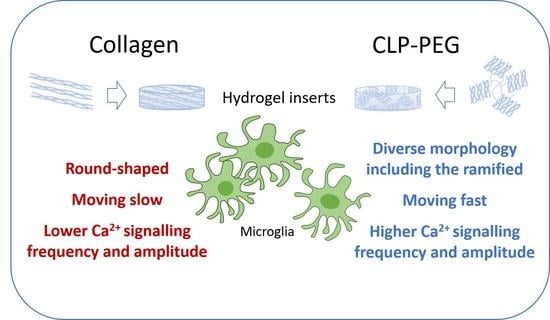
Comparison of Microglial Morphology and Function in Primary Cerebellar Cell Cultures on Collagen and Collagen-Mimetic Hydrogels
Abstract
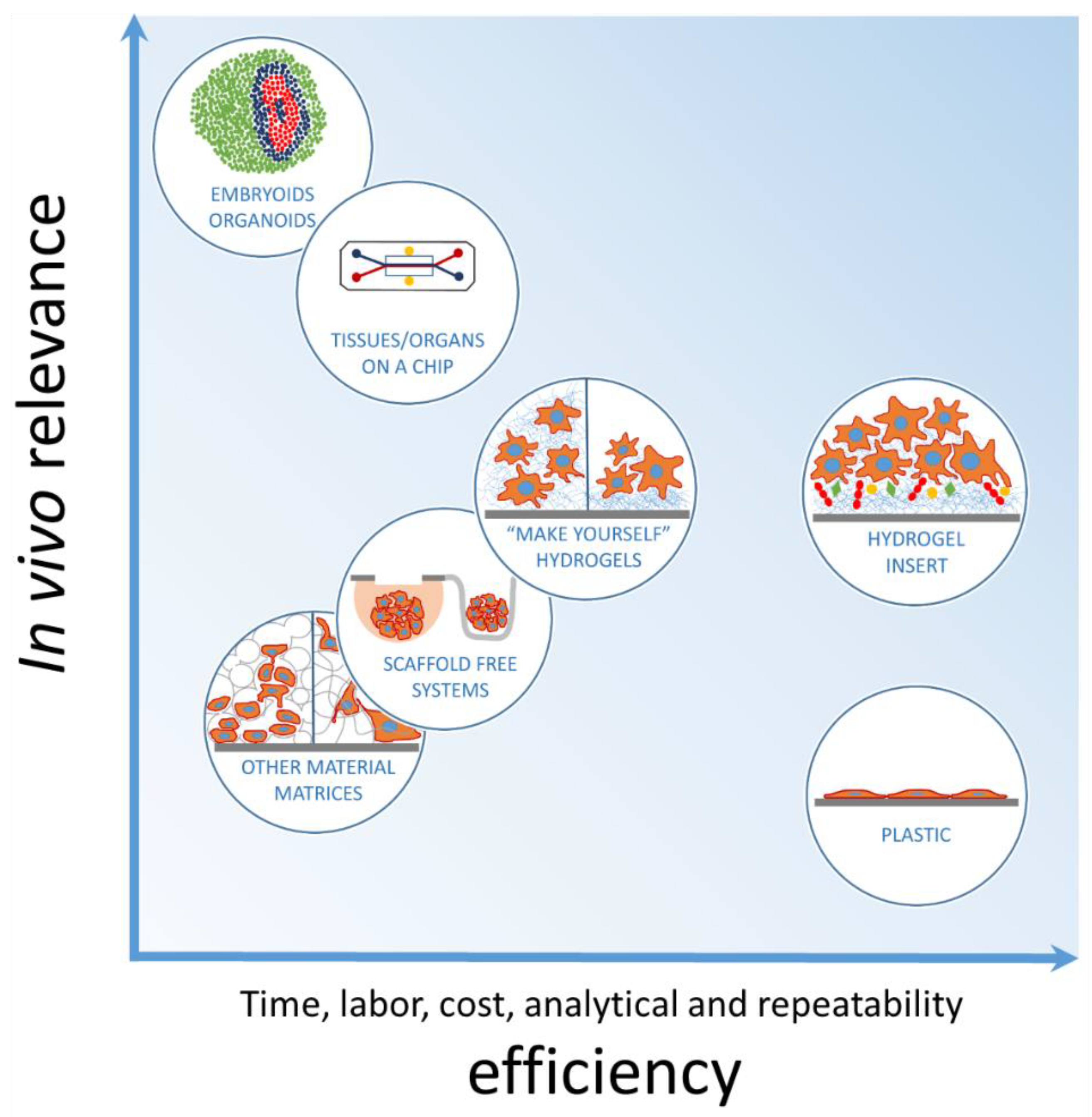
:1. Introduction
2. Materials and Methods
2.1. Hydrogel Synthesis
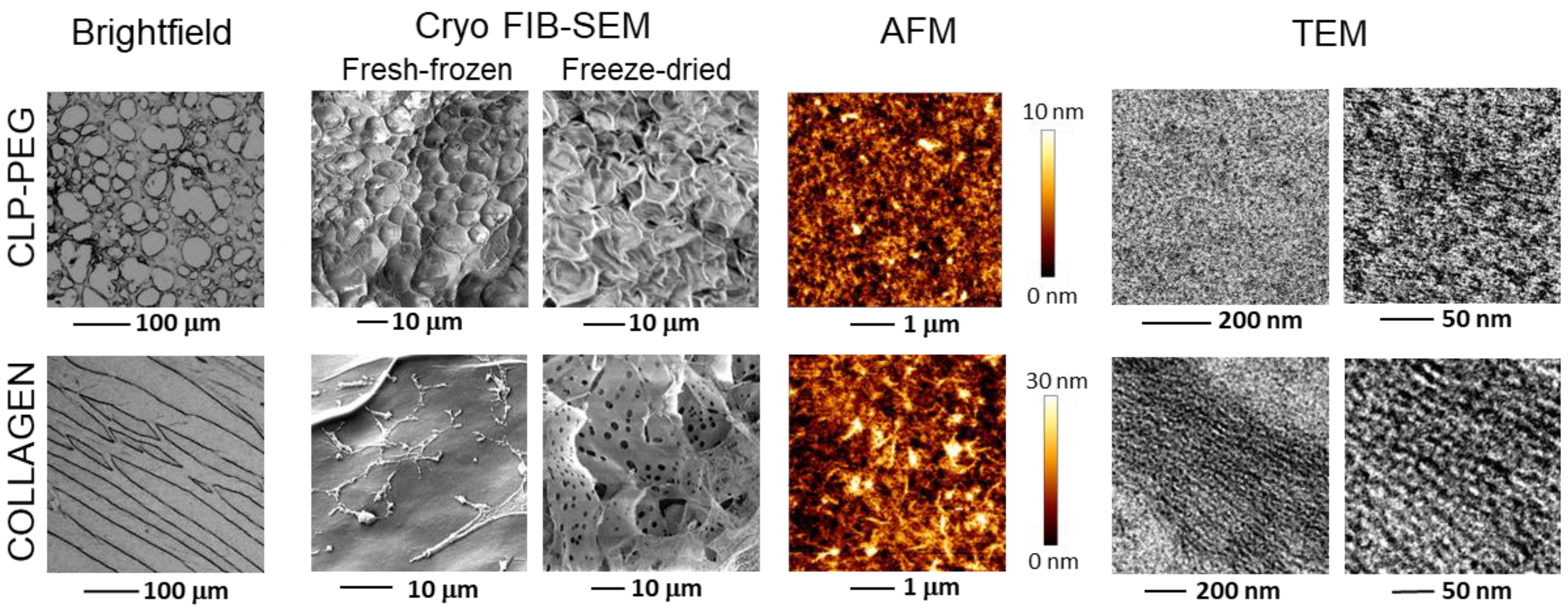
2.2. Visualization of Hydrogel Microslices
2.3. Transmission Electron Microscopy
2.4. Focused Ion Beam Scanning Electron Microscopy
2.5. Atomic Force Microscopy
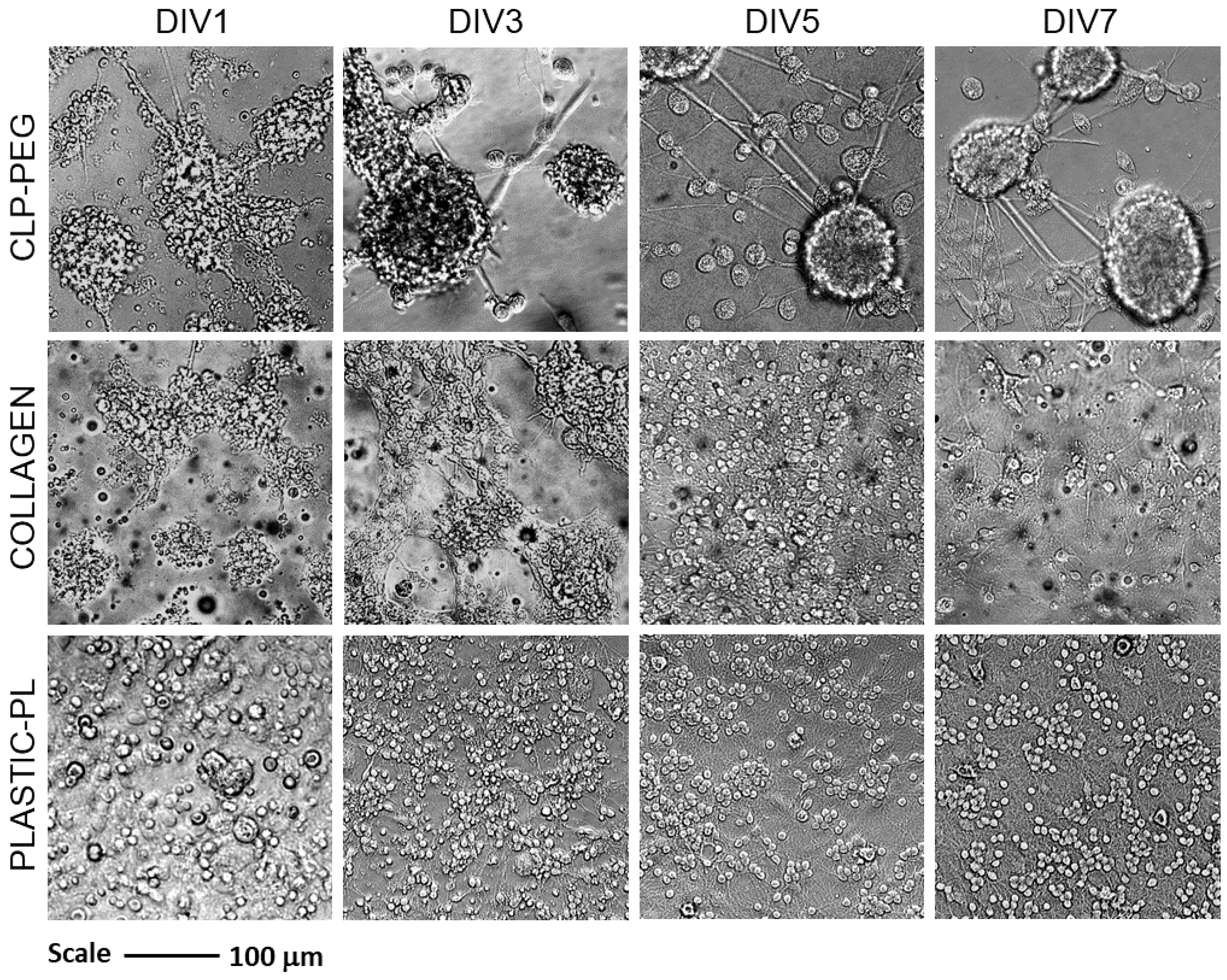
2.6. Neuronal-Glial Cell Culture
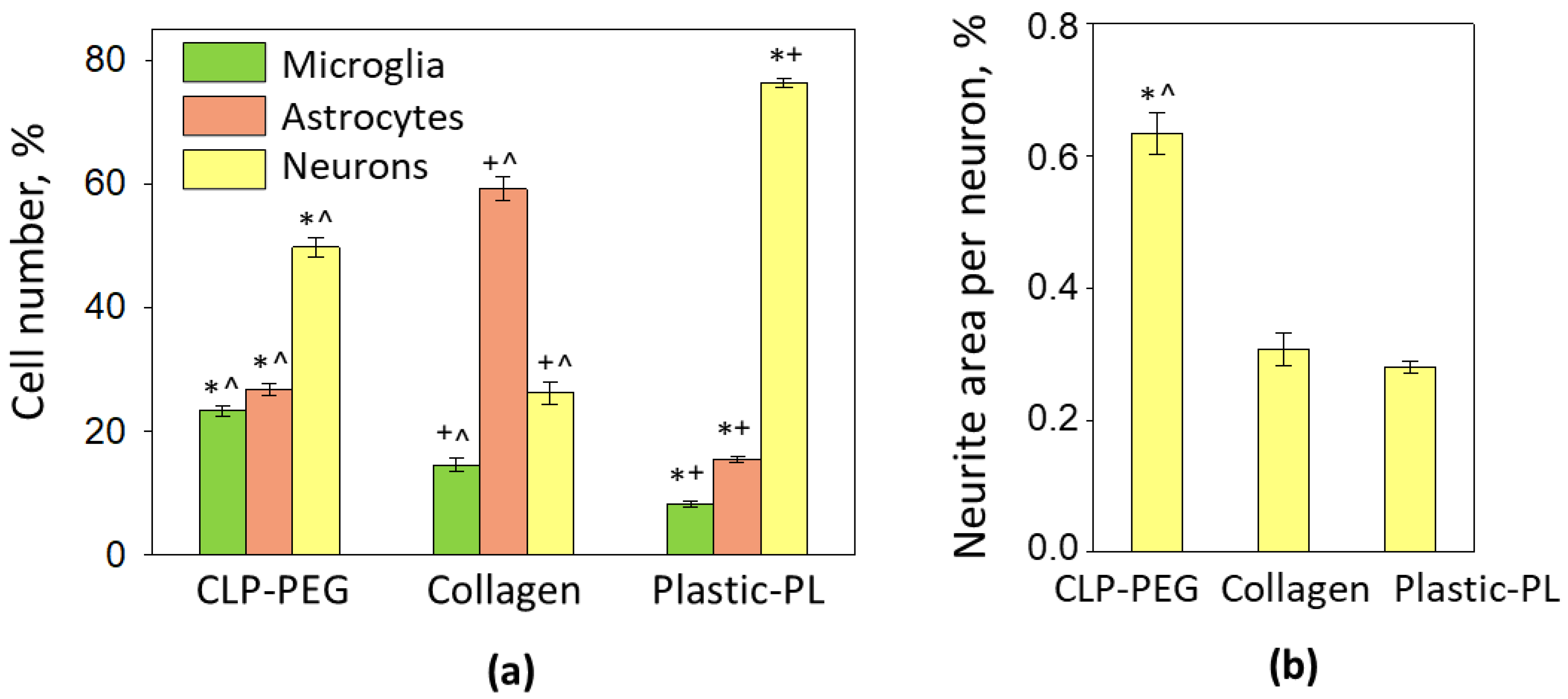
2.7. Immunostaining of Primary Cerebellar Cells, Cell Counting and Neurite Assessment
2.8. Evaluation of Microglial Motility
2.9. Ca2+-Signalling Recording
2.10. Statistical Analysis
3. Results
3.1. Comparison of Micro and Nanostructure of CLP-PEG and Collagen Hydrogels
3.2. The Effect of CLP-PEG and Collagen Hydrogel on Cerebellar Cell Culture Formation
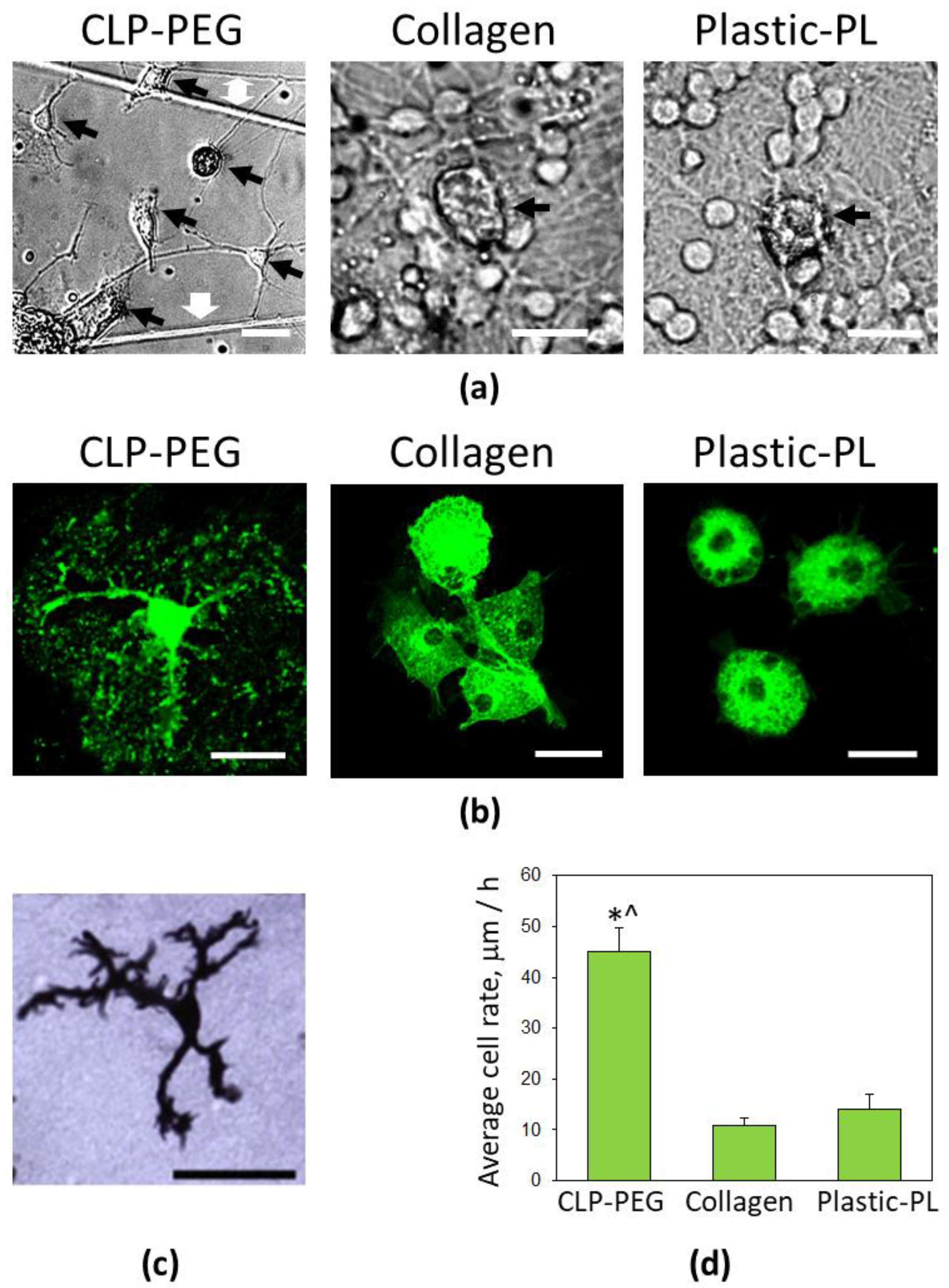
3.3. Comparison of Microglial Morphology and Motility on CLP-PEG and Collagen Hydrogels
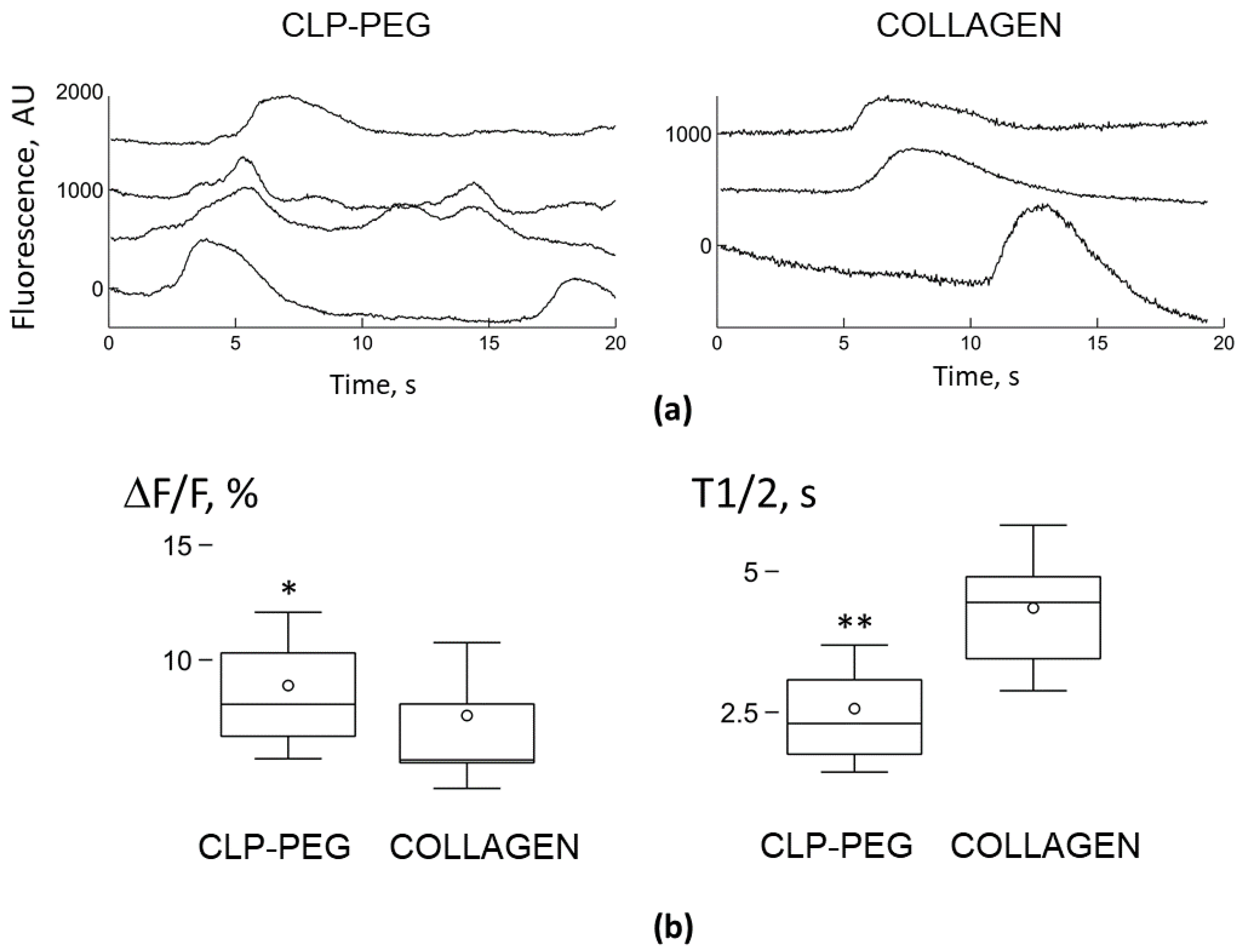
3.4. Comparison of Microglial Ca2+ Signalling on CLP-PEG and Collagen Hydrogels
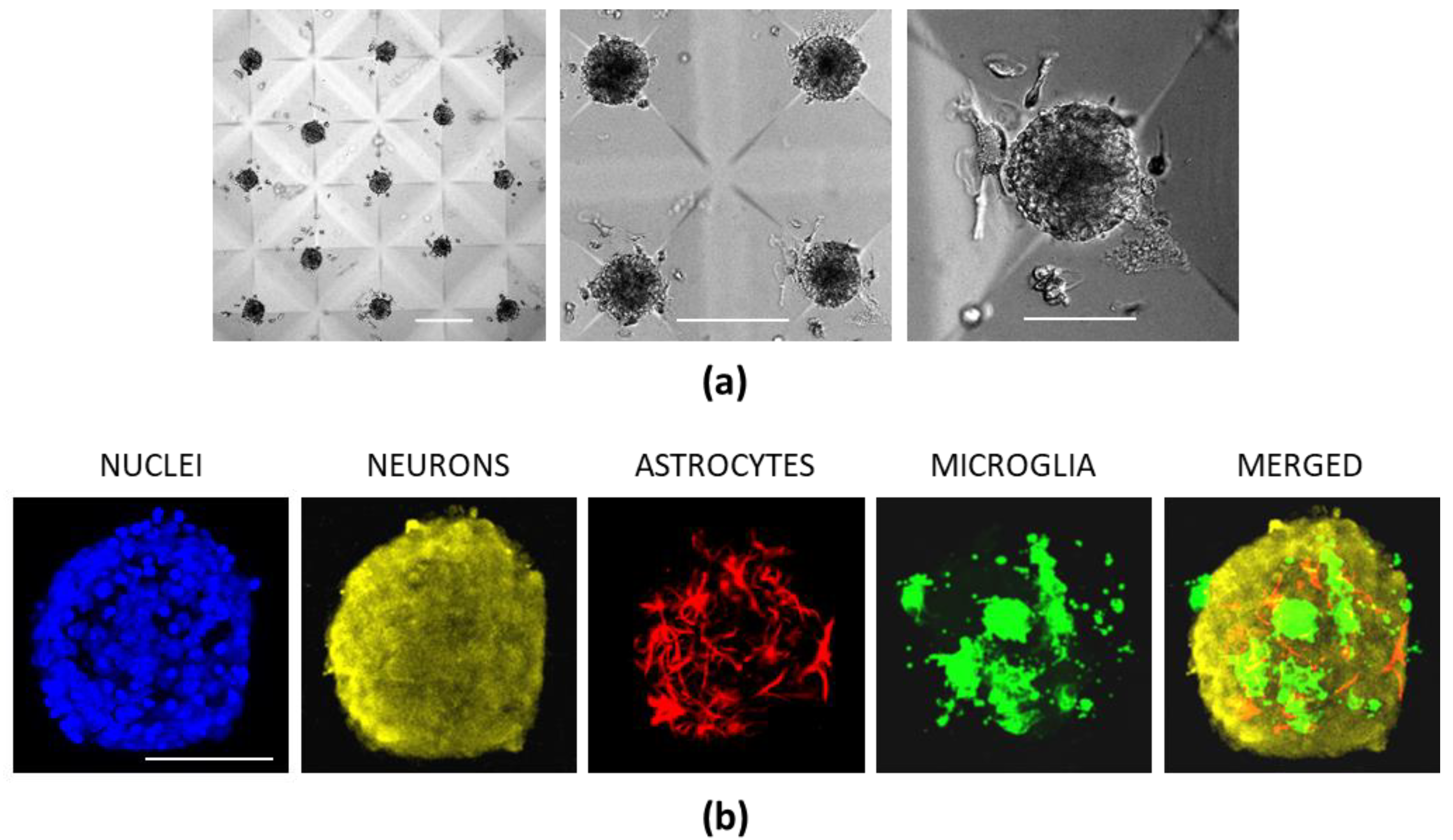
3.5. Cerebellar Cell Cultures in CLP-PEG Hydrogel Microwells
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, J.; Amini, S.; White, M.K. General overview of neuronal cell culture. Methods Mol. Biol. 2014, 1–6. [Google Scholar] [CrossRef]
- Yamasaki, R. Microglia in vivo and in vitro. Clin. Exp. Neuroimmunol. 2014, 5, 114–116. [Google Scholar] [CrossRef]
