In Vitro Model of Human Trophoblast in Early Placentation
Abstract
1. Introduction
2. Human Placenta Development
2.1. Trophoblast Invasion
2.2. Early Hypoxic Placental Environment
2.3. Cytotrophoblast Differentiation
2.4. Transcription Factors, Cytokines in Early Placentation
2.5. The Role of the TGFβ Superfamily in Placentation
2.6. Immunomodulation in Early Placentation
3. In Vitro Placental Models
3.1. Primoculture Trophoblasts Monolayer Cells
3.2. Human Cancer Cell Lines
3.3. Trophoblast Stem Cells of the Blastocyst
3.4. Induced Stem Cell Engineering Cell Fate
3.5. Trophoblast Organoids and Spheroids as Placental Model
3.5.1. Placental Tissue Culture
3.5.2. Organoids Mimic EVT
3.5.3. Spheroids of Placenta-Derived Mesenchymal Stem Cells
3.5.4. Characteristics Phenotype of Trophoblast Organoids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, L.; Brkić, J.; Liu, M.; Fu, G.; Peng, C. Placental trophoblast cell differentiation: Physiological regulation and pathological relevance to preeclampsia. Mol. Asp. Med. 2013, 34, 981–1023. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Y.; Turco, M.Y.; Burton, G.J.; Moffett, A. Investigation of human trophoblast invasion in vitro. Hum. Reprod. Update 2020, 26, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Oyen, M.L. Bioengineering Approaches for Placental Research. Ann. Biomed. Eng. 2021, 49, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.D.; Straszewski-Chavez, S.L.; Kalionis, B.; Dunk, C.; Morrish, D. Trophoblast differentiation: Progenitor cells, fusion and migration—A workshop report. Placenta 2006, 27, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling development and disease with organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Kojima, Y.; Yamashiro, C.; Murase, Y.; Yabuta, Y.; Okamoto, I.; Iwatani, C.; Saitou, M. GATA transcription factors, SOX17 and TFAP2C, drive the human germ-cell specification program. Life Sci. Alliance 2021, 4, 5. [Google Scholar] [CrossRef]
- Fujii, M.; Matano, M.; Toshimitsu, K.; Takano, A.; Mikami, Y. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell 2018, 23, 787–793. [Google Scholar] [CrossRef]
- Marti-Figueroa, C.R.; Ashton, R.S. The case for applying tissue engineering methodologies to instruct human organoid morphogenesis. Acta Biomater. 2017, 54, 35–44. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Dye, B.R.; Hill, D.R.; Ferguson, M.A.; Tsai, Y.H.; Nagy, M.S. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 2015, 4, 05098. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H. Longterm expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Nivet, E.; Sancho-Martinez, I.; Gallegos, T.; Suzuki, K.; Okamura, D. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat. Cell Biol. 2013, 15, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.W.; Shibata, M.; Lei, M.; Toivanen, R.; Barlow, L.J.; Bergren, S.K. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol. 2014, 16, 951–961. [Google Scholar] [CrossRef]
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–56. [Google Scholar] [CrossRef]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–109. [Google Scholar] [CrossRef]
