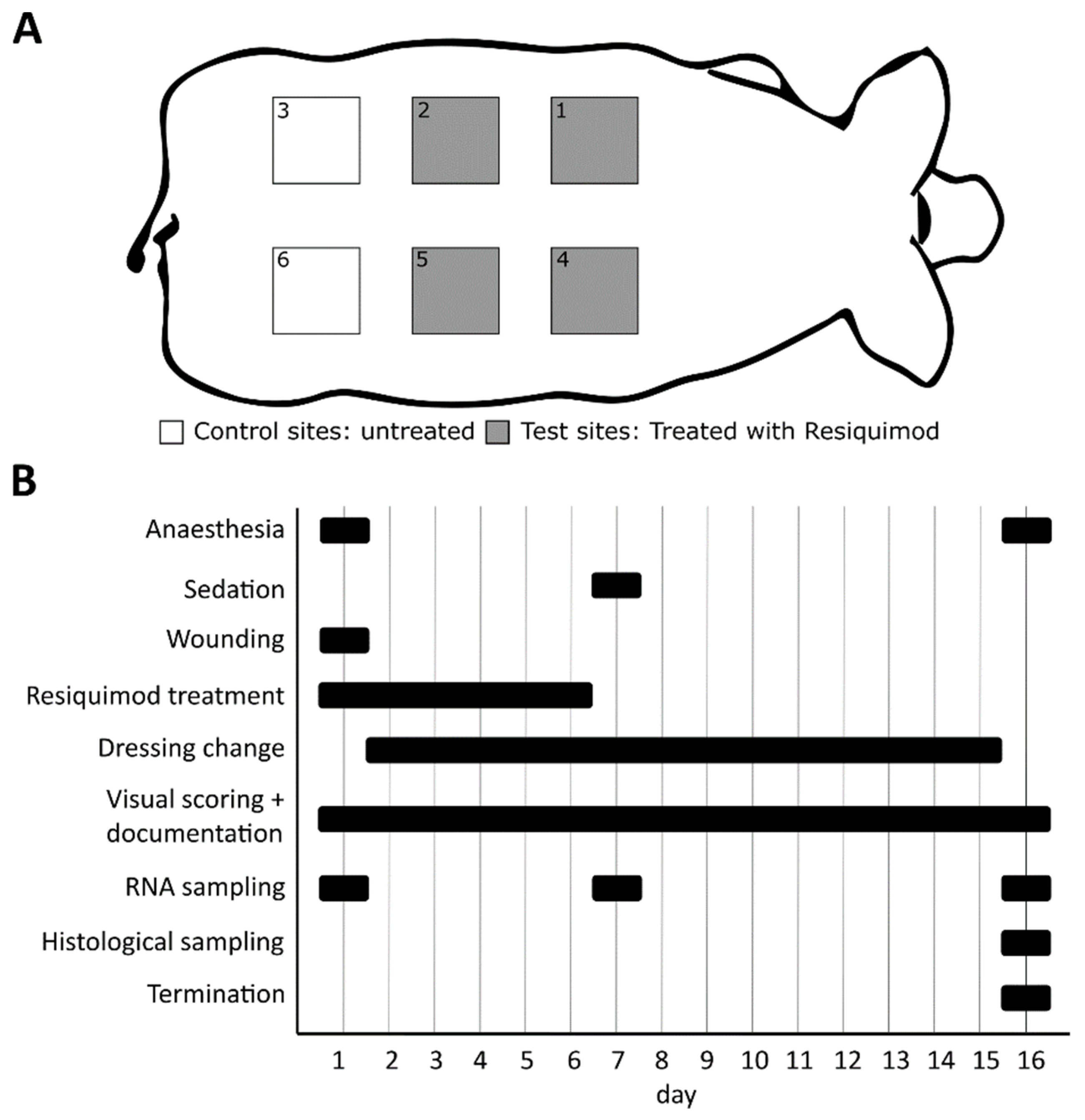
2.2. Animal Study
2.3. Wound Dressing Changes
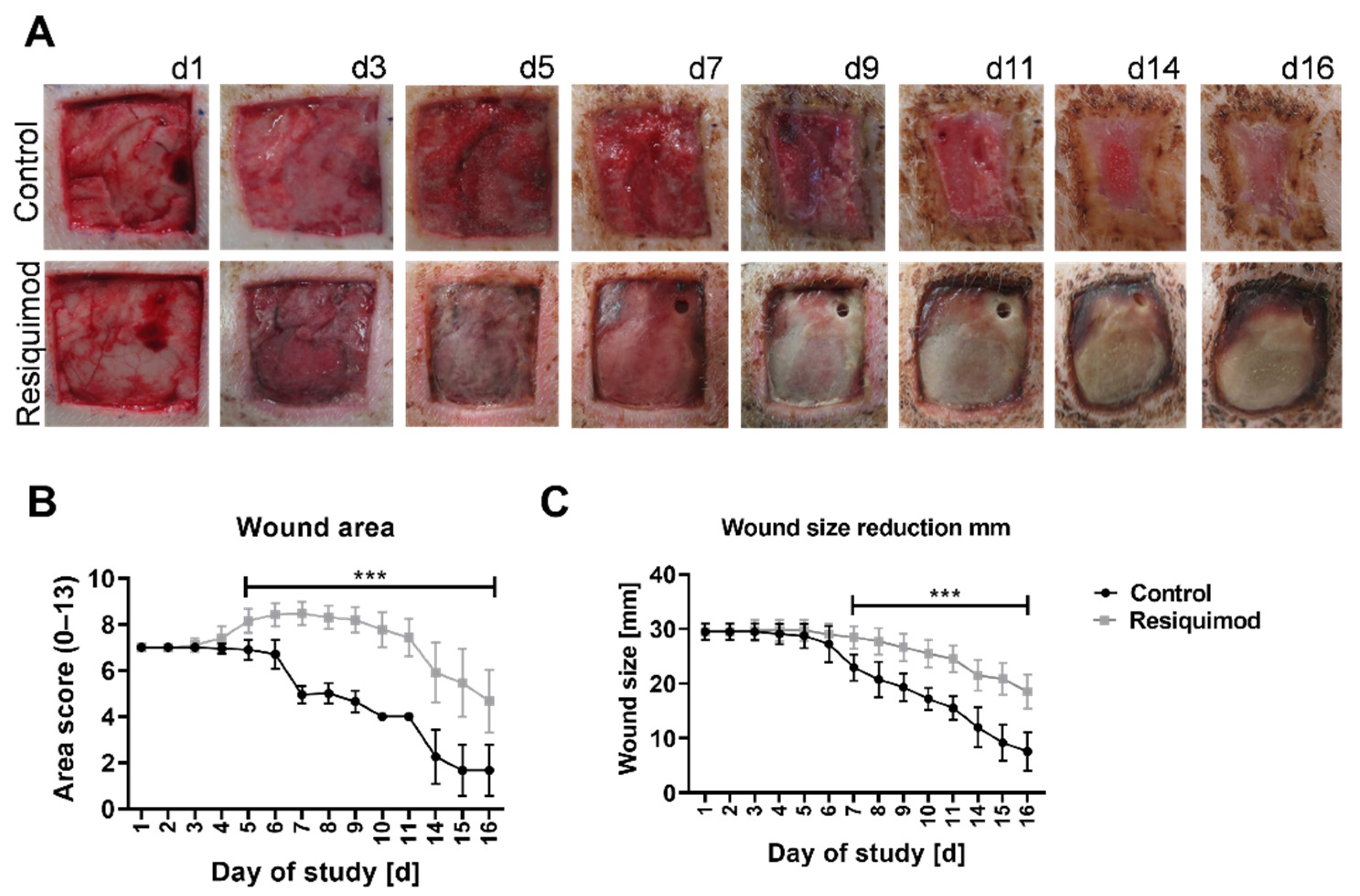
2.4. Visual Wound Scoring
2.5. Gene Expression Analysis
2.6. Histology
2.7. Quantification of Immune Cells
2.8. Open Flow Microperfusion and Cytokine Measurements in Dermal Wounds
2.9. Data Analysis and Statistics
3. Results
3.1. Topical Resiquimod Application Increased Wound Size and Delayed Wound Healing
3.2. Resiquimod Treatment Initiated Inflammation and Necrosis
3.3. Resiquimod-Induced Wounds Promoted Increased Expression of IL6, MMP1, CD68, IL8 and COX-2 Expression and Led to Immune Cell Infiltration
3.4. Open Flow Microperfusion (OFM) Showed That Short-Term Resiquimod Application Promoted Cytokine Release into the Dermis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lazarus, G.; Valle, M.F.; Malas, M.; Qazi, U.; Maruthur, N.M.; Doggett, D.; Fawole, O.A.; Bass, E.B.; Zenilman, J. Chronic venous leg ulcer treatment: Future research needs. Wound Repair Regen. 2014, 22, 34–42. [Google Scholar] [CrossRef]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Hunt, T.K.; Hopf, H.; Hussain, Z. Physiology of Wound Healing. Adv. Ski. Wound Care 2000, 13, 1–13, (Adv Skin Wound Care). [Google Scholar] [CrossRef]
- Ganesh, K.; Sinha, M.; Mathew-Steiner, S.S.; Das, A.K.; Roy, S.; Sen, C.K. Chronic Wound Biofilm Model. Adv. Wound Care 2015, 4, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.-W.; Fourcaudot, A.B.; Yamane, K.; You, T.; Chan, R.K.; Leung, K.P. Exacerbated and prolonged inflammation impairs wound healing and increases scarring. Wound Repair Regen. 2016, 24, 26–34. [Google Scholar] [CrossRef]
- Ramasastry, S.S. Acute wounds. Clin. Plast. Surg. 2005, 32, 195–208. [Google Scholar] [CrossRef]
- Zomer, H.D.; Trentin, A.G. Skin wound healing in humans and mice: Challenges in translational research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Dunn, L.; Prosser, H.C.G.; Tan, M.-J.; Vanags, L.Z.; Ng, M.K.C.; Bursill, C. Murine Model of Wound Healing. J. Vis. Exp. 2013, e50265. [Google Scholar] [CrossRef] [Green Version]