- Tremblay, M.-È.; Stevens, B.; Sierra, A.; Wake, H.; Bessis, A.; Nimmerjahn, A. The role of microglia in the healthy brain. J. Neurosci. 2011, 31, 16064–16069. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Ravichandran, R.; Olsen, D.; Ljunggren, M.K.; Fagerholm, P.; Lee, C.J.; Griffith, M.; Phopase, J. Self-assembled collagen-like-peptide implants as alternatives to human donor corneal transplantation. RSC Adv. 2016, 6, 55745–55749. [Google Scholar] [CrossRef] [Green Version]
- Balion, Z.; Cėpla, V.; Svirskiene, N.; Svirskis, G.; Druceikaitė, K.; Inokaitis, H.; Rusteikaitė, J.; Masilionis, I.; Stankevičienė, G.; Jelinskas, T.; et al. Cerebellar Cells Self-Assemble into Functional Organoids on Synthetic, Chemically Crosslinked ECM-Mimicking Peptide Hydrogels. Biomolecules 2020, 10, 754. [Google Scholar] [CrossRef]
- Helmut, K.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Färber, K.; Kettenmann, H. Functional role of calcium signals for microglial function. Glia 2006, 54, 656–665. [Google Scholar] [CrossRef]
- Sogkas, G.; Stegner, D.; Syed, S.N.; Vogtle, T.; Rau, E.; Gewecke, B.; Schmidt, R.E.; Nieswandt, B.; Engelbert Gessner, J. Cooperative and alternate functions for STIM1 and STIM2 in macrophage activation and in the context of inflammation. Immun. Inflamm. Dis. 2015, 3, 154–170. [Google Scholar] [CrossRef] [Green Version]
- Toulme, E.; Khakh, B.S. Imaging P2 × 4 receptor lateral mobility in microglia: Regulation by calcium and p38 MAPK. J. Biol. Chem. 2012, 287, 14734–14748. [Google Scholar] [CrossRef] [Green Version]