- McCracken, K.W.; Catá, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, 163428. [Google Scholar] [CrossRef]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy: Review articles. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef]
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef]
- Castellucci, M.; Kosanke, G.; Verdenelli, F.; Huppertz, B.; Kaufmann, P. Villous sprouting: Fundamental mechanisms of human placental development. Hum. Reprod. Update 2000, 6, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M.; Enders, A.C.; Pijnenborg, R. The role of invasive trophoblast in implantation and placentation of primates. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140070. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Meinhardt, G.; Saleh, L.; Kunihs, V.; Gamperl, M.; Kaindl, U. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep. 2018, 11, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Longtine, M.S.; Chen, B.; Odibo, A.O.; Zhong, Y. Villous trophoblast apoptosis is elevated and restricted to cytotrophoblasts in pregnancies complicated by preeclampsia, IUGR, or preeclampsia with IUGR. Placenta 2012, 33, 352–359. [Google Scholar] [CrossRef]
- Brkić, J.; Dunk, C.; O’Brien, J.; Fu, G.; Nadeem, L.; Wang, Y.L. MicroRNA-218-5p promotes endovascular trophoblast differentiation and spiral artery remodeling. Mol. Ther. 2018, 26, 2189–2205. [Google Scholar] [CrossRef] [PubMed]
- Lyall, F. Mechanisms regulating cytotrophoblast invasion in normal pregnancy and pre-eclampsia. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 266–273. [Google Scholar] [CrossRef]
- Lee, C.Q.; Turco, M.Y.; Gardner, L.; Simons, B.D.; Hemberger, M.; Moffett, A. Integrin α2 marks a niche of trophoblast progenitor cells in first trimester human placenta. Development 2018, 145, 16. [Google Scholar]
- Toki, T.; Horiuchi, A.; Ichikawa, N.; Mori, A.; Nikaido, T.; Fujii, S. Inverse relationship between apoptosis and Bcl-2 expression in syncytiotrophoblast and fibrin-type fibrinoid in early gestation. Mol. Hum. Reprod. 1999, 5, 246–251. [Google Scholar] [CrossRef][Green Version]
- Knöfler, M.; Pollheimer, J. IFPA Award in Placentology lecture: Molecular regulation of human trophoblast invasion. Placenta 2012, 33, 55–62. [Google Scholar] [CrossRef]
- Forristal, C.E.; Wright, K.L.; Hanley, N.A.; Oreffo, R.O. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 2010, 139, 85. [Google Scholar] [CrossRef]
- Red-Horse, K.; Zhou, Y.; Genbacev, O.; Prakobphol, A.; Foulk, R.; McMaster, M.; Fisher, S.J. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Investig. 2004, 114, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Yee, S.W.; Mitchell, M.D.; Rice, G.E. The possible role of extravillous trophoblast-derived exosomes on the uterine spiral arterial remodeling under both normal and pathological conditions. BioMed Res. Int. 2014, 2014, 693157. [Google Scholar] [CrossRef] [PubMed]
