Carbohydrate Metabolism Parameters of Adult Glial Neoplasms According to Immunohistochemical Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurement of Tumor Markers
2.3. Preparation of Tissue Homogenate for Biochemical Research
2.4. Analysis of the Biochemical Parameters of Carbohydrate Metabolism
2.5. Analysis of Protein–Protein Interactions
2.6. Statistical Analysis
3. Results
3.1. The Level of Molecular Genetic Markers of Gliomas with Varying Degrees of Anaplasia
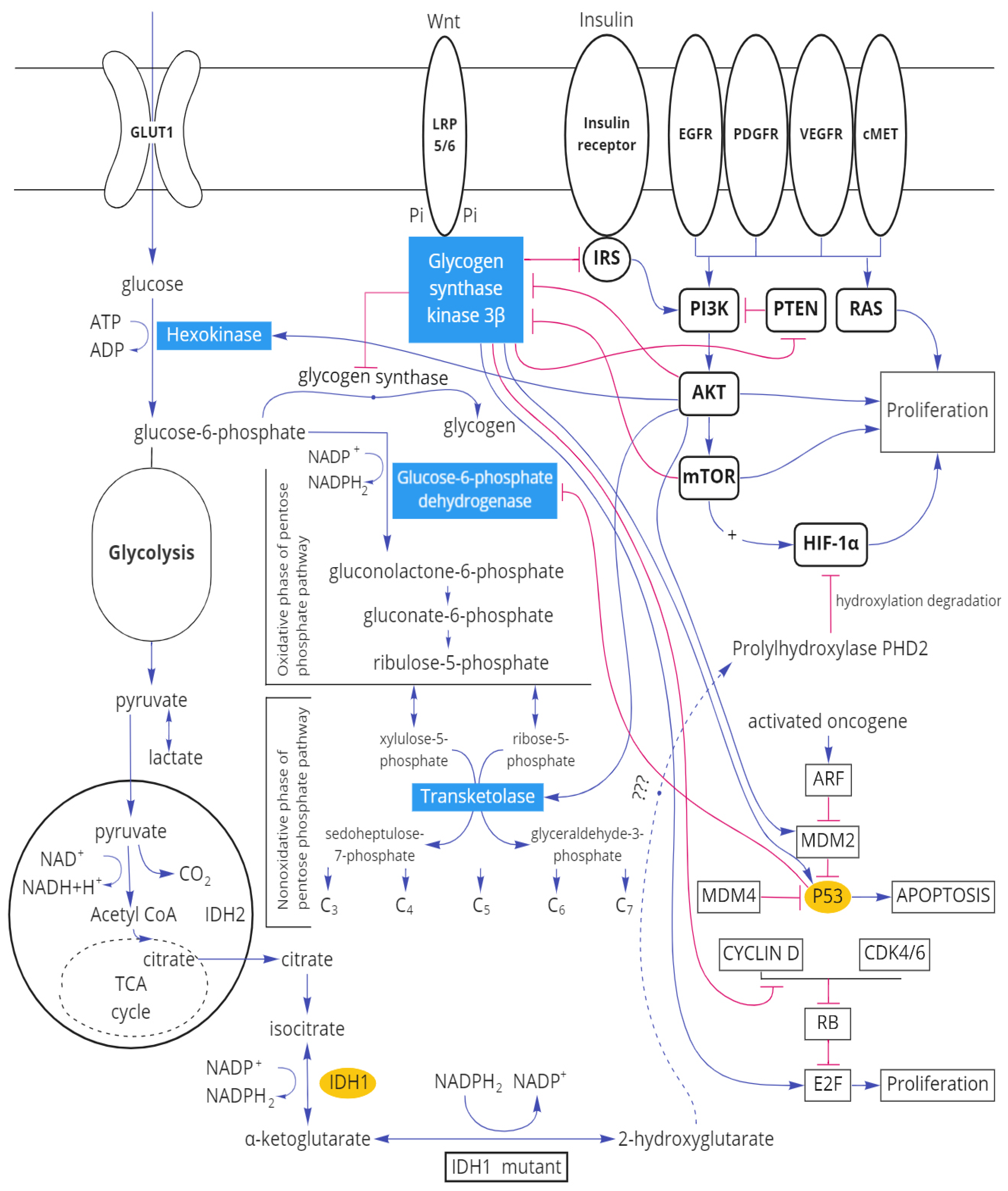
3.2. Protein–Protein Interactions between Immunohistochemical Markers of Gliomas and the Main Enzymes of Carbohydrate Metabolism
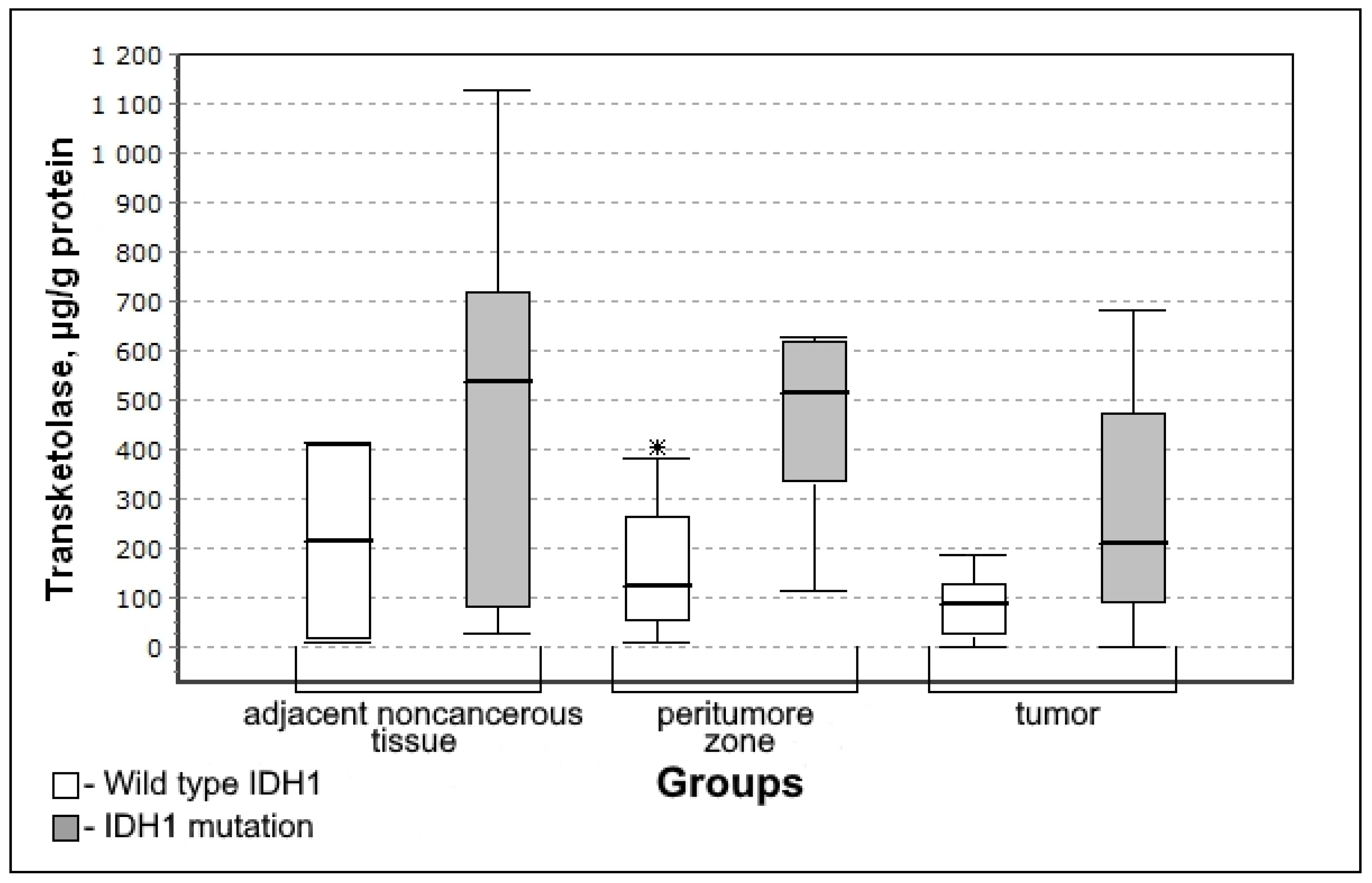
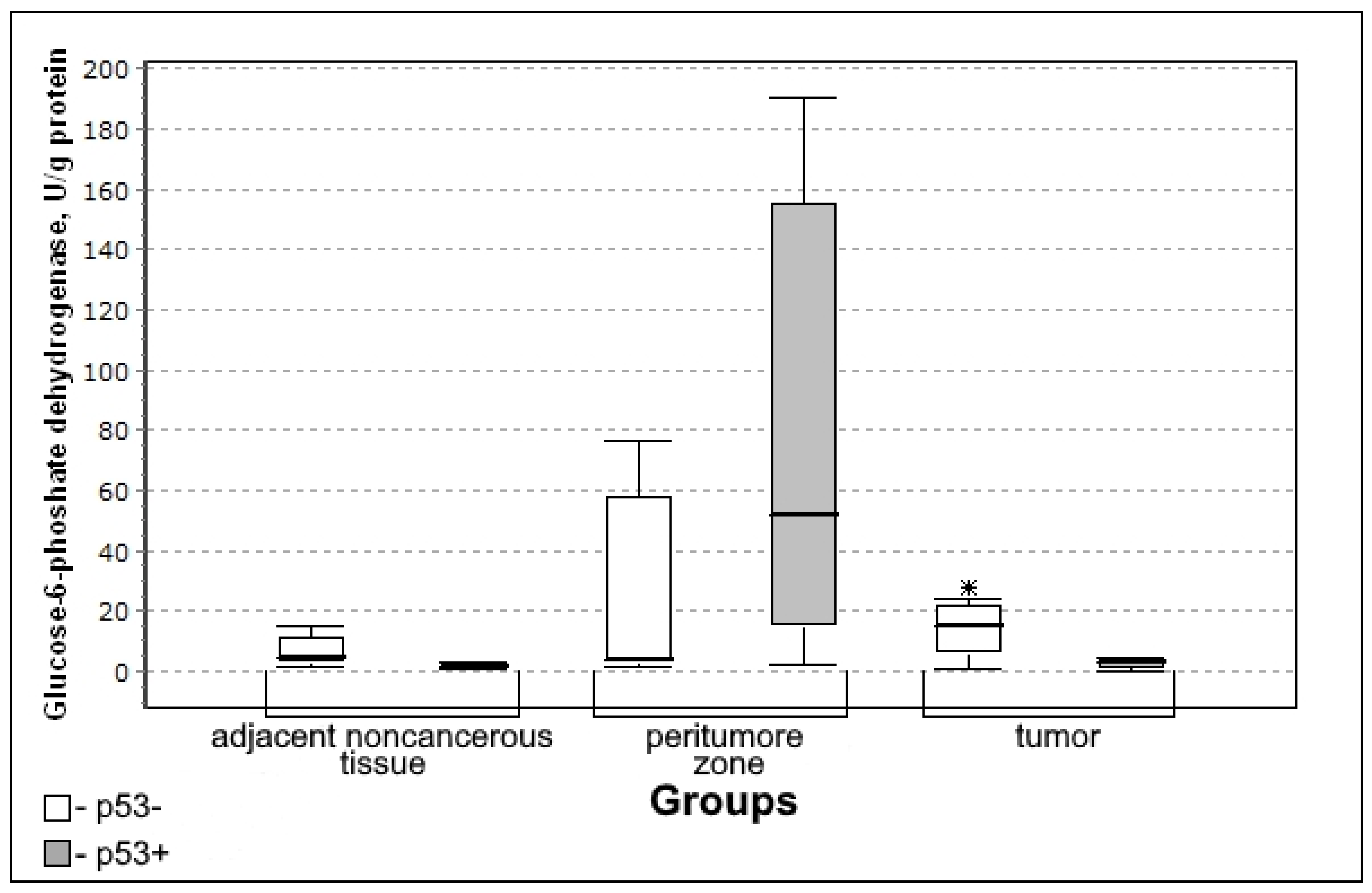
3.3. Parameters of Carbohydrate Metabolism Depending on the Zone of Glial Tumor
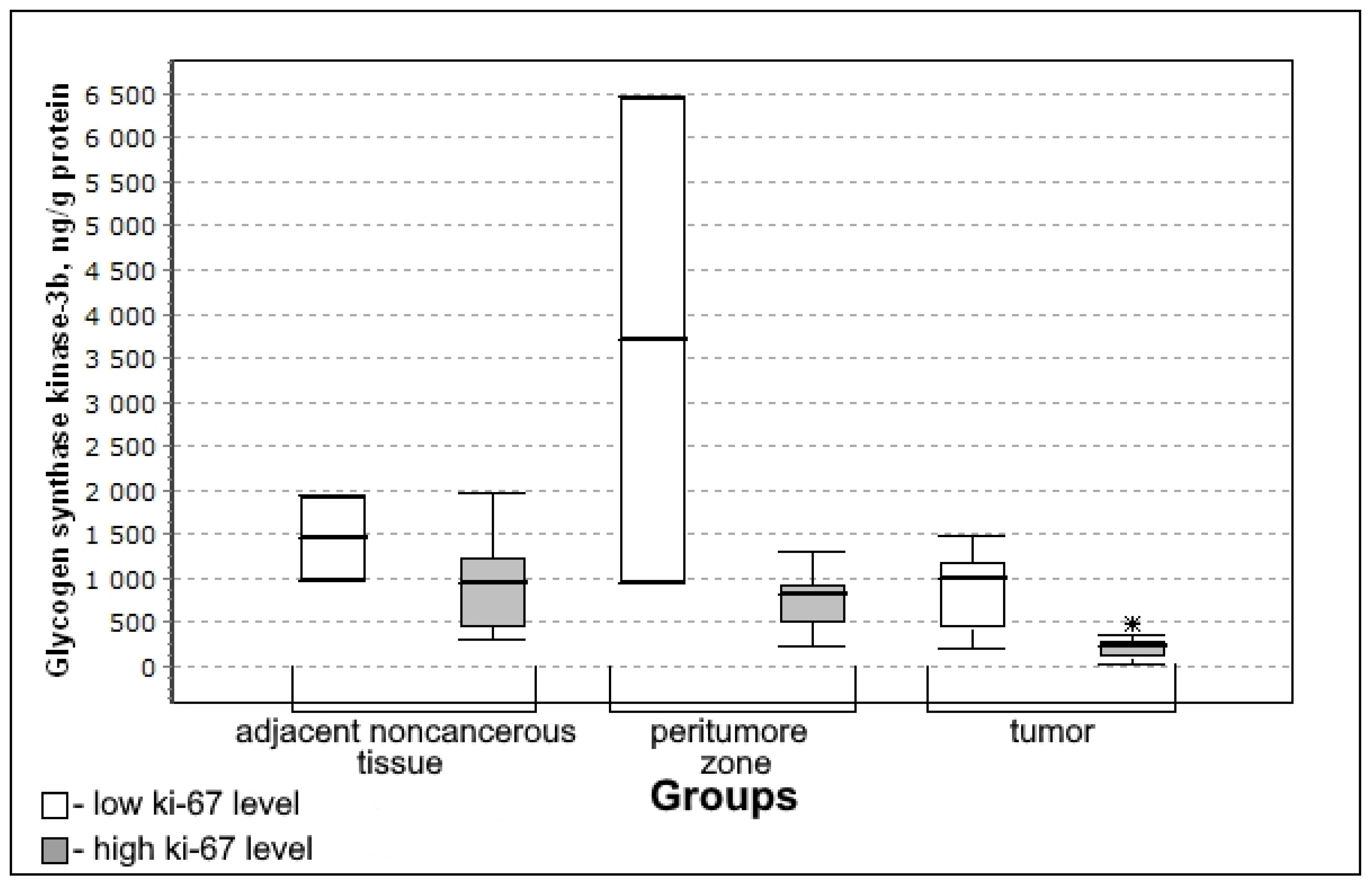
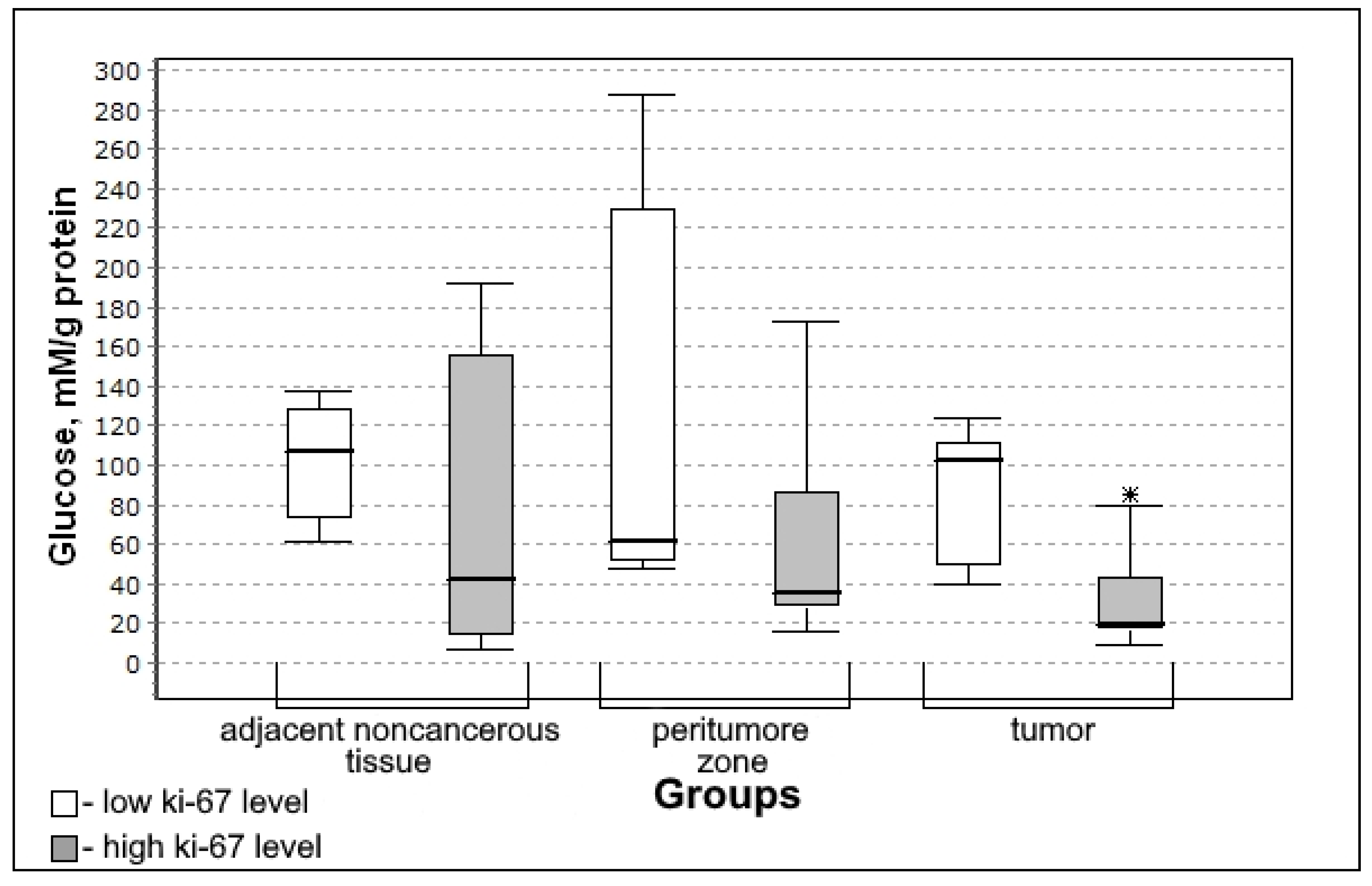
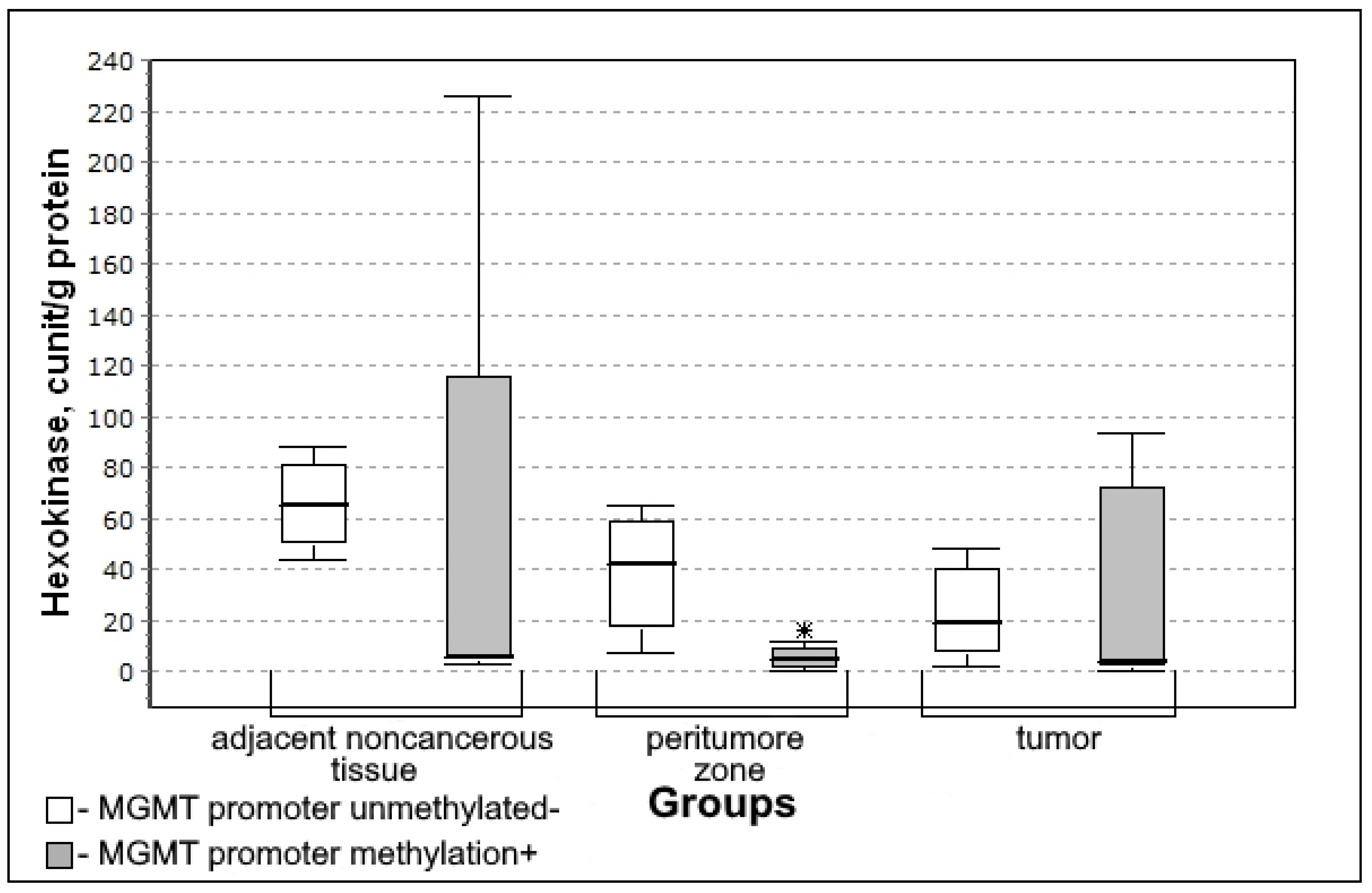
3.4. Carbohydrate Metabolism Characteristics Depending on the Immunohistochemical Profile of Gliomas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montemurro, N.; Perrini, P.; Rapone, B. Clinical Risk and Overall Survival in Patients with Diabetes Mellitus, Hyperglycemia and Glioblastoma Multiforme. A Review of the Current Literature. Int. J. Environ. Res. Public Health 2020, 17, 8501. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-F.; Chen, J. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes. Rev. 2009, 10, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; John, S.M.; Schmitz, G. Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: Lessons learnt from laron syndrome. Nutr. Metab. 2011, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Masur, K.; Vetter, C.; Hinz, A.; Tomas, N.; Henrich, H.; Niggemann, B.; Zänker, K.S. Diabetogenic glucose and insulin concentrations modulate transcriptom and protein levels involved in tumour cell migration, adhesion and proliferation. Br. J. Cancer 2010, 104, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Altieri, R.; Barbagallo, D.; Certo, F.; Broggi, G.; Ragusa, M.; Di Pietro, C.; Caltabiano, R.; Magro, G.; Peschillo, S.; Purrello, M.; et al. Peritumoral Microenvironment in High-Grade Gliomas: From FLAIRectomy to Microglia–Glioma Cross-Talk. Brain Sci. 2021, 11, 200. [Google Scholar] [CrossRef]
- Louis, D.N. WHO Classification of Tumours Editorial Board. In Central Nervous System Tumors Internet, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021; Volume 6, Available online: https://tumourclassification.iarc.who.int/chapters/45 (accessed on 20 February 2022).
- Dimitrov, L.; Hong, C.S.; Yang, C.; Zhuang, Z.; Heiss, J.D. New Developments in the Pathogenesis and Therapeutic Targeting of the IDH1 Mutation in Glioma. Int. J. Med. Sci. 2015, 12, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Tateishi, K.; Wakimoto, H.; Iafrate, A.J.; Tanaka, S.; Loebel, F.; Lelic, N.; Wiederschain, D.; Bedel, O.; Deng, G.; Zhang, B.; et al. Extreme Vulnerability of IDH1 Mutant Cancers to NAD+ Depletion. Cancer Cell 2015, 28, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Tamura, R.E.; De Vasconcellos, J.F.; Sarkar, D.; Libermann, T.A.; Fisher, P.B.; Zerbini, L.F. GADD45 Proteins: Central Players in Tumorigenesis. Curr. Mol. Med. 2012, 12, 634–651. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.; Strasser, A.; Kelly, G.L. Tumor-Suppressor Functions of the TP53 Pathway. Cold Spring Harb. Perspect. Med. 2016, 6, a026062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullwinkel, J.; Baron-Lühr, B.; Lüdemann, A.; Wohlenberg, C.; Gerdes, J.; Scholzen, T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J. Cell. Physiol. 2005, 206, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Theresia, E.; Malueka, R.G.; Pranacipta, S.; Kameswari, B.; Dananjoyo, K.; Asmedi, A.; Wicaksono, A.S.; Hartanto, R.A.; Dwianingsih, E.K. Association between Ki-67 Labeling index and Histopathological Grading of Glioma in Indonesian Population. Asian Pac. J. Cancer Prev. 2020, 21, 1063–1068. [Google Scholar] [CrossRef]