- Eichhoff, G.; Brawek, B.; Garaschuk, O. Microglial calcium signal acts as a rapid sensor of single neuron damage in vivo. Biochim. Biophys. Acta 2011, 1813, 1014–1024. [Google Scholar] [CrossRef] [Green Version]
- Brawek, B.; Garaschuk, O. Monitoring in vivo function of cortical microglia. Cell Calcium 2017, 64, 109–117. [Google Scholar] [CrossRef]
- Pozner, A.; Xu, B.; Palumbos, S.; Gee, J.M.; Tvrdik, P.; Capecchi, M.R. Intracellular calcium dynamics in cortical microglia responding to focal laser injury in the PC::G5-tdT reporter mouse. Front. Mol. Neurosci. 2015, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Del Moral, M.O.; Asavapanumas, N.; Uzcátegui, N.L.; Garaschuk, O. Healthy Brain Aging Modifies Microglial Calcium Signaling In Vivo. Int. J. Mol. Sci. 2019, 20, 589. [Google Scholar] [CrossRef] [Green Version]
- Umpierre, A.D.; Bystrom, L.L.; Ying, Y.; Liu, Y.U.; Worrell, G.; Wu, L.J. Microglial calcium signaling is attuned to neuronal activity in awake mice. eLife 2020, 9, e56502. [Google Scholar] [CrossRef]
- Wurm, J.; Konttinen, H.; Andressen, C.; Malm, T.; Spittau, B. Microglia Development and Maturation and Its Implications for Induction of Microglia-Like Cells from Human iPSCs. Int. J. Mol. Sci. 2021, 22, 3088. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, S.; Feinberg, P.A.; Johnson, K.M.; Schafer, D.P. A microglia-cytokine axis to modulate synaptic connectivity and function. Curr. Opin. Neurobiol. 2017, 47, 138–145. [Google Scholar] [CrossRef]