- Dockrell, D.H.; Kinghorn, G.R. Imiquimod and resiquimod as novel immunomodulators. J. Antimicrob. Chemother. 2001, 48, 751–755. [Google Scholar] [CrossRef] [Green Version]
- Sauder, D.N.; Smith, M.H.; Senta-McMillian, T.; Soria, I.; Meng, T.C. Randomized, Single-Blind, Placebo-Controlled Study of Topical Application of the Immune Response Modulator Resiquimod in Healthy Adults. Antimicrob. Agents Chemother. 2003, 47, 3846–3852. [Google Scholar] [CrossRef] [Green Version]
- Stockfleth, E.; Hofbauer, G.; Reinhold, U.; Popp, G.; Hengge, U.; Szeimies, R.M.; Brüning, H.; Anliker, M.; Hunger, T.; Dummer, R.; et al. Topical resiquimod dosing regimens in patients with multiple actinic keratoses: A multicentre, partly placebo-controlled, double-blind clinical trial. Br. J. Dermatol. 2019, 180, 297–305. [Google Scholar] [CrossRef]
- Fife, K.H.; Meng, T.C.; Ferris, D.G.; Liu, P. Effect of resiquimod 0.01% gel on lesion healing and viral shedding when applied to genital herpes lesions. Antimicrob. Agents Chemother. 2008, 52, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Rook, A.H.; Gelfand, J.M.; Wysocka, M.; Troxel, A.B.; Benoit, B.; Surber, C.; Elenitsas, R.; Buchanan, M.A.; Leahy, D.S.; Watanabe, R.; et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood 2015, 126, 1452–1461. [Google Scholar] [CrossRef] [Green Version]
- Gilliet, M.; Conrad, C.; Geiges, M.; Cozzio, A.; Thürlimann, W.; Burg, G.; Nestle, F.O.; Dummer, R. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch. Dermatol. 2004, 140, 1490–1495. [Google Scholar] [CrossRef] [Green Version]
- Dasu, M.R.; Isseroff, R.R. Toll-like receptors in wound healing: Location, accessibility, and timing. J. Investig. Dermatol. 2012, 132, 1955–1958. [Google Scholar] [CrossRef] [Green Version]
- Kluwe, J.; Mencin, A.; Schwabe, R. Toll-like Receptors, Wound healing and Carcinogenesis. J. Mol. Med. 2009, 87, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Singh, K.K.; Agrawal, N.K.; Gupta, S.K.; Mohan, G.; Chaturvedi, S.; Singh, K. Increased expression of endosomal members of toll-like receptor family abrogates wound healing in patients with type 2 diabetes mellitus. Int. Wound J. 2016, 13, 927–935. [Google Scholar] [CrossRef]