- Knöfler, M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int. J. Dev. Biol. 2010, 54, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Pollheimer, J.; Vondra, S.; Baltayeva, J.; Beristain, A.G.; Knöfler, M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front. Immunol. 2018, 9, 2597. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Moretto-Zita, M.; Leon-Garcia, S.; Parast, M.M. p63 inhibits extravillous trophoblast migration and maintains cells in a cytotrophoblast stem cell-like state. Am. J. Pathol. 2014, 184, 3332–3343. [Google Scholar] [CrossRef] [PubMed]
- Moffett, A.; Loke, C. Immunology of placentation in eutherian mammals. Nat. Rev. Immunol. 2006, 6, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, R.; Vercruysse, L.; Carter, A.M. Deep trophoblast invasion and spiral artery remodelling in the placental bed of the chimpanzee. Placenta 2011, 32, 400–408. [Google Scholar] [CrossRef]
- Hidaka, A.; Nakamoto, O. Retraction: Etiopathology of preeclampsia—Recent progress from the perspective of a poor/ischemic placenta. Hypertens. Res. Pregnancy 2014, 2, 98–107. [Google Scholar] [CrossRef][Green Version]
- Kliman, H.J. Uteroplacental blood flow. The story of decidualization, menstruation, and trophoblast invasion. Am. J. Pathol. 2000, 157, 1759–1768. [Google Scholar] [CrossRef]
- Schmidt, A.; Morales-Prieto, D.M.; Pastuschek, J.; Fröhlich, K.; Markert, U.R. Only humans have human placentas: Molecular differences between mice and humans. J. Reprod. Immunol. 2015, 108, 65–71. [Google Scholar] [CrossRef]
- Krendl, C.; Shaposhnikov, D.; Rishko, V.; Ori, C.; Ziegenhain, C.; Sass, S. GATA2/3-TFAP2A/C transcription factor network couples human pluripotent stem cell differentiation to trophectoderm with repression of pluripotency. Proc. Natl. Acad. Sci. USA 2017, 114, 9579–9588. [Google Scholar] [CrossRef] [PubMed]
- Beck, F. The role of Cdx genes in the mammalian gut. Gut 2004, 53, 1394–1396. [Google Scholar] [CrossRef] [PubMed]
- Soumen, P.; Home, P.; Bhattacharya, B.; Ray, S. GATA factors: Master regulators of gene expression in trophoblast progenitors. Placenta 2017, 60, 61–66. [Google Scholar]
- Chen, Y.; Wang, K.; Gong, Y.G.; Khoo, S.K.; Leach, R. Roles of CDX2 and EOMES in human induced trophoblast progenitor cells. Biochem. Biophys. Res. Commun. 2013, 431, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Morrisey, E.E.; Ip, H.S.; Tang, Z.; Lu, M.M. GATA-5: A transcriptional activator expressed in a novel temporally and spatim, GATA-5: A transcriptional activator expressed in a novel temporally and spatially-restricted pattern during embryonic development. Dev. Biol. 1997, 183, 21–36. [Google Scholar] [CrossRef]
- Saha, B.; Ganguly, A.; Home, P.; Bhattacharya, B.; Ray, S.; Ghosh, A. TEAD4 ensures postimplantation development by promoting trophoblast self-renewal: An implication in early human pregnancy loss. Proc. Natl. Acad. Sci. USA 2020, 117, 17864–17875. [Google Scholar] [CrossRef]
- Meinhardt, G.; Haider, S.; Kunihs, V.; Saleh, L.; Pollheimer, J.; Fiala, C. Pivotal role of the transcriptional co-activator YAP in trophoblast stemness of the developing human placenta. Proc. Natl. Acad. Sci. USA 2020, 117, 13562–13570. [Google Scholar] [CrossRef]
