The Clinical Utility of D-Dimer and Prothrombin Fragment (F1+2) for Peripheral Artery Disease: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Patient Selection
2.3. Baseline Measurements and Sample Processing
2.4. Biomarkers of Thrombin Activation and ELISA
2.5. Follow-Up and Measured Outcomes
2.6. Statistical Methods
3. Results
3.1. Overall Characteristics and Study Cohorts
3.2. Clinical Outcomes
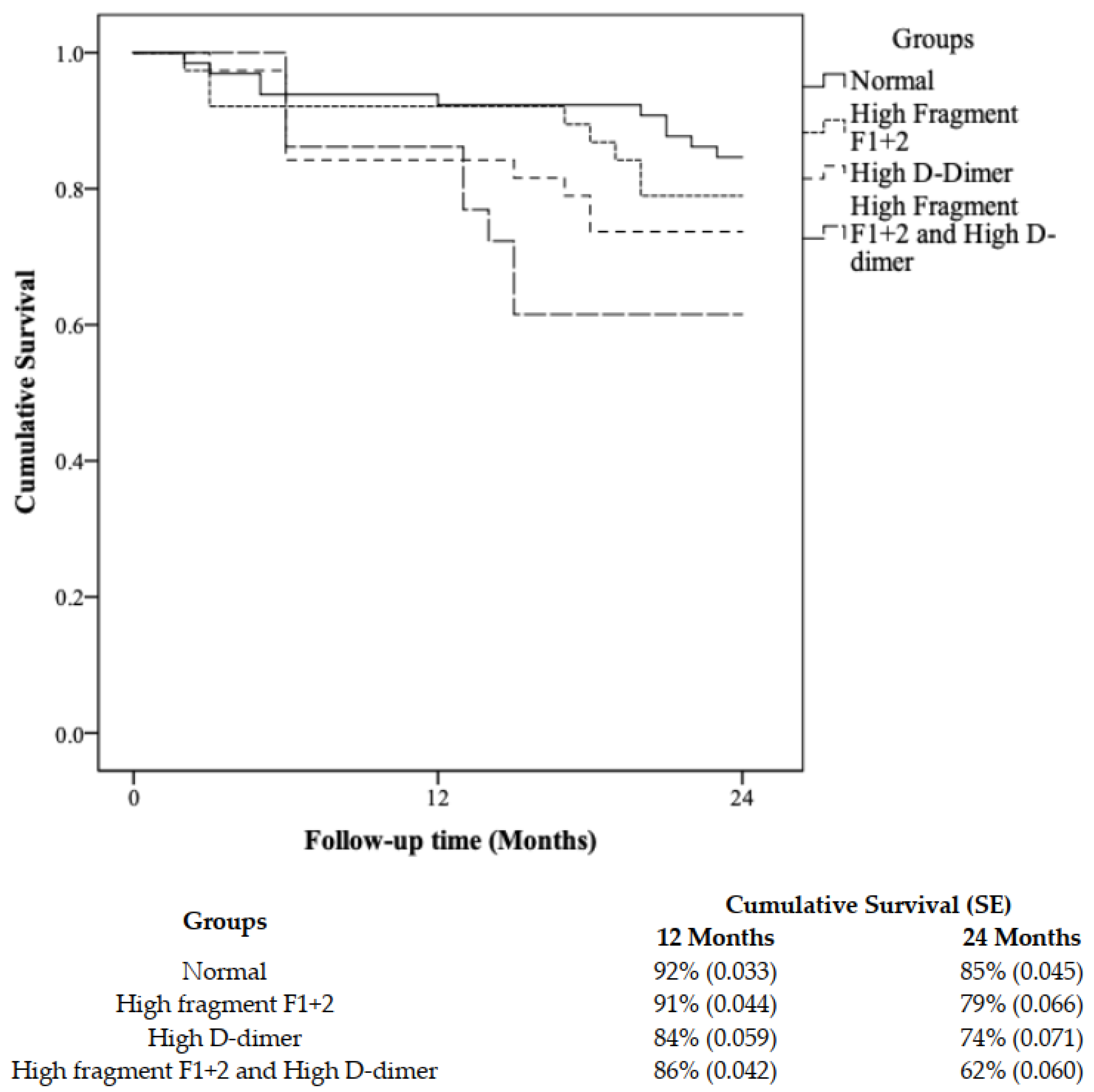
3.3. Survival Analysis and Risk Stratification Using a Combination of F1+2 and D-Dimer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fowkes, F.G.R.; Aboyans, V.; Fowkes, F.J.; McDermott, M.M.; Sampson, U.K.; Criqui, M.H. Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Criqui, M.H.; Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef]
- Diehm, C.; Allenberg, J.R.; Pittrow, D.; Mahn, M.; Tepohl, G.; Haberl, R.L.; Darius, H.; Burghaus, I.; Trampisch, H.J. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation 2009, 120, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef]
- Krishna, S.M.; Moxon, J.V.; Golledge, J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int. J. Mol. Sci. 2015, 16, 11294–11322. [Google Scholar] [CrossRef] [PubMed]
- Aday, A.W.; Matsushita, K. Epidemiology of peripheral artery disease and polyvascular disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef]
- Kleinegris, M.-C.F.; ten Cate, H.; Arina, J. D-dimer as a marker for cardiovascular and arterial thrombotic events in patients with peripheral arterial disease. Thromb. Haemost. 2013, 110, 233–243. [Google Scholar]
- Zamzam, A.; Syed, M.H.; Rand, M.L.; Singh, K.; Hussain, M.A.; Jain, S.; Khan, H.; Verma, S.; Al-Omran, M.; Abdin, R.; et al. Altered coagulation profile in peripheral artery disease patients. Vascular 2020, 28, 368–377. [Google Scholar] [CrossRef]
- Ana Paula Lucas, M.; Santos Maria Elizabeth Rennó de, C.; Silva Francisco das Chagas, L.; Schachnik Natalia Castro de, C.; Marinez de Oliveira, S.; Maria das Gracas, C. Hypercoagulability markers in patients with peripheral arterial disease: Association to ankle-brachial index. Angiology 2009, 60, 529–535. [Google Scholar] [CrossRef]
- Lee, A.J.; Fowkes, F.G.R.; Lowe, G.D.; Rumley, A. Fibrin D-dimer, haemostatic factors and peripheral arterial disease. Thromb. Haemost. 1995, 74, 828–832. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Liu, K.; Green, D.; Greenland, P.; Tian, L.; Kibbe, M.; Tracy, R.; Shah, S.; Wilkins, J.T.; Huffman, M.; et al. Changes in D-dimer and inflammatory biomarkers before ischemic events in patients with peripheral artery disease: The BRAVO Study. Vasc. Med. 2016, 21, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Komarov, A.; Panchenko, E.; Dobrovolsky, A.; Karpov, Y.A.; Deev, A.; Titaeva, E.; Davletov, K.; Eshkeeva, A.; Markova, L. D-dimer and platelet aggregability are related to thrombotic events in