- Yoda, R.A.; Marxen, T.; Longo, L.; Ene, C.; Wirsching, H.-G.; Keene, C.D.; Holland, E.C.; Cimino, P.J. Mitotic Index Thresholds Do Not Predict Clinical Outcome for IDH-Mutant Astrocytoma. J. Neuropathol. Exp. Neurol. 2019, 78, 1002–1010. [Google Scholar] [CrossRef]
- Tano, K.; Shiota, S.; Collier, J.; Foote, R.S.; Mitra, S. Isolation and structural characterization of a cDNA clone encoding the human DNA repair protein for O6-alkylguanine. Proc. Natl. Acad. Sci. USA 1990, 87, 686–690. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, M.; Gan, H.; Wang, H.; Lee, J.-H.; Fang, D.; Kitange, G.J.; He, L.; Hu, Z.; Parney, I.F.; et al. A novel enhancer regulates MGMT expression and promotes temozolomide resistance in glioblastoma. Nat. Commun. 2018, 9, 2949. [Google Scholar] [CrossRef] [Green Version]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Zhu, P.; Zhang, C.; Li, Q.; Wang, Z.; Li, G.; Wang, G.; Yang, P.; Li, J.; Han, B.; et al. Detection of ATRX and IDH1-R132H immunohistochemistry in the progression of 211 paired gliomas. Oncotarget 2016, 7, 16384–16395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaurasia, A.; Park, S.-H.; Seo, J.-W.; Park, C.-K. Immunohistochemical Analysis of ATRX, IDH1 and p53 in Glioblastoma and Their Correlations with Patient Survival. J. Korean Med. Sci. 2016, 31, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.C.Y.; Nielsen, T.O.; Zabaglo, L.; Arun, I.; Badve, S.S.; Bane, A.L.; Bartlett, J.M.S.; Borgquist, S.; Chang, M.C.; Dodson, A.; et al. Analytical validation of a standardized scoring protocol for Ki67: Phase 3 of an international multicenter collabo-ration. NPJ Breast Cancer 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, L.A.G.; Bangsø, J.A.; Lindahl, K.H.; Dahlrot, R.H.; Hjelmborg, J.V.B.; Hansen, S.; Kristensen, B.W. Evaluation of the proliferation marker Ki-67 in gliomas: Interobserver variability and digital quantification. Diagn. Pathol. 2018, 13, 38. [Google Scholar] [CrossRef]
