A Novel Approach to Enhance the Regenerative Potential of Circulating Endothelial Progenitor Cells in Patients with End-Stage Kidney Disease
Abstract
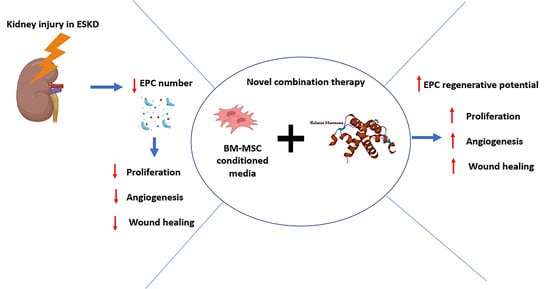
:1. Introduction
2. Materials and Methods
2.1. EPC Isolation and Culture from Peripheral Blood
2.2. Preparation of Conditioned Media (CM) from Cultured BM-MSC
2.3. Functional Assays
2.3.1. Treatment of Cultured Late EPCs
2.3.2. Viability and Proliferation Assay
2.3.3. Tube Formation Assay
2.3.4. Wound Healing Assay
2.3.5. Image Analyses
2.3.6. Immunofluorescence
2.4. Statistical Analyses
3. Results
3.1. Patient Demography
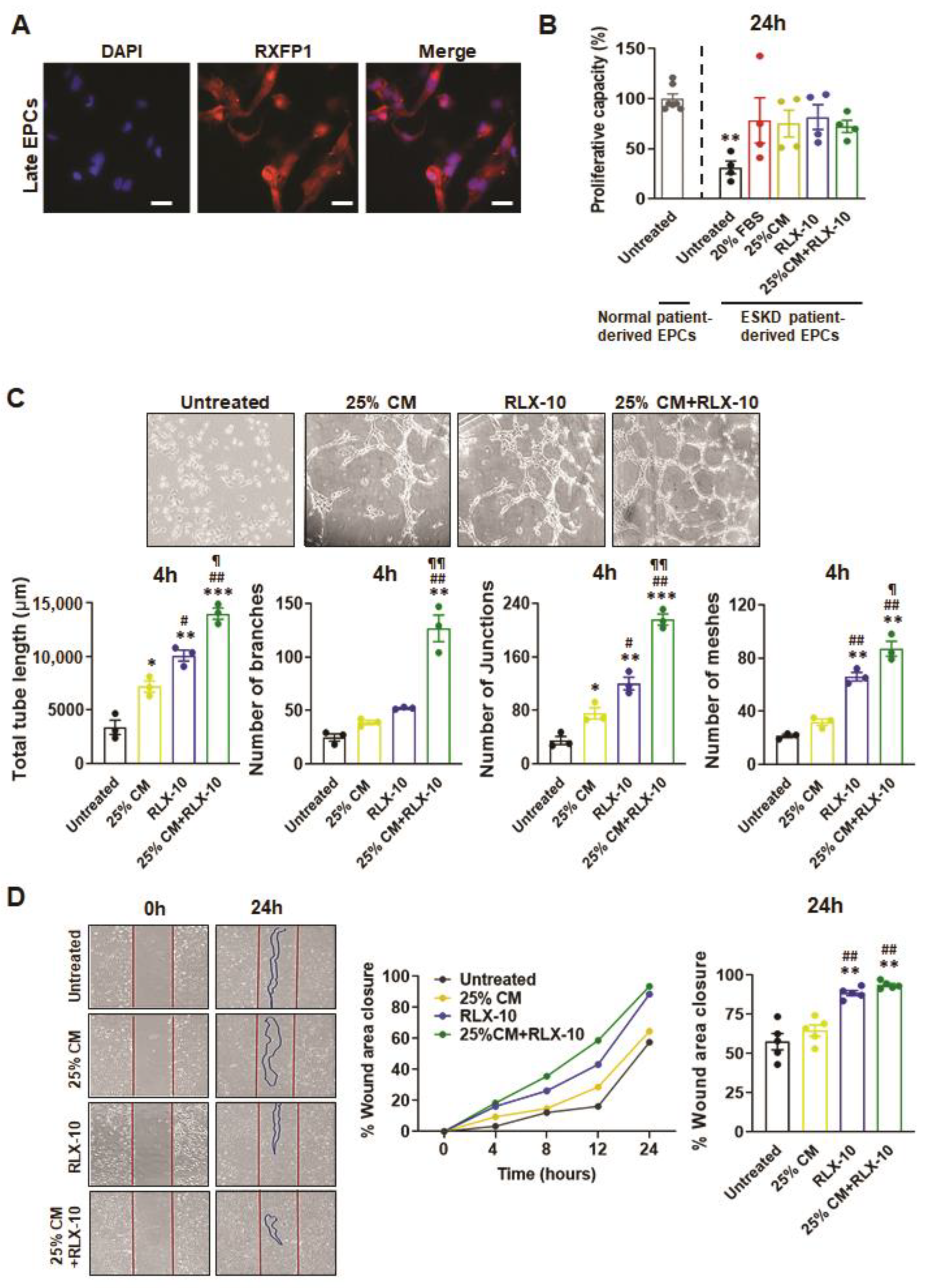
3.2. Functional Analysis of EPCs Derived from Control Patients
3.3. Functional Analyses of EPC’s Derived from ESKD Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic kidney disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; De Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statemen from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewitson, T.D. Fibrosis in the kidney: Is a problem shared a problem halved? Fibrogen. Tissue Rep. 2012, 5, S14. [Google Scholar] [CrossRef] [Green Version]
- Ruggenenti, P.; Cravedi, P.; Remuzzi, G. Mechanisms and treatment of CKD. J. Am. Soc. Nephrol. 2012, 23, 1917–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.K.; Molitoris, B.A. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin. Nephrol. 2015, 35, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Yuen, D.A.; Gilbert, R.E.; Marsden, P.A. Bone marrow cell therapies for endothelial repair and their relevance to kidney disease. Semin. Nephrol. 2012, 32, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.T.; Cheng, B.C.; Ko, S.F.; Chen, C.H.; Tsai, T.H.; Leu, S.; Chang, H.W.; Chung, S.Y.; Chua, S.; Yeh, S.C.; et al. Value and level of circulating endothelial progenitor cells, angiogenesis factors and mononuclear cell apoptosis in patients with chronic kidney disease. Clin. Exp. Nephrol. 2013, 17, 83–91. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, K.L.; Huh, W.; Kim, B.; Byuen, J.; Suh, W.; Sung, J.; Jeon, E.S.; Oh, H.K.; Kim, D.K. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1246–1252. [Google Scholar] [CrossRef] [Green Version]
- Ozkok, A.; Yildiz, A. Endothelial progenitor cells and kidney diseases. Kidney Blood Press. Res. 2018, 43, 701–718. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, W.; Kim, W.S.; Woo, J.S.; Kim, Y.G.; Moon, J.Y.; Lee, S.H.; Ihm, C.G.; Lee, T.W.; Jeong, K.H. Circulating endothelial progenitor cell levels predict cardiovascular events in end-stage renal disease patients on maintenance hemodialysis. Nephrology 2015, 130, 151–158. [Google Scholar] [CrossRef]
- Lu, C.L.; Leu, J.G.; Liu, W.C.; Zheng, C.M.; Lin, Y.F.; Shyu, J.F.; Wu, C.C.; Lu, K.C. Endothelial progenitor cells predict long-term mortality in hemodialysis patients. Int. J. Med. Sci. 2016, 13, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Almeida, D.C.; Donizetti-Oliveira, C.; Barbosa-Costa, P.; St Origassa, C.; Os Câmara, N. In search of mechanisms associated with mesenchymal stem cell-based therapies for acute kidney injury. Clin. Biochem. Rev. 2013, 34, 131–144. [Google Scholar] [PubMed]