patients with peripheral arterial occlusive disease. Eur. Heart J. 2002, 23, 1309–1316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simurda, T.; Vilar, R.; Zolkova, J.; Ceznerova, E.; Kolkova, Z.; Loderer, D.; Neerman-Arbez, M.; Casini, A.; Brunclikova, M.; Skornova, I.; et al. A novel nonsense mutation in FGB (c. 1421G> A; p. Trp474Ter) in the beta chain of fibrinogen causing hypofibrinogenemia with bleeding phenotype. Biomedicines 2020, 8, 605. [Google Scholar] [CrossRef] [PubMed]
- Brunclikova, M.; Simurda, T.; Zolkova, J.; Sterankova, M.; Skornova, I.; Dobrotova, M.; Kolkova, Z.; Loderer, D.; Grendar, M.; Hudecek, J.; et al. Heterogeneity of Genotype–Phenotype in Congenital Hypofibrinogenemia—A Review of Case Reports Associated with Bleeding and Thrombosis. J. Clin. Med. 2022, 11, 1083. [Google Scholar] [CrossRef]
- López, Y.; Paloma, M.J.; Rifón, J.; Cuesta, B.; Páramo, J.A. Measurement of prethrombotic markers in the assessment of acquired hypercoagulable states. Thromb. Res. 1999, 93, 71–78. [Google Scholar] [CrossRef][Green Version]
- Vidula, H.; Tian, L.; Liu, K.; Criqui, M.H.; Ferrucci, L.; Pearce, W.H.; Greenland, P.; Green, D.; Tan, J.; Garside, D.B.; et al. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: A cohort study. Ann. Intern. Med. 2008, 148, 85–93. [Google Scholar] [CrossRef]
- Kremers, B.; Wübbeke, L.; Mees, B.; Ten Cate, H.; Spronk, H.; ten Cate-Hoek, A. Plasma biomarkers to predict cardiovascular outcome in patients with peripheral artery disease: A systematic review and meta-analysis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2018–2032. [Google Scholar] [CrossRef]
- Gremmels, H.; Teraa, M.; de Jager, S.C.; Pasterkamp, G.; de Borst, G.J.; Verhaar, M.C. A pro-inflammatory biomarker-profile predicts amputation-free survival in patients with severe limb ischemia. Sci. Rep. 2019, 9, 10740. [Google Scholar] [CrossRef]
- Syed, M.H.; Zamzam, A.; Khan, H.; Singh, K.; Forbes, T.L.; Rotstein, O.; Abdin, R.; Eikelboom, J.; Qadura, M. Fatty acid binding protein 3 is associated with peripheral arterial disease. JVS Vasc. Sci. 2020, 1, 168–175. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. Editor’s Choice–2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef] [PubMed]
- Frank, U.; Nikol, S.; Belch, J.; Boc, V.; Brodmann, M.; Carpentier, P.H.; Chraim, A.; Canning, C.; Dimakakos, E.; Gottsäter, A.; et al. ESVM Guideline on peripheral arterial disease. Vasa 2019, 48, 1–79. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef] [PubMed]