- Younis, S.G.; Khedr, R.A.E.-G.; El-Shorbagy, S.H. Immunohistochemical analysis of O6-methylguanine-DNA methyltransferase (MGMT) protein expression as prognostic marker in glioblastoma patients treated with radiation therapy with concomitant and adjuvant Temozolomide. J. Egypt. Natl. Cancer Inst. 2016, 28, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Turcan, S.; Rohle, D.; Goenka, A.; Walsh, L.; Fang, F.; Yilmaz, E.; Campos, C.; Fabius, A.W.M.; Lu, C.; Ward, P.; et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 2012, 483, 479–483. [Google Scholar] [CrossRef]
- Kathagen-Buhmann, A.; Schulte, A.; Weller, J.; Holz, M.; Herold-Mende, C.; Glass, R.; Lamszus, K. Glycolysis and the pentose phosphate pathway are differentially associated with the dichotomous regulation of glioblastoma cell migration versus proliferation. Neuro-Oncology 2016, 18, 1219–1229. [Google Scholar] [CrossRef] [Green Version]
- Krell, D.; Assoku, M.; Galloway, M.; Mulholland, P.; Tomlinson, I.; Bardella, C. Screen for IDH1, IDH2, IDH3, D2HGDH and L2HGDH Mutations in Glioblastoma. PLoS ONE 2011, 6, e19868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, D.A.N.; Swagemakers, S.M.; Buise, L.; Van Der Spek, P.J.; Kros, J.M. Metabolic alterations due to IDH1 mutation in glioma: Opening for therapeutic opportunities? Acta Neuropathol. Commun. 2014, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Garcia, J.L.; Viswanath, P.; Eriksson, P.; Cai, L.; Radoul, M.; Chaumeil, M.M.; Blough, M.; Luchman, H.A.; Weiss, S.; Cairncross, J.G.; et al. IDH1 Mutation Induces Reprogramming of Pyruvate Metabolism. Cancer Res. 2015, 75, 2999–3009. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Garcia, J.L.; Cai, L.M.; Chaumeil, M.M.; Eriksson, P.; Robinson, A.E.; Pieper, R.O.; Phillips, J.J.; Ronen, S.M. Glioma Cells with the IDH1 Mutation Modulate Metabolic Fractional Flux through Pyruvate Carboxylase. PLoS ONE 2014, 9, e108289. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH mutation in glioma: Molecular mechanisms and potential therapeutic targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef]
- Jiang, P.; Du, W.; Wang, X.; Mancuso, A.; Gao, X.; Wu, M.; Yang, X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 2011, 13, 310–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vleeschouwer, S. Glioblastoma; University Hospitals Leuven: Leuven, Belgium, 2017; p. 432, ISBN-13: 978-0-9944381-2-6. [Google Scholar] [CrossRef]
- Majewska, E.; Szeliga, M. AKT/ GSK3β Signaling in Glioblastoma. Neurochem. Res. 2017, 42, 918–924. [Google Scholar] [CrossRef] [Green Version]
- Hermida, M.A.; Kumar, J.D.; Leslie, N.R. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv. Biol. Regul. 2017, 65, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.R.; Qian, L.; Zhou, X. Developmental origins and oncogenic pathways in malignant brain tumors. Wiley Interdiscip. Rev. Dev. Biol. 2019, 8, e342. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Deng, H.; Jian, Z.; Cui, H.; Guo, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; et al. Copper exposure induces hepatic G0/G1 cell-cycle arrest through suppressing the Ras/PI3K/Akt signaling pathway in mice. Ecotoxicol. Environ. Saf. 2021, 222, 112518. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Tago, K.; Funakoshi-Tago, M.; Matsugi, J.; Yanagisawa, K. Intracellular NF-HEV/IL-33 harbors essential roles in Ras-induced cellular transformation by contributing to cyclin D1 protein synthesis. Cell. Signal. 2016, 28, 1025–1036. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, C.; Gibert, M.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K.; et al. The p53 Pathway in Glioblastoma. Cancers 2018, 10, 297. [Google Scholar] [CrossRef] [Green Version]