- Badimon, A.; Strasburger, H.J.; Ayata, P.; Chen, X.; Nair, A.; Ikegami, A.; Hwang, P.; Chan, A.T.; Graves, S.M.; Uweru, J.O.; et al. Negative feedback control of neuronal activity by microglia. Nature 2020, 586, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Balion, Z.; Sipailaite, E.; Stasyte, G.; Vailionyte, A.; Mazetyte-Godiene, A.; Seskeviciute, I.; Bernotiene, R.; Phopase, J.; Jekabsone, A. Investigation of Cancer Cell Migration and Proliferation on Synthetic Extracellular Matrix Peptide Hydrogels. Front. Bioeng. Biotechnol. 2020, 8, 773. [Google Scholar] [CrossRef]
- Liu, Y.; Gan, L.; Carlsson, D.J.; Fagerholm, P.; Lagali, N.; Watsky, M.A.; Munger, R.; Hodge, W.G.; Priest, D.; Griffith, M. A Simple, Cross-linked Collagen Tissue Substitute for Corneal Implantation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1869–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinevez, J.-Y.; Perry, N.; Schindelin, J.; Hoopes, G.M.; Reynolds, G.D.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.L.; Eliceiri, K.W. TrackMate: An open and extensible platform for single-particle tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef]
- Islam, M.M.; Cepla, V.; He, C.; Edin, J.; Rakickas, T.; Kobuch, K.; Ružele, Ž.; Jackson, W.B.; Rafat, M.; Lohmann, C.P.; et al. Functional fabrication of recombinant human collagen–phosphorylcholine hydrogels for regenerative medicine applications. Acta Biomater. 2015, 12, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Perez-Pouchoulen, M.; VanRyzin, J.W.; McCarthy, M.M. Morphological and Phagocytic Profile of Microglia in the Developing Rat Cerebellum. eNeuro 2015, 2, 36–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korvers, L.; de