- Biadasiewicz, K.; Sonderegger, S.; Haslinger, P.; Haider, S.; Saleh, L.; Fiala, C. Transcription factor AP-2α promotes EGF-dependent invasion of human trophoblast. Endocrinology 2011, 152, 1458–1469. [Google Scholar] [CrossRef]
- Plessl, K.; Haider, S.; Fiala, C.; Pollheimer, J.; Knöfler, M. Expression pattern and function of Notch2 in different subtypes of first trimester cytotrophoblast. Placenta 2015, 36, 365–371. [Google Scholar] [CrossRef]
- Adu-Gyamfi, E.A.; Ding, Y.B.; Wang, Y.X. Regulation of placentation by the transforming growth factor beta superfamily. Biol. Reprod. 2020, 102, 18–26. [Google Scholar] [CrossRef]
- Van den Brûle, F.; Berndt, S.; Simon, N.; Coulon, C.; Le Goarant, J.; Munaut, C. Trophoblast invasion and placentation: Molecular mechanisms and regulation. Immunol. Gametes Embryo Implant. 2005, 88, 163–180. [Google Scholar]
- Zhou, Y.; Damsky, C.H.; Fisher, S.J. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J. Clin. Investig. 1997, 99, 2152–2164. [Google Scholar] [CrossRef] [PubMed]
- Wagers, A.J.; Allsopp, R.C.; Weissman, I.L. Changes in integrin expression are associated with altered homing properties of Lin−/loThy1. 1loSca-1+ c-kit+ hematopoietic stem cells following mobilization by cyclophosphamide/granulocyte colony-stimulating factor. Exp. Hematol. 2002, 30, 176–185. [Google Scholar] [CrossRef]
- Lysiak, J.J.; Hunt, J.; Pringle, G.A.; Lala, P.K. Localization of transforming growth factor β and its natural inhibitor decorin in the human placenta and decidua throughout gestation. Placenta 1995, 16, 221–231. [Google Scholar] [CrossRef]
- Itoh, S.; Itoh, F.; Goumans, M.J.; ten Dijke, P. Signaling of transforming growth factor-β family members through Smad proteins. Eur. J. Biochem. 2000, 267, 6954–6967. [Google Scholar] [CrossRef]
- Yi, Y.; Cheng, J.C.; Klausen, C.; Leung, P.C. TGF-β1 inhibits human trophoblast cell invasion by upregulating cyclooxygenase-2. Placenta 2018, 68, 44–51. [Google Scholar] [CrossRef]
- Chuva de Sousa Lopes, S.M.; Alexdottir, M.S.; Valdimarsdottir, G. The TGFβ family in human placental development at the fetal-maternal interface. Biomolecules 2020, 10, 453. [Google Scholar] [CrossRef]
- Bujold, E.; Morency, A.M.; Roberge, S.; Lacasse, Y.; Forest, J.C.; Giguère, Y. Acetylsalicylic acid for the prevention of preeclampsia and intra-uterine growth restriction in women with abnormal uterine artery Doppler: A systematic review and meta-analysis. J. Obstet. Gynaecol. Can. 2009, 31, 818–826. [Google Scholar] [CrossRef]
- Southcombe, J.; Tannetta, D.; Redman, C.; Sargent, I. The immunomodulatory role of syncytiotrophoblast microvesicles. PLoS ONE 2011, 6, e20245. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Okae, H.; Toh, H.; Sato, T.; Hiura, H.; Takahashi, S.; Shirane, K. Derivation of human trophoblast stem cells. Cell Stem Cell 2018, 22, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Ringler, G.E.; Strauss, J.F., III. In vitro systems for the study of human placental endocrine function. Endocr. Rev. 1990, 11, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Bangma, J.; Szilagyi, J.; Blake, B.E.; Plazas, C.; Kepper, S.; Fenton, S.E. An assessment of serum-dependent impacts on intracellular accumulation and genomic response of per-and polyfluoroalkyl substances in a placental trophoblast model. Environ. Toxicol. 