- D’arpa, P.; Leung, K.P. Toll-like receptor signaling in burn wound healing and scarring. Adv. Wound Care 2017, 6, 330–343. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, S.; Dhayani, A.; Vemula, P.K.; Mishra, R. Upregulated toll-like receptors 2 and 4 expression underlies delayed diabetic wound healing. bioRxiv 2019, 611053. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Bodenlenz, M.; Aigner, B.; Dragatin, C.; Liebenberger, L.; Zahiragic, S.; Höfferer, C.; Birngruber, T.; Priedl, J.; Feichtner, F.; Schaupp, L.; et al. Clinical applicability of dOFM devices for dermal sampling. Ski. Res. Technol. 2013, 19, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Pipper, C.; Bordag, N.; Reiter, B.; Economides, K.; Florian, P.; Birngruber, T.; Sinner, F.; Bodenlenz, M.; Eberl, A. LC-MS/MS analyses of open flow microperfusion samples quantify eicosanoids in a rat model of skin inflammation. J. Lipid Res. 2019, 60, 758–766. [Google Scholar] [CrossRef] [Green Version]
- Holmgaard, R.; Benfeldt, E.; Nielsen, J.B.; Gatschelhofer, C.; Sorensen, J.A.; Höfferer, C.; Bodenlenz, M.; Pieber, T.R.; Sinner, F. Comparison of open-flow microperfusion and microdialysis methodologies when sampling topically applied fentanyl and benzoic acid in human dermis ex vivo. Pharm. Res. 2012, 29, 1808–1820. [Google Scholar] [CrossRef]
- Chen, L.; Dipietro, L.A. Toll-like receptor function in acute wounds. Adv. Wound Care 2017, 6, 344–355. [Google Scholar] [CrossRef] [Green Version]
- Crompton, R.; Williams, H.; Ansell, D.; Campbell, L.; Holden, K.; Cruickshank, S.; Hardman, M. Oestrogen promotes healing in a bacterial LPS model of delayed cutaneous wound repair. Lab. Investig. 2016, 96, 439–449. [Google Scholar] [CrossRef]
- Schierle, C.F.; De la Garza, M.; Mustoe, T.A.; Galiano, R.D. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009, 17, 354–359. [Google Scholar] [CrossRef]
- Van Der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Franquesa, M.; Sarrias, M.R.; Borràs, F.E. Low doses of LPS exacerbate the inflammatory response and trigger death on TLR3-primed human monocytes article. Cell Death Dis. 2018, 9, 499. [Google Scholar] [CrossRef]