- Nicoloff, A.D.; Taylor Jr, L.M.; Sexton, G.J.; Schuff, R.A.; Edwards, J.M.; Yeager, R.A.; Landry, G.J.; Moneta, G.L.; Porter, J.M.; The Homocysteine and Progression of Atherosclerosis Study Investigators. Relationship between site of initial symptoms and subsequent progression of disease in a prospective study of atherosclerosis progression in patients receiving long-term treatment for symptomatic peripheral arterial disease. J. Vasc. Surg. 2002, 35, 38–47. [Google Scholar]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- McDermott, M.M.; Green, D.; Greenland, P.; Liu, K.; Criqui, M.H.; Chan, C.; Guralnik, J.M.; Pearce, W.H.; Ridker, P.M.; Taylor, L. Relation of levels of hemostatic factors and inflammatory markers to the ankle brachial index. Am. J. Cardiol. 2003, 92, 194–199. [Google Scholar] [CrossRef]
- Cortellaro, M.; Cofrancesco, E.; Boschetti, C.; Mussoni, L.; Donati, M.; Catalano, M.; Gabrielli, L.; Lambardi, B.; Specchia, G.; Tavazzi, L.; et al. Association of increased fibrin turnover and defective fibrinolytic capacity with leg atherosclerosis. Thromb. Haemost. 1994, 72, 292–296. [Google Scholar] [CrossRef]
- Fowkes, F.; Housley, E.; Rattray, A.; Lowe, G.; Rumley, A.; Elton, R.; MacGregor, I.; Dawes, J. Cross-linked fibrin degradation products, progression of peripheral arterial disease, and risk of coronary heart disease. Lancet 1993, 342, 84–86. [Google Scholar] [CrossRef]
- Boneu, B.; Leger, P.; Arnaud, C. Haemostatic system activation and prediction of vascular events in patients presenting with stable peripheral arterial disease of moderate severity. Royat Study Group. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 1998, 9, 129–135. [Google Scholar] [CrossRef]
- Smith, F.; Rumley, A.; Lee, A.; Leng, G.; Fowkes, F.; Lowe, G. Haemostatic factors and prediction of ischaemic heart disease and stroke in claudicants. Br. J. Haematol. 1998, 100, 758–763. [Google Scholar] [CrossRef]
- Criqui, M.H.; Ho, L.A.; Denenberg, J.O.; Ridker, P.M.; Wassel, C.L.; McDermott, M.M. Biomarkers in peripheral arterial disease patients and near-and longer-term mortality. J. Vasc. Surg. 2010, 52, 85–90. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall n = 206 (%) | Non-PAD n = 43 (%) | PAD n = 163 (%) | p-Value α |
|---|---|---|---|---|
| Demographics and Clinical Characteristics at Baseline | ||||
| Mean (SD) ‡ | ||||
| ABI | 0.71 (0.23) | 1.04 (0.07) | 0.62 (0.16) | 0.001 * |
| Age, years | 69 (10) | 67 (12) | 69 (10) | 0.249 |
| N (%) ¶ | ||||
| Sex, male | 141 (68) | 32 (74) | 109 (67) | 0.365 |
| Hypertension | 148 (72) | 29 (67) | 119 (74) | 0.448 |
| Hyperlipidemia | 152 (75) | 27 (63) | 125 (78) | 0.048 * |
| Diabetes | 72 (35) | 4 (9) | 68 (42) | 0.001 * |
| Smoking, current | 63 (31) | 10 (23) | 53 (33) | 0.213 |
| Smoking, past | 113 (55) | 24 (56) | 89 (55) | 0.213 |
| History of congestive heart failure | 7 (3) | 1 (2) | 6 (4) | 0.670 |
| History of coronary artery disease | 71 (35) | 11 (26) | 60 (38) | 0.205 |
| History of stroke | 28 (14) | 6 (14) | 22 (14) | 0.938 |
| Vascular events at 2-year follow-up | ||||
| N (%) ¶ | ||||
| Decrease in ABI (≥−0.15) | 53 (26) | 5 (12) | 48 (29) | 0.017 * |
| Arterial intervention | 25 (12) | 0 (0) | 25 (15) | 0.006 |