2020, 35, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Heaton, S.J.; Eady, J.J.; Parker, M.L.; Gotts, K.L.; Dainty, J.R. The use of BeWo cells as an in vitro model for placental iron transport. Am. J. Physiol.-Cell Physiol. 2008, 295, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Abou-Kheir, W.; Barrak, J.; Hadadeh, O.; Daoud, G. HTR-8/SVneo cell line contains a mixed population of cells. Placenta 2017, 50, 1–7. [Google Scholar] [CrossRef]
- Jingting, C.; Yangde, Z.; Yi, Z.; Huining, L.; Rong, Y. Heparanase expression correlates with metastatic capability in human choriocarcinoma. Gynecol. Oncol. 2007, 107, 22–29. [Google Scholar] [CrossRef]
- Apps, R.; Murphy, S.P.; Fernando, R.; Gardner, L.; Ahad, T.; Moffett, A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 2009, 127, 26–39. [Google Scholar] [CrossRef]
- Apps, R.; Sharkey, A.; Gardner, L.; Male, V.; Trotter, M.; Miller, N.; Moffett, A. Genome-wide expression profile of first trimester villous and extravillous human trophoblast cells. Placenta 2011, 32, 33–43. [Google Scholar] [CrossRef]
- Manaster, I.; Goldman-Wohl, D.; Greenfield, C.; Nachmani, D.; Tsukerman, P.; Hamani, Y. MiRNA-mediated control of HLA-G expression and function. PLoS ONE 2012, 7, e33395. [Google Scholar]
- Kubaczka, C.; Kaiser, F.; Schorle, H. Breaking the first lineage barrier–many roads to trophoblast stem cell fate. Placenta 2017, 60, 52–56. [Google Scholar] [CrossRef]
- Castel, G.; Meistermann, D.; Bretin, B.; Firmin, J.; Blin, J.; Loubersac, S. Induction of Human Trophoblast Stem Cells from Somatic Cells and Pluripotent Stem Cells. Cell Rep. 2020, 33, 108419. [Google Scholar] [CrossRef] [PubMed]
- Genbacev, O.D.; Prakobphol, A.; Foulk, R.A.; Krtolica, A.R.; Ilic, D. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science 2003, 299, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Zdravkovic, T.; Nazor, K.L.; Larocque, N.; Gormley, M.; Donne, M.; Giritharan, G.; Fisher, S.J. Human stem cells from single blastomeres reveal pathways of embryonic or trophoblast fate specification. Development 2015, 142, 4010–4025. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Parker, G.C.; Puscheck, E.E.; Rappolee, D.A. Direct reprogramming to multipotent trophoblast stem cells, and is pluripotency needed for regenerative medicine either? Stem Cell Investig. 2016, 3, 24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deglincerti, A.; Croft, G.F.; Pietila, L.N.; Zernicka-Goetz, M.; Siggia, E.D.; Brivanlou, A.H. Self-organization of the in vitro attached human embryo. Nature 2016, 533, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.M.; Fisher, S.J. Trophoblast stem cells. Biol. Reprod. 2011, 84, 412–421. [Google Scholar] [CrossRef]
- Lee, C.Q.; Gardner, L.; Turco, M.; Zhao, N.; Murray, M.J.; Coleman, N. What is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem Cell Rep. 2016, 6, 257–272. [Google Scholar] [CrossRef]