- Gu, Q.; Wang, D.; Gao, Y.; Zhou, J.; Peng, R.; Cui, Y.; Xia, G.; Qing, Q.; Yang, H.; Liu, J.; et al. Expression of MMP1 in surgical and radiation-impaired wound healing and its effects on the healing process. J. Environ. Pathol. Toxicol. Oncol. Off. Organ Int. Soc. Environ. Toxicol. Cancer 2002, 21, 71–78. [Google Scholar] [CrossRef]
- Krisp, C.; Jacobsen, F.; McKay, M.J.; Molloy, M.P.; Steinstraesser, L.; Wolters, D.A. Proteome analysis reveals antiangiogenic environments in chronic wounds of diabetes mellitus type 2 patients. Proteomics 2013, 13, 2670–2681. [Google Scholar] [CrossRef] [PubMed]
- Bergant Suhodolčan, A.; Luzar, B.; Kecelj Leskovec, N. Matrix metalloproteinase (MMP)-1 and MMP-2, but not COX-2 serve as additional predictors for chronic venous ulcer healing. Wound Repair Regen. 2021, 29, 725–731. [Google Scholar] [CrossRef]
- Sutcliffe, J.E.S.; Thrasivoulou, C.; Serena, T.E.; Madden, L.; Richards, T.; Phillips, A.R.; Becker, D.L. Changes in the extracellular matrix surrounding human chronic wounds revealed by 2-photon imaging. Int. Wound J. 2017, 14, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Ligi, D.; Maniscalco, R.; Khalil, R.A.; Mannello, F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J. Clin. Med. 2020, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.A.; Dipietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Livingstone, J.P.; Lautze, J.; Murray, P.C. Measuring In-Vivo Foot Perfusion Distal to a Near-Circumferential Negative Pressure Wound Therapy Dressing via Thermal Imaging. Cureus 2021, 13, e17720. [Google Scholar] [CrossRef]
- Holmer, A.; Marotz, J.; Wahl, P.; Dau, M.; Kämmerer, P.W. Hyperspectral imaging in perfusion and wound diagnostics-methods and algorithms for the determination of tissue parameters. Biomed. Tech. 2018, 63, 587–594. [Google Scholar] [CrossRef]
- Bacci, S. Fine Regulation during Wound Healing by Mast Cells, a Physiological Role Not Yet Clarified. Int. J. Mol. Sci. 2022, 23, 1820. [Google Scholar] [CrossRef]