- Perico, N.; Casiraghi, F.; Remuzzi, G. Clinical translation of mesenchymal stromal cells therapies in nephrology. J. Am. Soc. Nephrol. 2018, 29, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.S.; Royce, S.G.; Hewitson, T.D.; Denton, K.M.; Cooney, T.E.; Bennett, R.G. Anti-fibrotic actions of relaxin. Br. J. Pharmacol. 2017, 174, 962–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huuskes, B.M.; Wise, A.F.; Cox, A.J.; Lim, E.X.; Payne, N.L.; Kelly, D.J.; Samuel, C.S.; Ricardo, S.D. Combination therapy of mesenchymal stem cells and serelaxin effectively attenuates renal fibrosis in obstructive nephropathy. FASEB J. 2015, 29, 540–553. [Google Scholar] [CrossRef]
- Li, Y.; Shen, M.; Ferens, D.; Broughton, B.R.S.; Murthi, P.; Saini, S.; Widdop, R.E.; Ricardo, S.D.; Pinar, A.A.; Samuel, C.S. Combining mesenchymal stem cells with serelaxin provides enhanced renoprotection against 1K/DOCA/salt-induced hypertension. Br. J. Pharmacol. 2021, 178, 1164–1181. [Google Scholar] [CrossRef]
- Li, Y.; Chakraborty, A.; Broughton, B.R.S.; Ferens, D.; Widdop, R.E.; Ricardo, S.D.; Samuel, C.S. Comparing the renoprotective effects of BM-MSCs versus BM-MSC-exosomes, when combined with an anti-fibrotic drug, in hypertensive mice. Biomed. Pharmacother. 2021, 144, 112256. [Google Scholar] [CrossRef]
- Segal, M.S.; Sautina, L.; Li, S.; Diao, Y.; Agoulnik, A.I.; Kielczewski, J.; McGuane, J.T.; Grant, M.B.; Conrad, K.P. Relaxin increases human endothelial progenitor cell NO and migration and vasculogenesis in mice. Blood 2012, 119, 629–636. [Google Scholar] [CrossRef] [Green Version]
- Huuskes, B.M.; DeBuque, R.J.; Polkinghorne, K.R.; Samuel, C.S.; Kerr, P.G.; Ricardo, S.D. Endothelial progenitor cells and vascular health in dialysis patients. Kidney Int. Reps. 2018, 3, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Huuskes, B.M.; DeBuque, R.; Kerr, P.G.; Samuel, C.S.; Ricardo, S.D. The use of live cell imaging and automated image analysis to assist with determining optimal parameters for angiogenic assay in vitro. Front. Cell Dev. Biol. 2019, 7, 45. [Google Scholar] [CrossRef]
- Bell, R.J.; Eddie, L.W.; Lester, A.R.; Wood, E.C.; Johnston, P.D.; Niall, H.D. Relaxin in human pregnancy serum measured with a homologous radioimmunoassay. Obstet. Gynecol. 1987, 69, 585–589. [Google Scholar] [PubMed]
- Stam, F.; van Guldener, C.; Becker, A.; Dekker, J.M.; Heine, R.J.; Bouter, L.M.; Stehouwer, C.D. Endothelial dysfunction contributes to renal function–associated cardiovascular mortality in a population with mild renal insufficiency: The Hoorn study. J. Am. Soc. Nephrol. 2006, 17, 537–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbrig, K.; Pistrosch, F.; Oelschlaegel, U.; Wichmann, G.; Wagner, A.; Foerster, S.; Richter, S.; Gross, P.; Passauer, J. Increased total number but impaired migratory activity and adhesion of endothelial progenitor cells in patients on long-term hemodialysis. Am. J. Kidney Dis. 2004, 44, 840–849. [Google Scholar] [CrossRef]