- Miller, R.K.; Genbacev, O.; Turner, M.A.; Aplin, J.D.; Caniggia, I. Human placental explants in culture: Approaches and assessments. Placenta 2005, 26, 439–448. [Google Scholar] [CrossRef]
- Turco, M.Y.; Gardner, L.; Kay, R.G.; Hamilton, R.S.; Prater, M.; Hollinshead, M.S. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature 2018, 564, 263–267. [Google Scholar] [CrossRef]
- Kretzschmar, K.; Clevers, H. Organoids: Modeling development and the stem cell niche in a dish. Dev. Cell 2016, 38, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Horii, M.; Touma, O.; Bui, T.; Parast, M.M. Modeling human trophoblast, the placental epithelium at the maternal fetal interface. Reproduction 2020, 160, R1–R11. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Beltcheva, M.; Gontarz, P.; Zhang, B.; Popli, P.; Fischer, L.A.; Theunissen, T.W. Derivation of trophoblast stem cells from naïve human pluripotent stem cells. eLife 2020, 9, 52504. [Google Scholar] [CrossRef] [PubMed]
- Wooding, F.B.P. The synepitheliochorial placenta of ruminants: Binucleate cell fusions and hormone production. Placenta 1992, 13, 101–113. [Google Scholar] [CrossRef]
- Kadyrov, M.; Garnier, Y.; Gantert, M.; Kramer, B.W.; Kaufmann, P.; Huppertz, B. Cytokeratin antibodies as differential markers of trophoblast and fetomaternal syncytial plaques in the sheep placentome. Placenta 2007, 28, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Highet, A.R.; Zhang, V.J. Use of Matrigel in culture affects cell phenotype and gene expression in the first trimester trophoblast cell line HTR8/SVneo. Placenta 2012, 33, 586–588. [Google Scholar] [CrossRef] [PubMed]
- Bárcia, R.N.; Santos, J.M.; Teixeira, M.; Filipe, M.; Pereira, A.R.S.; Ministro, A. Umbilical cord tissue–derived mesenchymal stromal cells maintain immunomodulatory and angiogenic potencies after cryopreservation and subsequent thawing. Cytotherapy 2017, 19, 360–370. [Google Scholar] [CrossRef]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Bühring, H.J.; Evangelista, M. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef]
- Bačenková, D.; Rosocha, J.; Tóthová, T.; Rosocha, L.; Šarisský, M. Isolation and basic characterization of human term amnion and chorion mesenchymal stromal cells. Cytotherapy 2011, 13, 1047–1056. [Google Scholar] [CrossRef]
- Bačenková, D.; Trebuňová, M.; Zachar, L.; Hudák, R.; Ižaríková, G.; Šurínová, K.; Živčák, J. Analysis of same selected immunomodulatory properties of chorionic mesenchymal stem cells. Appl. Sci. 2020, 10, 9040. [Google Scholar] [CrossRef]
- Janockova, J.; Slovinska, L.; Harvanova, D.; Spakova, T.; Rosocha, J. New therapeutic approaches of mesenchymal stem cells-derived exosomes. J. Biomed. Sci. 2021, 28, 39. [Google Scholar] [CrossRef] [PubMed]
- Lankford, L.; Chen, Y.J.; Saenz, Z.; Kumar, P.; Long, C.; Farmer, D.; Wang, A. Manufacture and preparation of human placenta-derived mesenchymal stromal cells for local tissue delivery. Cytotherapy 2017, 19, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Rettinger, C.L.; Fourcaudot, A.B.; Hong, S.J.; Mustoe, T.A.; Hale, R.G.; Leung, K.P. In vitro characterization of scaffold-free three-dimensional mesenchymal stem cell aggregates. Cell Tissue Res. 2014, 358, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Pampalone, M.; Vella, S.; Carreca, A.P.; Amico, G.; Conaldi, P.G. Comparison of immunosuppressive and angiogenic properties of human amnion-derived mesenchymal stem cells between 2D and 3D culture systems. Stem Cells Int. 2019, 2019, 7486279. [Google Scholar] [CrossRef] [PubMed]