Andrade Costa, A.; Mersch, M.; Matyash, V.; Kettenmann, H.; Semtner, M. Spontaneous Ca2+ transients in mouse microglia. Cell Calcium 2016, 60, 396–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankeviciute, S.; Svirskiene, N.; Svirskis, G.; Borutaite, V. Effects of Metformin on Spontaneous Ca2+ Signals in Cultured Microglia Cells under Normoxic and Hypoxic Conditions. Int. J. Mol. Sci. 2021, 22, 9493. [Google Scholar] [CrossRef]
- Zhang, T.; Sanguramath, R.A.; Israel, S.; Silverstein, M.S. Emulsion Templating: Porous Polymers and Beyond. Macromolecules 2019, 52, 5445–5479. [Google Scholar] [CrossRef] [Green Version]
- Tang-Schomer, M.D.; White, J.D.; Tien, L.W.; Schmitt, L.I.; Valentin, T.M.; Graziano, D.J.; Hopkins, A.M.; Omenetto, F.G.; Haydon, P.G.; Kaplan, D.L. Bioengineered functional brain-like cortical tissue. Proc. Natl. Acad. Sci. USA 2014, 111, 13811–13816. [Google Scholar] [CrossRef] [Green Version]
- Muguruma, K.; Nishiyama, A.; Kawakami, H.; Hashimoto, K.; Sasai, Y. Self-Organization of Polarized Cerebellar Tissue in 3D Culture of Human Pluripotent Stem Cells. Cell Rep. 2015, 10, 537–550. [Google Scholar] [CrossRef] [Green Version]
- Hattori, N. Cerebral organoids model human brain development and microcephaly. Mov. Disord. 2014, 29, 185. [Google Scholar] [CrossRef] [PubMed]
- Sgodda, M.; Dai, Z.; Zweigerdt, R.; Sharma, A.D.; Ott, M.; Cantz, T. A Scalable Approach for the Generation of Human Pluripotent Stem Cell-Derived Hepatic Organoids with Sensitive Hepatotoxicity Features. Stem Cells Dev. 2017, 26, 1490–1504. [Google Scholar] [CrossRef]