- Boyer, M.; Townsend, L.E.; Vogel, L.M.; Falk, J.; Reitz-Vick, D.; Trevor, K.T.; Villalba, M.; Bendick, P.J.; Glover, J.L. Isolation of endothelial cells and their progenitor cells from human peripheral blood. J. Vasc. Surg. 2000, 31, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Hur, J.; Yoon, C.H.; Kim, H.S.; Choi, J.H.; Kang, H.J.; Hwang, K.K.; Oh, B.H.; Lee, M.M.; Park, Y.B. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 288–293. [Google Scholar] [CrossRef]
- Mead, L.E.; Prater, D.; Yoder, M.C.; Ingram, D.A. Isolation and characterization of endothelial progenitor cells from human blood. Curr. Prot. Stem. Cell Biology 2008, 6, 2C-1. [Google Scholar] [CrossRef]
- Mukai, N.; Akahori, T.; Komaki, M.; Li, Q.; Kanayasu-Toyoda, T.; Ishii-Watabe, A.; Kobayashi, A.; Yamaguchi, T.; Abe, M.; Amagasa, T.; et al. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp. Cell Res. 2008, 314, 430–440. [Google Scholar] [CrossRef]
- Minami, Y.; Nakajima, T.; Ikutomi, M.; Morita, T.; Komuro, I.; Sata, M.; Sahara, M. Angiogenic potential of early and late outgrowth endothelial progenitor cells is dependent on the time of emergence. Int. J. Cardiol. 2015, 186, 305–314. [Google Scholar] [CrossRef]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef]
- Eun, L.Y.; Song, H.; Choi, E.; Lee, T.G.; Moon, D.W.; Hwang, D.; Byun, K.H.; Sul, J.H.; Hwang, K.C. Implanted bone marrow-derived mesenchymal stem cells fail to metabolically stabilize or recover electromechanical function in infarcted hearts. Tissue Cell. 2011, 43, 238–245. [Google Scholar] [CrossRef]
- Papazova, D.A.; Oosterhuis, N.R.; Gremmels, H.; Van Koppen, A.; Joles, J.A.; Verhaar, M.C. Cell-based therapies for experimental chronic kidney disease: A systematic review and meta-analysis. Dis. Model. Mech. 2015, 8, 281–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tögel, F.; Weiss, K.; Yang, Y.; Hu, Z.; Zhang, P.; Westenfelder, C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am. J. Physiol. Renal. Physiol. 2007, 292, F1626–F1635. [Google Scholar] [CrossRef] [Green Version]
- Ninichuk, V.; Gross, O.; Segerer, S.; Hoffmann, R.; Radomska, E.; Buchstaller, A.; Huss, R.; Akis, N.; Schlöndorff, D.; Anders, H.J. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3- deficient mice. Kidney Int. 2006, 70, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Lie, P.; Miao, T.; Yu, M.; Lu, Q.; Feng, T.; Li, J.; Zu, T.; Liu, X.; Li, H. Conditioned medium from umbilical cord mesenchymal stem cells induces migration and angiogenesis. Mol. Med. Rep. 2015, 12, 20–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unemori, E.N.; Lewis, M.; Constant, J.; Arnold, G.; Grove, B.H.; Normand, J.; Deshpande, U.; Salles, A.; Pickfoed, L.B.; Erikson, M.E.; et al. Relaxin induces vascular endothelial growth factor expression and angiogenesis selectively at wound sites. Wound Repair Regen. 