- Watcharasit, P.; Bijur, G.N.; Song, L.; Zhu, J.; Chen, X.; Jope, R.S. Glycogen Synthase Kinase-3β (GSK3β) Binds to and Promotes the Actions of p53. J. Biol. Chem. 2003, 278, 48872–48879. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Wang, H.; Liu, Y.; Shui, W.; Wang, J.; Cao, P.; Wang, H.; You, R.; Zhang, Y. Dendrobium officinale polysaccharide attenuates type 2 diabetes mellitus via the regulation of PI3K/Akt-mediated glycogen synthesis and glucose metabolism. J. Funct. Foods 2018, 40, 261–271. [Google Scholar] [CrossRef]
- Pandith, A.A.; Qasim, I.; Zahoor, W.; Shah, P.; Bhat, A.R.; Sanadhya, D.; Shah, Z.A.; Naikoo, N.A. Concordant association validates MGMT methylation and protein expression as favorable prognostic factors in glioma patients on alkylating chemotherapy (Temozolomide). Sci. Rep. 2018, 8, 6704. [Google Scholar] [CrossRef] [Green Version]
- Dahlrot, R.; Larsen, P.; Boldt, H.B.; Kreutzfeldt, M.S.; Hansen, S.; Hjelmborg, J.B.; Kristensen, B.W. Posttreatment Effect of MGMT Methylation Level on Glioblastoma Survival. J. Neuropathol. Exp. Neurol. 2019, 78, 633–640. [Google Scholar] [CrossRef]
- Aoki, K.; Natsume, A. Overview of DNA methylation in adult diffuse gliomas. Brain Tumor Pathol. 2019, 36, 84–91. [Google Scholar] [CrossRef]

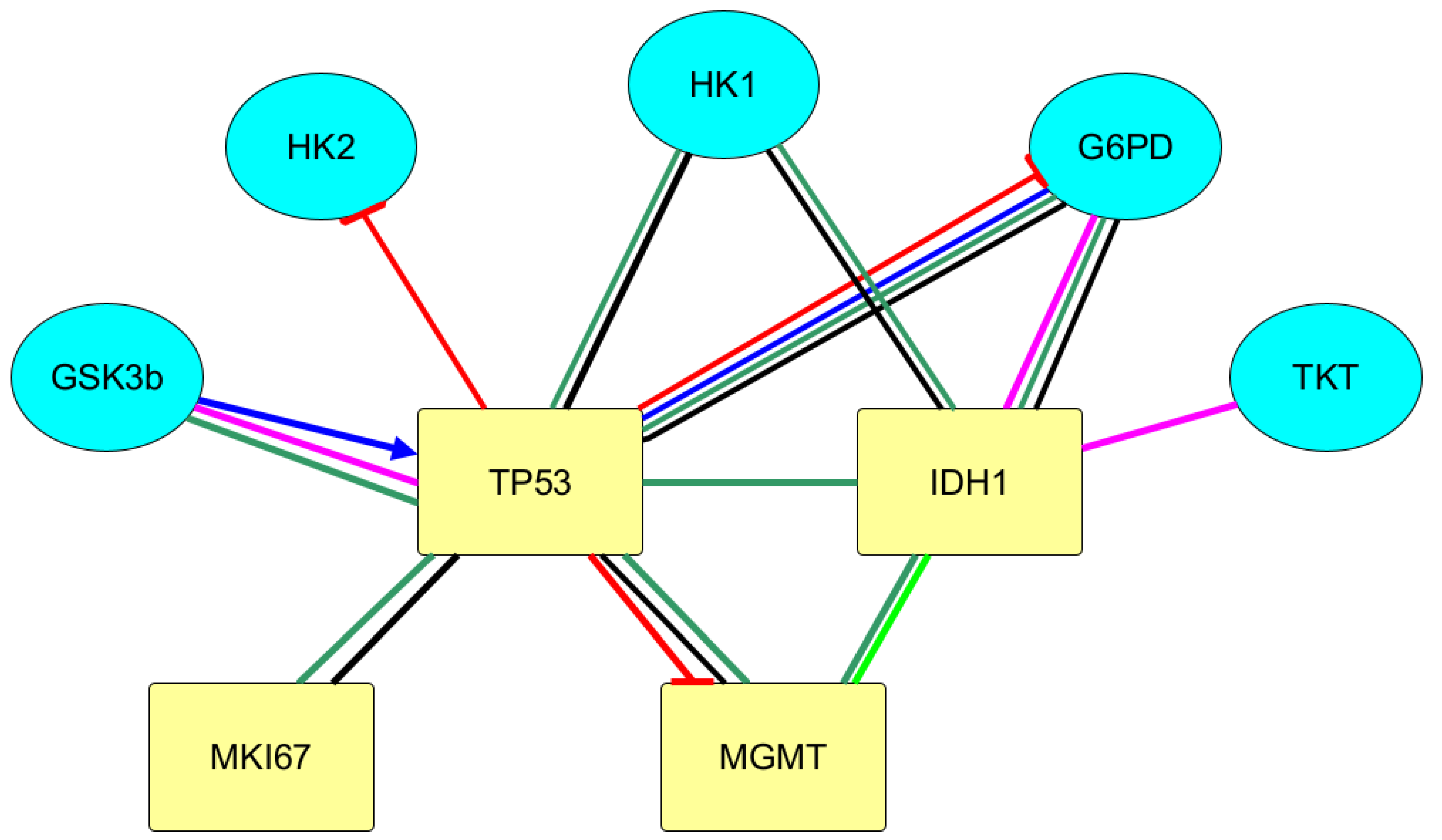

| Grade | I | II | III | IV |
|---|---|---|---|---|
| Number of patients in group | 1 | 6 | 3 | 10 |
| IDH1—% of mutation presence | 0% | 100% | 100% | 30% |
| MGMT—% of methylation found | 100% | 83% | 100% | 30% |
| p53—% of presence | 0% | 66% | 0% | 80% |
| Ki-67 high (more than 10%)—% of presence | 0% | 0% | 100% | 100% |
| Ki-67 low (less than 10%)—% of presence | 100% | 100% | 0% | 0% |
| Median Ki-67 | - | 2% | 12% | 37% |
| Interquartile range of Ki-67 (25–75) | - | 1.5–3% | - | 25–40% |
| The Adjacent Noncancerous Tissues (Median; Quartiles) | Peritumoral Zone | Tumor | |||
|---|---|---|---|---|---|