- Vikartovska, Z.; Humenik, F.; Maloveska, M.; Farbakova, J.; Hornakova, L.; Murgoci, A.N.; Cizkova, D. Adult Stem Cells Based Therapies in Veterinary Medicine. Arch. Vet. Sci. Med. 2020, 3, 40–50. [Google Scholar] [CrossRef]
- Trebuňova, M.; Gromošová, S.; Bačenková, D.; Rosocha, J.; Živčák, J. Biocompatibility of the human mesenchymal stem cells with bovine bone tissue at the cellular level in vitro. Lékař A Tech. Clin. Technol. 2018, 48, 59–65. [Google Scholar]
- Morrish, D.W.; Bhardwaj, D.; Paras, M.T. Transforming growth factor β1 inhibits placental differentiation and human chorionic gonadotropin and human placental lactogen secretion. Endocrinology 1991, 129, 22–26. [Google Scholar] [CrossRef]
- Chawengsaksophak, K.; de Graaff, W.; Rossant, J.; Deschamps, J.; Beck, F. Cdx2 is essential for axial elongation in mouse development. Proc. Natl. Acad. Sci. USA 2004, 101, 7641–7645. [Google Scholar] [CrossRef]
- Crum, C.P.; McKeon, F.D. p63 in epithelial survival, germ cell surveillance, and neoplasia. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 349–371. [Google Scholar] [CrossRef]
- Alzamil, L.; Nikolakopoulou, K.; Turco, M.Y. Organoid systems to study the human female reproductive tract and pregnancy. Cell Death Differ. 2021, 28, 35–51. [Google Scholar] [CrossRef]
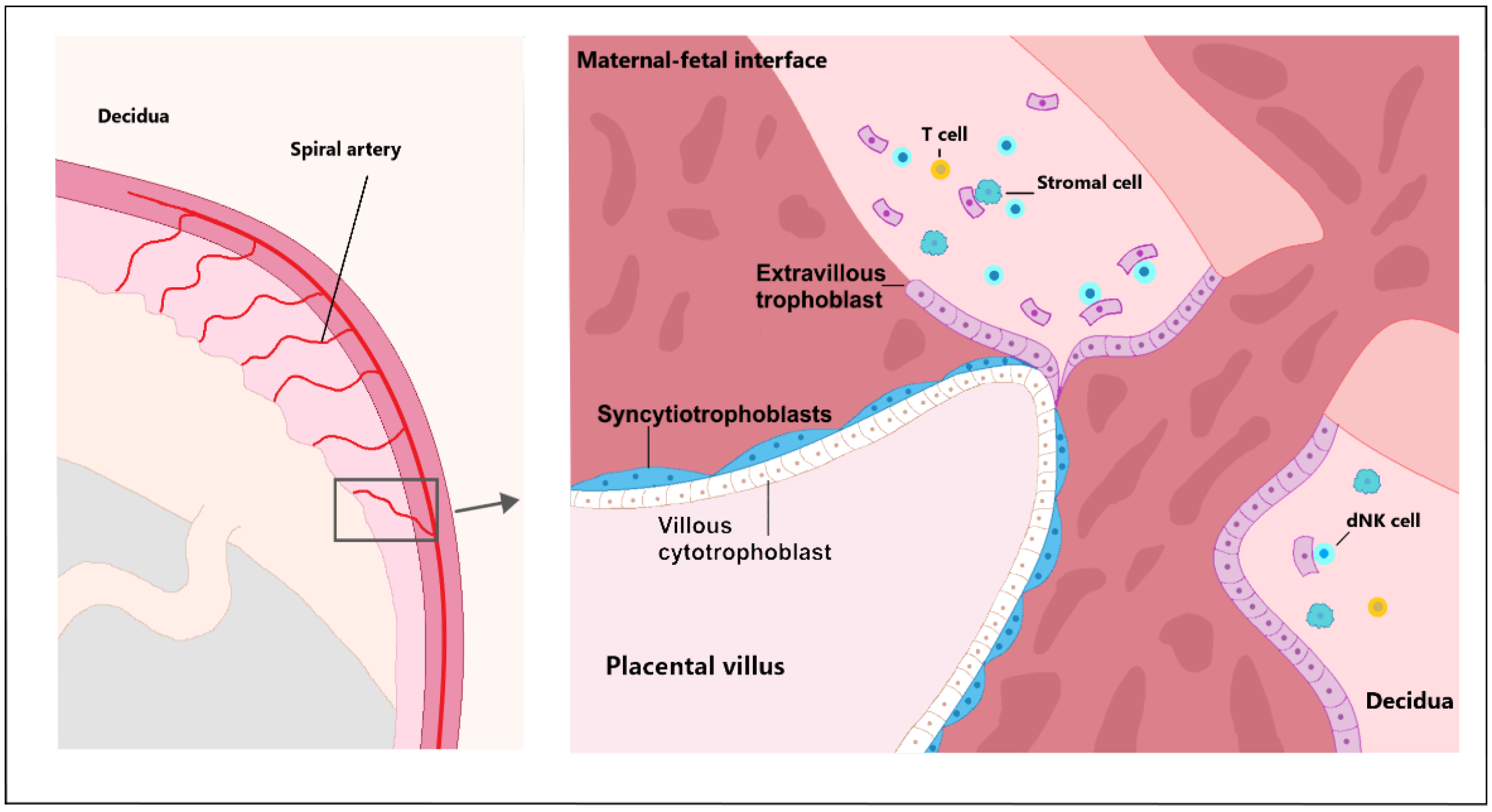
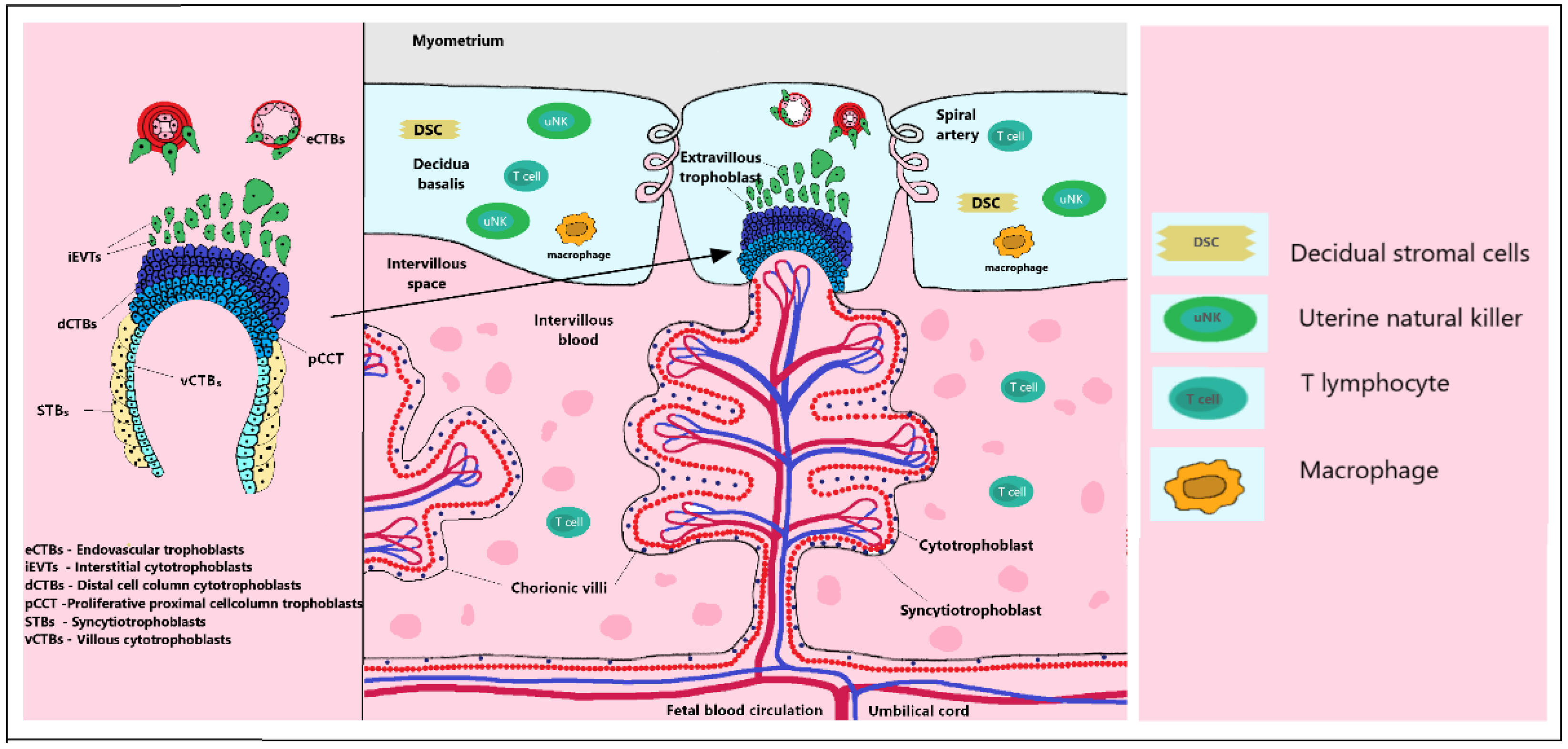


| Abbreviation/Glossary | Acronym |
|---|---|
| Adult stem cells | ASCs |
| Cell column trophoblasts | CCTs |
| Cytotrophoblast | CTBs |
| Decidual natural killer | dNK |
| Distal cell column cytotrophoblast | dCTBs |
| Embryonic stem cells | ESCs |
| Endovascular cytotrophoblasts | eCTBs |
| Extravillous trophoblasts | EVTs |
| Human embryonic stem | hESCs |
| Inner cell mass | ICM |
| Interstitial cytotrophoblasts | iEVTs |
| Mesenchymal stem cells | MSCs |
| Placental villous stromal cells | PVSCs |
| Pluripotent stem cells | PSCs |
| Primary villi | PV |
| Primitive syncytium | PS |
| Proliferative proximal cell column trophoblasts | pCCTs |
| Syncytiotrophoblasts | STBs |
| Syncytiotrophoblast microvesicles | STBMs |
| Trophectoderm | TE |
| Trophoblast progenitor cells | TPCs |
| Trophoblast stem cells | TSCs |
| Villous cytotrophoblasts | vCTBs |
| Cell Type | Phenotype | References |
|---|---|---|
| Morula cells (Mrl) | Elf5, EOMES GATA6, BMP4, SOX17 | [6] |
| Inner Cell Mass cells (ICM) | Nanog, Oct4, SOX2 | [71] |
| Trophectoderm cells (TE) | FGF4 | [46] |
| Cytotrophoblasts (CTBs) | Tpbpa, CDX2, GATA3, TFAP2C, TEAD4, E-cadherin, CK7 | [23,41] |
| Villous Cytotropboblasts (vCTBs) | GATA3, CDX2, TP63, TEAD4, K167, ITGB1, E-cadherin | [18,23,34] |
| Syncytiotrophoblast (STBs) | GATA3, TFAP2A, TFAP2C, hCG, EGFR, hCG | [18,23] |