- Thompson, W.L.; Takebe, T. Generation of multi-cellular human liver organoids from pluripotent stem cells. Methods Cell Biol. 2020, 159, 47–68. [Google Scholar] [CrossRef]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front. Cell. Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinet, J.; Weering, H.R.; Heinrich, A.; Kalin, R.E.; Wegner, A.; Brouwer, N.; Heppner, F.L.; Rooijen, N.; Boddeke, H.W.; Biber, K. Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J. Neuroinflamm. 2012, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Xavier, A.L.; Menezes, J.R.L.; Goldman, S.A.; Nedergaard, M. Fine-tuning the central nervous system: Microglial modelling of cells and synapses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampe, K.J.; Antaris, A.L.; Heilshorn, S.C. Design of three-dimensional engineered protein hydrogels for tailored control of neurite growth. Acta Biomater. 2013, 9, 5590–5599. [Google Scholar] [CrossRef] [Green Version]
- Dalmau, I.; Vela, J.M.; González, B.; Finsen, B.; Castellano, B. Dynamics of microglia in the developing rat brain. J. Comp. Neurol. 2003, 458, 144–157. [Google Scholar] [CrossRef]
- Giulian, D.; Young, D.G.; Woodward, J.; Brown, D.C.; Lachman, L.B. Interleukin-1 is an astroglial growth factor in the developing brain. J. Neurosci. 1988, 8, 709–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeles Muñoz-Fernández, M.; Fresno, M. The role of tumour necrosis factor, interleukin 6, interferon-γ and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog. Neurobiol. 1998, 56, 307–340. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nugent, B.M.; Haliyur, R.; McCarthy, M.M. Microglia Are Essential to Masculinisation of Brain and Behavior. J. Neurosci. 2013, 33, 2761. [Google Scholar] [CrossRef] [Green Version]