2000, 8, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Irrera, N.; Minutoli, L.; Calò, M.; Lo Cascio, P.; Caccia, P.; Pizzino, G.; Pallio, G.; Micali, A.; Vaccaro, M.; et al. Relaxin improves multiple markers of wound healing and ameliorates the disturbed healing pattern of genetically diabetic mice. Clin. Sci. 2013, 125, 575–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royce, S.G.; Shen, M.; Patek, K.P.; Huuskes, B.M.; Ricardo, S.D.; Samuel, C.S. Mesenchymal stem cells and serelaxin synergistically abrogate established airway fibrosis in an experimental model of chronic allergic airways disease. Stem Cell Res. 2015, 15, 495–505. [Google Scholar] [CrossRef]
- O’Neill, A.C.; Ricardo, S.D. Human kidney cell reprogramming: Applications for disease modeling and personalized medicine. J. Am. Soc. Nephrol. 2013, 24, 1347–1356. [Google Scholar] [CrossRef] [Green Version]
- Westerweel, P.E.; Hoefer, I.E.; Blankestijn, P.J.; de Bree, P.; Groeneveld, D.; van Oostrom, B.; Koomans, H.A.; Verhaar, M.C. End-stage renal disease causes an imbalance between endothelial and smooth muscle progenitor cells. Am. J. Physiol. Renal. Physiol. 2007, 292, F1132–F1140. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.J. Cardiovascular mortality in end-stage-renal disease. Am. J. Med. Sci. 2003, 325, 163–167. [Google Scholar] [CrossRef]

| Parameter | Healthy Control | ESKD Patients |
|---|---|---|
| Sample size (n) | 9 | 11 |
| Age (years) | 49 ± 5 | 57 ± 6 |
| Body height (cm) | Not Recorded | 182 ± 5 |
| Body weight (kg) | 85 ± 6 | |
| BMI | 24.7 + 0.6 | |
| Dialysis vintage (months) | Not Applicable | 2.3 ± 0.7 |
| Disease etiology: | ||
| Chronic glomerulonephritis (%) | 91 | |
| Amyloidosis (%) | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badawi, A.; Jefferson, O.C.; Huuskes, B.M.; Ricardo, S.D.; Kerr, P.G.; Samuel, C.S.; Murthi, P. A Novel Approach to Enhance the Regenerative Potential of Circulating Endothelial Progenitor Cells in Patients with End-Stage Kidney Disease. Biomedicines 2022, 10, 883. https://doi.org/10.3390/biomedicines10040883
Badawi A, Jefferson OC, Huuskes BM, Ricardo SD, Kerr PG, Samuel CS, Murthi P. A Novel Approach to Enhance the Regenerative Potential of Circulating Endothelial Progenitor Cells in Patients with End-Stage Kidney Disease. Biomedicines. 2022; 10(4):883. https://doi.org/10.3390/biomedicines10040883
Chicago/Turabian StyleBadawi, Amrilmaen, Osfred C. Jefferson, Brooke M. Huuskes, Sharon D. Ricardo, Peter G. Kerr, Chrishan S. Samuel, and Padma Murthi. 2022. "A Novel Approach to Enhance the Regenerative Potential of Circulating Endothelial Progenitor Cells in Patients with End-Stage Kidney Disease" Biomedicines 10, no. 4: 883. https://doi.org/10.3390/biomedicines10040883
APA StyleBadawi, A., Jefferson, O. C., Huuskes, B. M., Ricardo, S. D., Kerr, P. G., Samuel, C. S., & Murthi, P. (2022). A Novel Approach to Enhance the Regenerative Potential of Circulating Endothelial Progenitor Cells in Patients with End-Stage Kidney Disease. Biomedicines, 10(4), 883. https://doi.org/10.3390/biomedicines10040883