- Ban, E.; Jeong, S.; Park, M.; Kwon, H.; Park, J.; Song, E.J.; Kim, A. Accelerated wound healing in diabetic mice by miRNA-497 and its anti-inflammatory activity. Biomed. Pharmacother. 2020, 121, 109613. [Google Scholar] [CrossRef] [PubMed]
- Halin, C.; Fahrngruber, H.; Meingassner, J.G.; Bold, G.; Littlewood-Evans, A.; Stuetz, A.; Detmar, M. Inhibition of chronic and acute skin inflammation by treatment with a vascular endothelial growth factor receptor tyrosine kinase inhibitor. Am. J. pathol. 2008, 173, 265–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, Y.; Furue, M.; Sanada, H.; Tachibana, T.; Nakayama, T.; Sugama, J.; Furuta, K.; Tachi, M.; Tokunaga, K.; Miyachi, Y. Development of the DESIGN-R with an observational study: An absolute evaluation tool for monitoring pressure ulcer wound healing. Wound Repair Regen. 2011, 19, 309–315. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holzer-Geissler, J.C.J.; Schwingenschuh, S.; Zacharias, M.; Einsiedler, J.; Kainz, S.; Reisenegger, P.; Holecek, C.; Hofmann, E.; Wolff-Winiski, B.; Fahrngruber, H.; et al. The Impact of Prolonged Inflammation on Wound Healing. Biomedicines 2022, 10, 856. https://doi.org/10.3390/biomedicines10040856
Holzer-Geissler JCJ, Schwingenschuh S, Zacharias M, Einsiedler J, Kainz S, Reisenegger P, Holecek C, Hofmann E, Wolff-Winiski B, Fahrngruber H, et al. The Impact of Prolonged Inflammation on Wound Healing. Biomedicines. 2022; 10(4):856. https://doi.org/10.3390/biomedicines10040856
Chicago/Turabian StyleHolzer-Geissler, Judith C. J., Simon Schwingenschuh, Martin Zacharias, Johanna Einsiedler, Sonja Kainz, Peter Reisenegger, Christian Holecek, Elisabeth Hofmann, Barbara Wolff-Winiski, Hermann Fahrngruber, and et al. 2022. "The Impact of Prolonged Inflammation on Wound Healing" Biomedicines 10, no. 4: 856. https://doi.org/10.3390/biomedicines10040856
APA StyleHolzer-Geissler, J. C. J., Schwingenschuh, S., Zacharias, M., Einsiedler, J., Kainz, S., Reisenegger, P., Holecek, C., Hofmann, E., Wolff-Winiski, B., Fahrngruber, H., Birngruber, T., Kamolz, L.-P., & Kotzbeck, P. (2022). The Impact of Prolonged Inflammation on Wound Healing. Biomedicines, 10(4), 856. https://doi.org/10.3390/biomedicines10040856