| Major limb amputation | 5 (2) | 0 (0) | 5 (3) | NA |
| MALE | 29 (14) | 0 (0) | 29 (18) | 0.003 * |
| F1+2 nmol/mL | D-Dimer μg/mL | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical Event | Unadjusted HR (95% CI) | p-Value | Adjusted HR ‡ (95% CI) | p-Value | Unadjusted HR (95% CI) | p-Value | Adjusted HR ‡ (95% CI) * | p-Value |
| Change in ABI (≥−0.15) | 1.33 (1.19–1.61) | 0.004 | 1.28 (1.14–1.58) | 0.019 | 1.28 (1.16–1.53) | 0.008 | 1.27 (1.15–1.54) | 0.013 |
| Clinical Characteristics | Group 1: Normal (n = 65) | Group 2: High F1+2 (n = 38) | Group 3: High D-Dimer (n = 38) | Group 4: High and D-Dimer (n = 65) | p (Trend) |
|---|---|---|---|---|---|
| Mean (SD) ‡ | |||||
| Age, years | 69 (11) | 68 (10) | 67 (10) | 68 (10) | 0.856 |
| N (%) ¶ | |||||
| Peripheral artery disease | 39 (60) | 31 (82) | 29 (76) | 64 (99) | 0.001 |
| Sex, male | 44 (68) | 25 (66) | 30 (79) | 42 (65) | 0.092 |
| Hypertension | 43 (66) | 30 (79) | 28 (74) | 47 (72) | 0.562 |
| Hyperlipidemia | 43 (66) | 31 (82) | 30 (79) | 48 (74) | 0.299 |
| Diabetes | 22 (34) | 12 (32) | 15 (40) | 23 (35) | 0.903 |
| Smoking, current | 18 (28) | 14 (37) | 12 (32) | 19 (29) | 0.888 |
| History of congestive heart failure | 1 (2) | 1 (3) | 2 (5) | 3 (5) | 0.694 |
| History of coronary artery disease | 16 (25) | 16 (42) | 19 (50) | 20 (31) | 0·042 |
| History of stroke | 10 (15) | 6 (16) | 2 (5) | 10 (15) | 0.0431 |
| Event Rates | |||||
| ABI change (≥−0.15) event | 10 (15) | 8 (21) | 10 (26) | 25 (26) | 0.022 |
| Arterial intervention | 5 (8) | 4 (11) | 7 (18) | 9 (14) | 0.412 |
| Major limb amputation | 1 (2) | 1 (3) | 1 (3) | 2 (3) | 0.951 |
| MALE | 6 (9) | 5 (13) | 8 (21) | 10 (15) | 0.406 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arfan, S.; Zamzam, A.; Syed, M.H.; Jain, S.; Jahanpour, N.; Abdin, R.; Qadura, M. The Clinical Utility of D-Dimer and Prothrombin Fragment (F1+2) for Peripheral Artery Disease: A Prospective Study. Biomedicines 2022, 10, 878. https://doi.org/10.3390/biomedicines10040878
Arfan S, Zamzam A, Syed MH, Jain S, Jahanpour N, Abdin R, Qadura M. The Clinical Utility of D-Dimer and Prothrombin Fragment (F1+2) for Peripheral Artery Disease: A Prospective Study. Biomedicines. 2022; 10(4):878. https://doi.org/10.3390/biomedicines10040878
Chicago/Turabian StyleArfan, Sara, Abdelrahman Zamzam, Muzammil H. Syed, Shubha Jain, Niousha Jahanpour, Rawand Abdin, and Mohammad Qadura. 2022. "The Clinical Utility of D-Dimer and Prothrombin Fragment (F1+2) for Peripheral Artery Disease: A Prospective Study" Biomedicines 10, no. 4: 878. https://doi.org/10.3390/biomedicines10040878
APA StyleArfan, S., Zamzam, A., Syed, M. H., Jain, S., Jahanpour, N., Abdin, R., & Qadura, M. (2022). The Clinical Utility of D-Dimer and Prothrombin Fragment (F1+2) for Peripheral Artery Disease: A Prospective Study. Biomedicines, 10(4), 878. https://doi.org/10.3390/biomedicines10040878