| Median; Quartiles | U-Criterion of Mann–Whitney; Kolmogorov–Smirnov Criterion | Median; Quartiles | U-Criterion of Mann–Whitney; Kolmogorov–Smirnov Criterion | ||
| Glucose, mmol/g of protein | 94.08 (44.36–164.84) | 56.01 (38.53–163.89) | 0.9181; 0.9738 | 47.81 (24.10–101.46) | 0.1195; 0.1359 |
| Lactate, mmol/g of protein | 2.07 (1.02–4.43) | 1.56 (0.36–4.67) | 0.7576; 0.7502 | 0.49 * (0.15–1.53) | 0.0942; 0.0299 |
| Hexokinase, milliunit/mL/g of protein | 6.21 (5.84–54.90) | 8.93 (3.24–38.10) | 0.8370; 0.5888 | 12.56 (1.63–37.35) | 0.5463; 0.4360 |
| Glucose-6-phosphate dehydrogenase, U/L/g of protein | 4.59 (1.67–16.44) | 11.76 (2.87–29.19) | 0.3036; 0.7503 | 10.78 (2.88–28.44) | 0.6128; 0.9737 |
| Transketolase, µg/g of protein | 266.44 (45.92–549.63) | 237.08 (87.74–480.29) | 0.9590; 0.7503 | 145.37 (57.83–366.79) | 0.3305; 0.3684 |
| Glycogen synthase kinase-3β, pg/mL/g of protein | 998.17 (513.51–1944.6) | 918.91 (725.92–1218.97) | 0.7576; 0.8011 | 347.57 * (211.10–745.30) | 0.0323; 0.0091 |
| Parameter of Carbohydrate Metabolism | Markers of Gliomas | ||||
|---|---|---|---|---|---|
| IDH | Ki-67 | MGMT | p53 | ||
| Glucose Rho (p) | Adjacent noncancerous tissue | −0.194 (0.568) | 0.234 (0.544) | −0.504 (0.203) | 0.109 (0.797) |
| Peritumoral zone | −0.211 (0.488) | 0.012 (0.973) | −0.142 (0.695) | 0.0001(0.993) | |
| Tumor tissue | 0.073 (0.780) | −0.558 * (0.031) | 0.163 (0.595) | 0.116 (0.735) | |
| Lactate Rho (p) | Adjacent noncancerous tissue | 0.065 (0.851) | −0.042 (0.915) | −0.630 * (0.044) | 0.109 (0.797) |
| Peritumoral zone | 0.127 (0.680) | −0.055 (0.881) | 0.0001 (1) | 0.109 (0.797) | |
| Tumor tissue | 0.122 (0.641) | −0.189 (0.501) | −0.096 (0.756) | 0.346 (0.297) | |
| Glycogen synthase kinase-3β Rho (p) | Adjacent noncancerous tissue | 0.194 (0.568) | −0.201 (0.604) | −0.630 * (0.044) | 0.218 (0.604) |
| Peritumoral zone | 0.296 (0.327) | −0.407 (0.243) | 0.071 (0.845) | 0.218 (0.604) | |
| Tumor tissue | 0.024 (0.926) | −0.417 (0.123) | 0.067 (0.828) | −0.116 (0.735) | |