- Tam, W.Y.; Ma, C.H.E. Bipolar/rod-shaped microglia are proliferating microglia with distinct M1/M2 phenotypes. Sci. Rep. 2014, 4, 7279. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Knight, C.G.; Morton, L.F.; Peachey, A.R.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. The Collagen-binding A-domains of Integrins α1β1 and α2β1Recognize the Same Specific Amino Acid Sequence, GFOGER, in Native (Triple-helical) Collagens. J. Biol. Chem. 2000, 275, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.A.; Pappas, I.S.; Parnavelas, J.G. Collagen type IV promotes the differentiation of neuronal progenitors and inhibits astroglial differentiation in cortical cell cultures. Brain Res. Dev. Brain Res. 1998, 110, 31–38. [Google Scholar] [CrossRef]
- Wang, W.; Fang, H.; Groom, L.; Cheng, A.; Zhang, W.; Liu, J.; Wang, X.; Li, K.; Han, P.; Zheng, M.; et al. Superoxide flashes in single mitochondria. Cell 2008, 134, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, X.; Wu, D.; Huang, Z.; Hou, T.; Jian, C.; Yu, P.; Lu, F.; Zhang, R.; Sun, T.; et al. Mitochondrial flashes regulate ATP homeostasis in the heart. eLife 2017, 6, e23908. [Google Scholar] [CrossRef] [PubMed]