| Extravillous Trophoblasts (EVTs) | NOTCH1, CEA adhesion molecule 1, EGFR | [34] |
| Cell Column Trophoblasts (CCTs) | NOTCH1, CK7, E-cadherin, VE-cadherin | [1,34] |
| Distal Cell Column Trophoblasts (dCCTs) | NOTCH1, NOTCH2, HLA-G | [34,49] |
| Endovascular Cytotrophoblasts (eCTBs) | CK7, VE-cadherin, PECAM | [1] |
| Intersticial Cytotrophoblasts (iCTBs) | ITGA1, MMP 12, CK7, HLA-G, ITGBA1B1 | [1,34] |
| Type | Cell Type | Advantages | Disadvantages |
|---|---|---|---|
| Primo Culture Monolayer Trophoblasts | Trophoblast cells | Simple isolation | Differences in cell morphology in vivo and in vitro data |
| High viability during cultivation | Lost characteristics during culture periods | ||
| Large number of cells by subculturing | Time-limited cell growth data | ||
| Human Cancer Cell Lines | Choriocarcinoma cell lines | Unlimited cell growth | Chromosomal aberrations Abnormal number of chromosomes |
| Large numbers of cells by subculturing | |||
| JAGs | HLA-G expression | ||
| JEG-3 | Trophoblast invasion in vitro | ||
| BeWo | Testing of metabolism | ||
| HTR-8/Svneo | Cell fusion, migration and invasion | ||
| Trophoblast Stem Cells of Mouse Blastocyst | XEN | Self-renewing and retaining their fate-specific development potential in vitro vCTBs and STBs differentiating | Embryo is destroyed after isolation |
| ESCs | |||
| TSCs | |||
| Induced iPSCs | Induced human trophoblast stem cells (iTPs) | Differentiating into a wide range of body tissue types Suitable for the study of disease mechanisms | Efficiency of reprogramming is generally low |
| Spheroids of Placenta Cells | Placenta-derived MSCs Amnion MSCs Chorion MSCs Wharton-Jelly-derived MSCs | Easy-to-use protocol Co-culture ability High reproducibility | Simplified architecture |
| Placental Organoids | Human CTB-ORGs Human TSCs | Organoid mimic tissue architecture relatively easy to grow Suitable for the study of the human placenta An organoid ideal for development and function mimics EVT | Heterogeneity of organoids, problem with the uniformity of cell population Lack vasculature |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bačenková, D.; Trebuňová, M.; Čížková, D.; Hudák, R.; Dosedla, E.; Findrik-Balogová, A.; Živčák, J. In Vitro Model of Human Trophoblast in Early Placentation. Biomedicines 2022, 10, 904. https://doi.org/10.3390/biomedicines10040904
Bačenková D, Trebuňová M, Čížková D, Hudák R, Dosedla E, Findrik-Balogová A, Živčák J. In Vitro Model of Human Trophoblast in Early Placentation. Biomedicines. 2022; 10(4):904. https://doi.org/10.3390/biomedicines10040904
Chicago/Turabian StyleBačenková, Darina, Marianna Trebuňová, Daša Čížková, Radovan Hudák, Erik Dosedla, Alena Findrik-Balogová, and Jozef Živčák. 2022. "In Vitro Model of Human Trophoblast in Early Placentation" Biomedicines 10, no. 4: 904. https://doi.org/10.3390/biomedicines10040904
APA StyleBačenková, D., Trebuňová, M., Čížková, D., Hudák, R., Dosedla, E., Findrik-Balogová, A., & Živčák, J. (2022). In Vitro Model of Human Trophoblast in Early Placentation. Biomedicines, 10(4), 904. https://doi.org/10.3390/biomedicines10040904