| Glucose-6-phosphate dehydrogenase Rho (p) | Adjacent noncancerous tissue | −0.194 (0.568) | −0.167 (0.667) | 0.0001 (0.974) | −0.546 (0.162) |
| Peritumoral zone | −0.127 (0.680) | 0.480 (0.160) | −0.497 (0.144) | 0.109 (0.797) | |
| Tumor tissue | −0.366 (0.149) | 0.294 (0.287) | −0.278 (0.359) | −0.635 (0.036) * | |
| Hexokinase Rho (p) | Adjacent noncancerous tissue | −0.323 (0.333) | 0.209 (0.589) | −0.474 (0.282) | 0.0001(0.968) |
| Peritumoral zone | −0.085 (0.784) | 0.219 (0.544) | −0.693 (0.039) * | −0.109 (0.797) | |
| Tumor tissue | −0.293 (0.254) | 0.058 (0.866) | 0.056 (0.863) | 0.058 (0.866) | |
| Transketolase Rho (p) | Adjacent noncancerous tissue | 0.281 (0.443) | −0.2845 (0.458) | 0.0001 (0.946) | 0.109 (0.797) |
| Peritumoral zone | 0.507 (0.077) | −0.164 (0.651) | −0.142 (0.695) | 0.218 (0.604) | |
| Tumor tissue | 0.268 (0.298) | −0.368 (0.177) | −0.057 (0.852) | −0.404 (0.218) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obukhova, L.; Nikiforova, O.; Kontorshchikova, C.; Medyanik, I.; Orlinskaya, N.; Grishin, A.; Kontorshchikov, M.; Shchelchkova, N. Carbohydrate Metabolism Parameters of Adult Glial Neoplasms According to Immunohistochemical Profile. Biomedicines 2022, 10, 1007. https://doi.org/10.3390/biomedicines10051007
Obukhova L, Nikiforova O, Kontorshchikova C, Medyanik I, Orlinskaya N, Grishin A, Kontorshchikov M, Shchelchkova N. Carbohydrate Metabolism Parameters of Adult Glial Neoplasms According to Immunohistochemical Profile. Biomedicines. 2022; 10(5):1007. https://doi.org/10.3390/biomedicines10051007
Chicago/Turabian StyleObukhova, Larisa, Olga Nikiforova, Claudia Kontorshchikova, Igor Medyanik, Natalya Orlinskaya, Artem Grishin, Michael Kontorshchikov, and Natalya Shchelchkova. 2022. "Carbohydrate Metabolism Parameters of Adult Glial Neoplasms According to Immunohistochemical Profile" Biomedicines 10, no. 5: 1007. https://doi.org/10.3390/biomedicines10051007