- Haagdorens, M.; Cėpla, V.; Melsbach, E.; Koivusalo, L.; Skottman, H.; Griffith, M.; Valiokas, R.; Zakaria, N.; Pintelon, I.; Tassignon, M.J. In vitro cultivation of limbal epithelial stem cells on surface-modified crosslinked collagen scaffolds. Stem Cells Int. 2019, 2019, 7867613. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balion, Z.; Svirskienė, N.; Svirskis, G.; Inokaitis, H.; Cėpla, V.; Ulčinas, A.; Jelinskas, T.; Eimont, R.; Paužienė, N.; Valiokas, R.; et al. Comparison of Microglial Morphology and Function in Primary Cerebellar Cell Cultures on Collagen and Collagen-Mimetic Hydrogels. Biomedicines 2022, 10, 1023. https://doi.org/10.3390/biomedicines10051023
Balion Z, Svirskienė N, Svirskis G, Inokaitis H, Cėpla V, Ulčinas A, Jelinskas T, Eimont R, Paužienė N, Valiokas R, et al. Comparison of Microglial Morphology and Function in Primary Cerebellar Cell Cultures on Collagen and Collagen-Mimetic Hydrogels. Biomedicines. 2022; 10(5):1023. https://doi.org/10.3390/biomedicines10051023
Chicago/Turabian StyleBalion, Zbigniev, Nataša Svirskienė, Gytis Svirskis, Hermanas Inokaitis, Vytautas Cėpla, Artūras Ulčinas, Tadas Jelinskas, Romuald Eimont, Neringa Paužienė, Ramūnas Valiokas, and et al. 2022. "Comparison of Microglial Morphology and Function in Primary Cerebellar Cell Cultures on Collagen and Collagen-Mimetic Hydrogels" Biomedicines 10, no. 5: 1023. https://doi.org/10.3390/biomedicines10051023
APA StyleBalion, Z., Svirskienė, N., Svirskis, G., Inokaitis, H., Cėpla, V., Ulčinas, A., Jelinskas, T., Eimont, R., Paužienė, N., Valiokas, R., & Jekabsone, A. (2022). Comparison of Microglial Morphology and Function in Primary Cerebellar Cell Cultures on Collagen and Collagen-Mimetic Hydrogels. Biomedicines, 10(5), 1023. https://doi.org/10.3390/biomedicines10051023